Feasibility of Intraoperative Normothermic Intraperitoneal Chemotherapy Following Optimal Cytoreduction in Advanced Ovarian Carcinoma: A Pilot Study
Download
Abstract
Introduction: Based on the results of studies using HIPEC, EPIC, and normothermic intraperitoneal chemotherapy, we hypothesized that the instillation of normothermic chemotherapy after optimal cytoreduction would improve survival in patients undergoing this procedure. To test this hypothesis, we conducted a feasibility study to evaluate the effectiveness of intraoperative normothermic intraperitoneal chemotherapy in patients with advanced-stage epithelial ovarian carcinoma.
Methods: This single institutional feasibility study aimed to assess the primary objective of progression-free survival (PFS) following the instillation of normothermic chemotherapy after optimal cytoreduction in cases of advanced ovarian cancer following neoadjuvant chemotherapy. The secondary objective was to evaluate tolerability and toxicity. The study received clearance from the institutional ethical committee.
Results: A total of 24 patients were included in the pilot study, which spanned two years. Short-term analysis was conducted by comparing these patients with a group of 45 individuals who underwent interval cytoreductive surgery (ICS) with CC 0 and 1 during the same period. The most common side effect observed was prolonged post-operative ileus, which occurred in six patients. The median PFS among patients who received intraperitoneal (IP) chemotherapy was significantly longer compared to patients who received ICS alone (34.0 vs. 13.0 months, p=0.018).
Conclusion: Due to global resource limitations, the implementation of uniform state- of-the-art management for advanced epithelial ovarian cancer may not be accessible to all patients. Although the evidence is limited by the small sample size and short follow-up duration, the findings of this feasibility study are encouraging. The study provides substantial evidence to support further exploration of this approach within our institute and to plan a randomized controlled trial to gather more conclusive evidence.
Introduction
Epithelial carcinoma of the ovary primarily presents at an advanced stage, and most women recur within 1-2 years [1]. Optimal debulking surgery as interval cytoreductive surgery (ICS) or primary cytoreductive surgery (PCS) is the cornerstone in ensuring long term progression-free survival (PFS) in these patients. Many studies have shown ICS and PCS to have similar overall survival (OS) [2-4]. When compared to other chemotherapeutic medications, platinum-based chemotherapy has shown the greatest tumour regression when either cisplatin or carboplatin is combined with paclitaxel [5]. However, despite good response after optimal cytoreduction and chemotherapy, most patients recur, which signifies the inherent unfavourable nature of the advanced disease [6]. Various studies were undertaken to increase the PFS and OS of such patients. Studies conducted using Intraperitoneal Chemotherapy (IP) showed better survival than systemic chemotherapy alone [7-9]. The rationale for using IP chemotherapy has been based on the feasibility of IP instillation and the preponderance for intraperitoneal localization even in advanced stages, which makes it possible to bathe tumour cells with chemotherapy directly [10]. However, the benefit is only seen when the tumour has been optimally debulked, as IP chemotherapy does not diffuse in large bulky tumour nodules. Heated Intraperitoneal chemotherapy given immediately after surgery has been introduced and has shown to be of benefit both in the setting of PCS and ICS [11, 12]. Hyperthermia has been postulated to increase penetration of chemotherapy and sensitivity to chemotherapy by impairing DNA repair [13]. Intraoperative intraperitoneal chemotherapy has also been proposed to kill the floating tumour cells and prevent them from getting entrapped in the resection sites [14]. However, HIPEC is not freely available in most low-resource setting centres due to its high cost and requirement of specific types of equipment. Normothermic intraperitoneal chemotherapy (NIPEC) has also proven beneficial as adjuvant chemotherapy and when introduced in the early post-operative period [15-17]. Early postoperative intraperitoneal chemotherapy (EPIC) has certain conceptual benefits, as it is applied shortly after cytoreductive surgery when the tumor burden is minimal. Further-more, drug distribution is reduced as the adhesions are not formed and there is prevention of entrapment of residual cancer cells in postoperative fibrin deposits. EPIC does not entail hyperthermia and is administered in the postoperative period, usually on days 1 to 5, through an inflow catheter and outflow drains inserted during CRS. This approach can be employed with or without HIPEC [18]. Nevertheless, the drawbacks associated with EPIC, such as increased infection risks and postoperative complications, including catheter-related issues, have resulted in its diminished popularity compared to HIPEC [19].
Extrapolating the results from the studies using HIPEC and EPIC, we proposed that normothermic chemotherapy instilled after optimal cytoreduction will improve survival in patients who underwent optimal cytoreduction. We undertook this feasibility study with the aim of studying the effectiveness of modified EPIC using normothermic chemotherapy in patients with advanced-stage epithelial carcinoma ovary.
Materials and Methods
Methodology and study design
This was a single institutional feasibility study to evaluate the activity and tolerability of intraoperative normothermic intraperitoneal chemotherapy instilled as a modified EPIC regimen after optimal cytoreduction in advanced EOC after neoadjuvant chemotherapy. The study received clearance from the Institutional Ethical Committee. Recruitment of patients was done after ethical committee clearance. Patients who fulfilled the criteria were given patient information sheet and consent forms at the time of assessment for ICS, which was approximately 2-3 weeks before surgery.
The primary objective was to study the short-term efficacy by estimating the progression-free survival (PFS) following the instillation of normothermic chemotherapy following optimal cytoreduction in cases of advanced ovarian cancer after neoadjuvant chemotherapy.
The secondary objective was to study tolerability and toxicity.
Patients with advanced Ca Ovary (stage IIIC disease) were included in the study. A total of 24 patients were included in the study. Informed consent was taken from every patient. After optimal cytoreduction (defined by Cytoreductive scores 0 and 1) cisplatin (75 mg/m²) was instilled intraperitoneally with the help of intra-abdominal drains placed in Morrison’s pouch and pelvis. Drains were unclamped after 24 hours for any unabsorbed fluid to be removed. Preloading with potassium chloride and magnesium sulphate was done. Adequate hydration was maintained. Antiemetics were given for control of emesis associated with cisplatin. Monitoring and correcting dyselectrolytemia were done on the day of surgery for up to 3 days. Adverse effects were monitored and recorded per Common Terminology Criteria for Adverse Events (CTCAE) version 5 [20].
Eligibility criteria
Inclusion Criteria:
1. Patients with advanced (stage IIIC) epithelial carcinoma ovary, fallopian tube carcinoma or primary peritoneal cancer who received neoadjuvant chemotherapy.
2. Age: 20-70 years.
3. World Health Organization performance status 0-2.
4. Adequate organ function with the following criteria: White blood cell (WBC) ≥4,000/ul; Absolute neutrophil count (ANC) ≥1,500/ul; Platelet ≥100×103/ul; Aspartate aminotransferase (AST) ≤100 IU/L; Alanine aminotransferase (ALT) ≤100 IU/L; Serum total bilirubin ≤1.5 mg/dL; Creatinine clearance ≥ 60 mL/ min.
Exclusion Criteria:
1. Suboptimal debulking
2. Failure to give consent
3. Pre-existing renal disease
4. Benign ovarian disease, borderline ovarian malignancy, non-epithelial ovarian carcinoma or carcinosarcoma
5. Cirrhosis of liver
6. Known hypersensitivity to any of the study drugs, study drug classes, or excipients in the formulation
7. Auditory impairment
8. Dehydration or intercurrent disease that contraindicates hyperhydration (including cardio-respiratory disease)
9. Other uncontrolled intercurrent diseases: diabetes; hypertension; symptomatic congestive heart or pulmonary failure; renal, hepatic or severe gastrointestinal (associated with diarrhoea) chronic disease
10. Any unresolved NCI-CTCAE Grade ≥ 2 toxicities from previous anticancer therapy (excluding alopecia). Being a feasibility study, the results were analyzed for
PFS at the end of one year of recruitment of the patients (Median followup 22 months). They were compared with patients who underwent interval cytoreductive surgery (ICS) as standard of care for stage IIIC epithelial ovarian cancer at out institute with a cytoreductive score of 0 or 1. PFS was calculated from the date of ICS till the recurrence of the disease.
Role of the funding source
There was no sponsor involved in the study design, in the collection, analysis, and interpretation of data, writing of the report, and in the decision to submit the paper for publication.
Results
A total of 24 patients were included in the pilot study over two years (Dec 2019 to Dec 2021). The median age of the patients undergoing ICS+ IP was 49 years. The short-term analysis was made by comparing 45 patients undergoing ICS only who underwent interval cytoreductive surgery (ICS) with CC 0 and 1 during the same period. The median age of the patients was 48 years. The predominant histopathology in the ICS+IP arm was high-grade serous carcinoma (24/24). In the patients who underwent ICS alone, the predominant HPE was high-grade serous carcinoma (40/45). The intraoperative parameters are shown in Table 1.
| Intra-operative Parameters | |
| Number of surgical procedures performed | |
| Abdominal/pelvic peritonectomy | 4 |
| Bowel resection | 0 |
| Diaphragmatic stripping and or resection | 6 |
| Splenectomy | 0 |
| Aortic/pelvic lymphadenectomy/sampling | 20 |
| Cholecystectomy | 1 |
| Median operating time | 6 hours |
| Completeness of cytoreduction | CC-0 (21) |
| CC-1 (3) |
The mean PCI in the patients receiving intraoperative normothermic chemotherapy was 9.7 (range 0-28), whereas it was 6.48 (range 0-27) in patients undergoing ICS only.
There was acceptable tolerability and toxicity following intraperitoneal cisplatin instillation, with no grade IV toxicity or mortality. The most common side effect was prolonged post-operative ileus which was seen in 6 patients. Twelve patients received intraoperative blood transfusion of up to two units due to intraoperative blood loss. Although all patients received prophylaxis with dexamethasone and serotonin receptor uptake inhibitors, four patients had grade 2 nausea and vomiting lasting four days (Table 2).
| Post-operative Parameters | Number of patients |
| Number of patients transfused | |
| Blood | 12 |
| Plasma | 8 |
| 30-day mortality | 0 |
| Re-laparotomy | 0 |
| Post-operative ileus (>72 hours) | 6 |
In post-procedural period, one patient had leakage of intraperitoneal fluid after 4 hours from the stitch line (required unclamping of the drains, and the wound redressing; there is no post-operative wound complications). None of the patients had post-operative wound sepsis following intraperitoneal chemotherapy instillation. There were no incidences of pleural effusion, fistula (uretero-bowel), lung complication, arrhythmia, heart failure, tissue necrosis, central venous thrombosis, pulmonary embolism, septicaemia, intravenous line sepsis, wound seroma, urinary infections in the post-operative period.
Grade 2 anaemia was the most common haematological toxicity in the post-operative period, which developed in six patients, whereas grade 2 neutropenia was observed in one patient. One patient had grade 3 thrombocytopenia but recovered spontaneously (Table 3).
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Haematological: | ||||
| Leukopenia | 0 | 0 | 0 | 0 |
| Neutropenia | 0 | 1 | 0 | 0 |
| Anaemia | 6 | 6 | 0 | 0 |
| Thrombocytopenia | 0 | 0 | 1 | 0 |
| Non-haematological: | ||||
| Diarrhoea | 0 | 0 | 0 | 0 |
| Nausea | 0 | 4 | 0 | 0 |
| Vomiting | 0 | 4 | 0 | 0 |
With a median follow-up period of 22 months, nine patients (9/24) had disease recurrence in ICS plus intraperitoneal chemotherapy group, whereas 27 (27/44) patients had disease recurrence in ICS alone group (Table 4).
| Number of patients | |
| Type of recurrence | |
| Single- site | 3 |
| Multiple-site | 6 |
| Diffuse carcinomatosis | 0 |
| Site of recurrence | |
| 1) Intraperitoneal | 1 |
| 2) Hepatic/splenic | 0 |
| 3) Intraperitoneal + hepatic/splenic | 3 |
| 4) Retroperitoneal Lymph nodes | 2 |
| 5) Retroperitoneal Lymphnodes + with intraperitoneal/hepatic | 1 |
| 6) Distant mets | 2 (Brain/SCLN) |
PFS in patients who received standard treatment (ICS alone) & IP chemotherapy was 52.0% & 83.3%, respectively (Table 5).
| 1 Year | 3 Year | |||||||
| ICS alone | 52.00% | 22.00% | ||||||
| ICS plus IP chemotherapy | 83.30% | 30.50% | ||||||
| Means and Medians for Survival Time (PFS) | ||||||||
| Group | Meana | Median | ||||||
| Estimate | Std. Error | 95% Confidence Interval | Estimate | Std. Error | 95% Confidence Interval | |||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||
| ICS alone | 17.873 | 1.893 | 14.162 | 21.583 | 13 | 1.136 | 10.773 | 15.227 |
| ICS + IP | 26.56 | 2.338 | 21.977 | 31.143 | 34 | 7.532 | 19.238 | 48.762 |
| Overall | 21.17 | 1.612 | 18.011 | 24.329 | 18 | 2.046 | 13.989 | 22.011 |
a. Estimation is limited to the largest survival time if it is censored
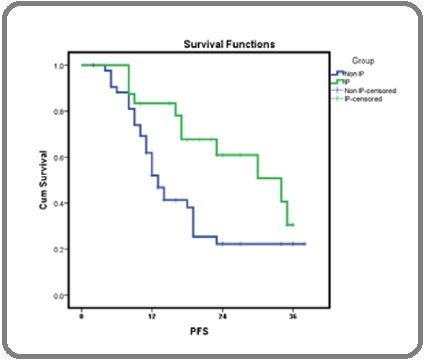
The median PFS among patients who received IP chemotherapy was significantly better in comparison to patients who recieved ICS alone (34.0 vs. 13.0 months, p=0.018) (Figure 1).
Figure 1. PFS ICS+IP Versus ICS Only.
Discussion
In patients with advanced epithelial ovarian cancer, the use of cisplatin-based chemotherapy, targeted therapy in the adjuvant setting, cytoreductive surgery with the goal of no residual disease, improved intensive care, and the use of HIPEC have resulted in modest improvement in overall survival [21, 22]. However, many factors affect the uniform delivery of optimal treatment, including high costs of newer drugs, specifically HIPEC and targeted therapy, lack of infrastructure, human resources, and ICU backup. Therefore, there is a need for innovative approaches in cancer treatment delivery aimed at improving survival at affordable costs. With the background knowledge and evidence from using normothermic intraperitoneal chemotherapy in adjuvant settings EPIC and HIPEC in intraoperative settings, this pilot project was undertaken to build up evidence for starting a randomized controlled trial. Our preliminary results show the feasibility of prolonged intraoperative instillation of normothermic cisplatin. The toxicity profile following intraperitoneal normothermic cisplatin was acceptable, with no grade IV toxicity or mortality. The most common side effect was prolonged postoperative ileus (25%), nausea and vomiting (16%). However, early feeds could be started in the majority of patients. The most common haematological toxicity was grade 2 anaemia in the postoperative period (25%). Grade 2 neutropenia and grade 3 thrombocytopenia (recovered spontaneously) were seen in one patient each. In the post-operative period, one patient had leakage at the stitch line after 4 hours, which was managed by unclamping the drain and redressing the wound. None of the patients had postoperative wound sepsis who received IP chemotherapy. Being a pilot project, we did a short follow-up (median 22 months) and compared the results with patients undergoing ICS only during the same period.
At the end of the follow-up, nine patients (37.5%) had a recurrence with ICS and intraperitoneal chemotherapy, whereas 27 (61.3%) had a recurrence with ICS only.
PFS in patients who received ICS alone versus ICS plus IP chemotherapy was 52.0% & 83.3%, respectively. The median PFS among patients who received ICS alone & ICS plus IP chemotherapy was 13.0 & 34.0 months, respectively (p=0.018).
Uniform state-of-the-art management of advanced epithelial ovarian cancer may not be accessible to all due to global resource limitations. Although the evidence is weak due to the small sample size and short follow-up of patients, the findings are encouraging. This feasibility study has provided substantial evidence to work further with this approach in our institute and plan an RCT to gain more evidence.
Disclosure
The authors declare no conflict of interest.
Acknowledgements
I wish to express my gratitude for the support and help provided during the tenure of my project to all the faculty and staff of the gynecologic oncology and medical oncology department. I would especially like to mention the names of Dr Mahendra, Manoj (Statistician BBCI) and Dr Dimpy Begum.
References
- Treatment for recurrent ovarian cancer-at first relapse Ushijima K. Journal of Oncology.2010;2010. CrossRef
- Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer Vergote I, Tropé CG , Amant F, Kristensen GB , Ehlen T, Johnson N, Verheijen RHM , et al . The New England Journal of Medicine.2010;363(10). CrossRef
- Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial Kehoe S, Hook J, Nankivell M, Jayson GC , Kitchener H, Lopes T, Luesley D, et al . Lancet (London, England).2015;386(9990). CrossRef
- Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602 Onda T, Satoh T, Saito T, Kasamatsu T, Nakanishi T, Nakamura K, Wakabayashi M, et al . European Journal of Cancer (Oxford, England: 1990).2016;64. CrossRef
- A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer Bois A, Lück H, Meier W, Adams H, Möbus V, Costa S, Bauknecht T, et al . Journal of the National Cancer Institute.2003;95(17). CrossRef
- First-line treatment of advanced ovarian cancer: current research and perspectives Marchetti C, Pisano C, Facchini G, Bruni GS , Magazzino FP , Losito S, Pignata S. Expert Review of Anticancer Therapy.2010;10(1). CrossRef
- Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer Jaaback K, Johnson N, Lawrie TA . The Cochrane Database of Systematic Reviews.2016;2016(1). CrossRef
- Principles and practice of intraperitoneal chemotherapy for ovarian cancer Fujiwara K, Armstrong D, Morgan M, Markman M. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society.2007;17(1). CrossRef
- Use and Effectiveness of Intraperitoneal Chemotherapy for Treatment of Ovarian Cancer Wright AA , Cronin A, Milne , Bookman MA , Burger RA , Cohn DE , Cristea MC , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2015;33(26). CrossRef
- Intraperitoneal Chemotherapy of Ovarian Cancer: A Review, With a Focus on Practical Aspects of Treatment. 2006 [cited 2023 Mar 5]; Available from: www.jco.org Markman M , Walker JL . .
- Current status and future prospects of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) clinical trials in ovarian cancer Cowan RA , O'Cearbhaill RE , Zivanovic O, Chi DS . International Journal of Hyperthermia: The Official Journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group.2017;33(5). CrossRef
- Hyperthermic intraperitoneal chemotherapy for epithelial ovarian cancer: A meta-analysis Kim SI , Kim JH , Lee S, Cho H, Driel WJ , Sonke GS , Bristow RE , Park S, Fotopoulou C, Lim MC . Gynecologic Oncology.2022;167(3). CrossRef
- Combined hyperthermia and chemotherapy as a synergistic anticancer treatment Truong DH , Trần H. Journal of Pharmaceutical Investigation.2019;49. CrossRef
- Cytoreductive surgery with intraperitoneal chemotherapy in the management of peritoneal surface malignancy: a pharmacist's perspective Mistry P, Mohamed F, Dayal S, Cecil TD , Moran BJ . European Journal of Hospital Pharmacy.2016;23(4). CrossRef
- Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group Markman M, Bundy BN , Alberts DS , Fowler JM , Clark-Pearson DL , Carson LF , Wadler S, Sickel J. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2001;19(4). CrossRef
- Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer Alberts DS , Liu PT , Hannigan EV , O'Toole R, Williams SD , Young JA , Franklin EW , et al . The New England Journal of Medicine.1996;335(26). CrossRef
- Intraperitoneal cisplatin and paclitaxel in ovarian cancer Armstrong DK , Bundy B, Wenzel L, Huang HQ , Baergen R, Lele S, Copeland LJ , Walker JL , Burger RA . The New England Journal of Medicine.2006;354(1). CrossRef
- Overview of the optimal perioperative intraperitoneal chemotherapy regimens used in current clinical practice Van der Speeten K, Lemoine L, Sugarbaker P. Pleura and Peritoneum.2017;2(2). CrossRef
- Does early post-operative intraperitoneal chemotherapy (EPIC) for patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) make a difference? Tan GHC , Ong WZ , Chia CS , Tham CK , Soo KC , Teo MCC . International Journal of Hyperthermia: The Official Journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group.2016;32(3). CrossRef
- Common terminology criteria for adverse events - UpToDate [Internet]. [cited 2023 Apr 14]. Available from: https://www.uptodate.com/contents/common-terminology-criteria-for-adverse-events#! .
- Improved survival in ovarian cancer, with widening survival gaps of races and socioeconomic status: a period analysis, 1983-2012 Wu J, Sun H, Yang L, Deng Y, Yan Y, Wang S, Yang G, Ma H. Journal of Cancer.2018;9(19). CrossRef
- HIPEC improves survival in stage III epithelial ovarian cancer Stirrups R. The Lancet. Oncology.2018;19(3). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2024
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times