Solitary Fibrous Tumor of the Mediastinum: A Rare Clinical Entity and Review of the Literature
Download
Abstract
Solitary fibrous tumor (SFT) is an uncommon mesenchymal tumor, generally exhibiting indolent clinical behavior compared to its malignant counterpart. There are very few reported cases of extrapleural malignant SFT arising from the mediastinum. This report presents a rare case of solitary fibrous tumor originating in the mediastinum and reviews the relevant literature on this entity.
Introduction
A solitary fibrous tumour (SFT) is an uncommon intrathoracic fibroblastic tumour of intermediate malignant potential originally described to arise from pleura. Approximately 1–8% of intrathoracic SFT are reported from the mediastinum [1, 2]. Malignant SFTs are furthermore extremely unusual in the mediastinal region, with a relatively aggressive course and inferior disease-specific survival [3, 4]. The peculiar characteristics of increased cellularity, cellular pleomorphism, mitoses > 4 per 10 high-power fields, and necrosis are strikingly seen in malignant SFT [3, 4]. We hereby present a rare case report of an SFT of the mediastinum and review the literature on this entity.
Methods
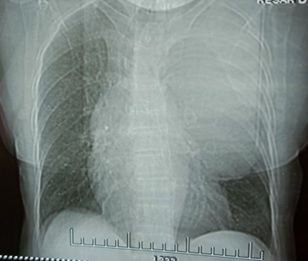
A 55-year-old man presented to our clinic with gradually progressive, productive cough for the last 1 year and shortness of breath for the last 2 months. On clinical evaluation, he was found to have decreased breath sounds on left side of chest. On further evaluation, Chest X-ray revealed large opacity in left side of chest (Figure 1).
Figure 1. Chest X Ray -Radioopacity in Left Chest.
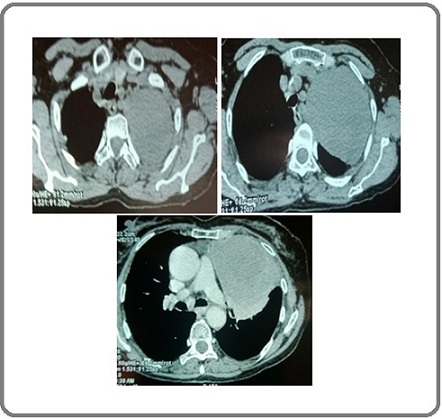
Contrast-enhanced computed tomography (CECT) scan revealed a left sided well-defined heterogeneously enhancing large mediastinal soft tissue mass with internal prominent vessels measuring 9.8× 8.6 x 16cm with compressed and collapsed left upper lobe and few enlarged mediastinal nodes. Laterally,the mass extends to subpleural margins (Figure 2).
Figure 2. CT Chest Showing Left Sided Large Heterogenous Enhancing Mediastinal Mass.
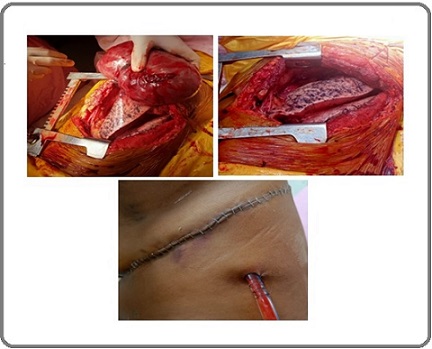
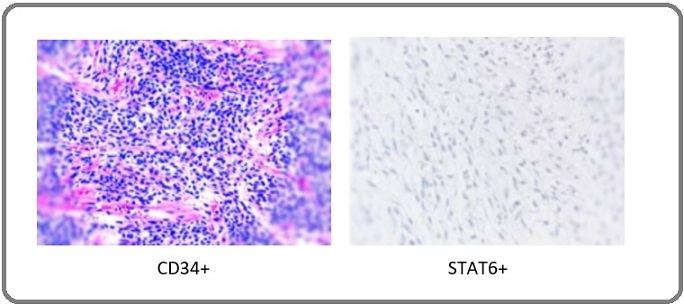
Routine blood investigations including serum tumour marker levels were within normal range. A CT- guided transthoracic core biopsy of the mediastinal mass was performed, which revealed a moderately cellular spindle cell tumor in a collagenous stroma with prominent dilated staghorn vasculature and no mitosis and tumor necrosis was seen. On subsequent IHC studies,the tumor cells were found to have strong positivity for CD34 and STAT6 and negative for SOX10, S100 and CK (Figure 4). Final diagnosis of solitary fibrous tumor was reached at. Case was discussed in multidisciplinary tumor board where plan for surgical excision of mass was taken. Patient was optimized for surgery and he subsequently underwent a left anterolateral thoracotomy with excision of the anterior mediastinal mass along with sleeve of upper lobe lung to which it was adhered to its apicoposterior segment (Figure 3).
Figure 3. Intraoperative Picture with Large Left Side Mediastinal Mass Adhered to Left Upper Lobe Lung.
Results
Patient was shifted to Intensive care unit and was monitored there for 2 days. He was allowed oral diet from POD1 and was started on incentive spirometry. His recovery was eventful and was discharged on POD5 after the removal of intercostal drain. Final histopathological evaluation of tumor revealed single unencapsulated well circumscribed mass of size 15 x 10 x 8cm with no areas of tumor necrosis on cut section of tumor. Microscopically, it revealed moderately cellular spindle shaped cells with ill defined fascicles. Presence of dilated staghorn like vasculature was seen with no cytologic atypia and / necrosis and mitosis. Resected margins were free of tumor. On Immunohistochemistry (IHC), the tumor cells were positive for CD34 (strongly and diffusely) and STAT6 (Figure 4).
Figure 4. IHC Studies with Different Markers Reactivity to Tumor Cells.
Hence, final diagnosis of benign counterpart of SFT was reached at. Patient remained on regular follow up with no evidence of recurrence seen till date.He is disease free from last 9 months post surgery.
Discussion
SFT is defined to be fibroblastic tumor with ubiquitous neoplasm most commonly affecting the adult patients with age range from 20 to 70 years. In one of the series, the distribution of extrameningeal SFT cases in abdominal cavity is 31% while in limbs it is 29%, pleura 22% and in other region being 18% [5]. The median age of presentation ranged from 50 to 60 years [5, 6]. Clinically, SFTs present as an asymptomatic, well-defined mass in the primary pleural region as compared to extra-pleural SFT. While 2013 WHO classification integrated under the SFT nomenclature the hemangiopericytoma denomination, and the 2020 WHO classification excluded the terms of “typical” or “malignant” owing to the fact that typical SFT does not look synonymous with benign disease [7, 8]. 10% of the patients may have paraneoplastic syndromes like hypertrophic osteoarthropathy, hypoglycemia are seen more often in pleural SFT. The underlying pathogenesis is related to the overproduction of insulin-like growth factor-II [9].
Radiologically, these tumors show an avid contrast enhancement with collateral feeding vessels in 65% of cases in CT scans [10]. Heterogeneity after contrast injection was seen seen more commonly in malignant SFT than in indolent SFTs, 76.5% vs. 40.0% [11]. In other series, heterogeneous enhancement was seen in 88% of cases. In our case report,tumor showed heterogeneous enhancement with feeder vessels on CT scan chest. In MRI, SFT appears as isointense in T1 weighted images due to collagen content and low cellularity and variable in T2. Strong enhancement with gadolinium is usually seen with vascular tumors [12]. The 18F-FDG PET-CT scan has not shown to distinguish indolent SFT (the old fashioned typical SFT) from aggressive SFT (the old-fashioned malignant SFT) in a series of 17 patients with confirmed SFT diagnosis [13]. In our case report,we did not perform PET-CT scan as patient had financial constraints. Risk classification models have been developed to distinguish three different subsets of SFT: low aggressive, high aggressive and dedifferentiated SFT, for localized resected extrameningeal SFT [5, 6, 14, 16]. Among these, there were two reliable models to accurately predict the risk of metastasis [5, 6, 14, 16]. One model included parameters like patient age (< or ≥ 55 years), mitotic count (0, 1–3 and > 4 mitosis/10 high power field), tumour size (< 5, 5- < 10,10- ≤ 15, > 15 cm) with each factor assigned a score of 0–3. The overall score predicted low (0–2 points), intermediate (3–4 points) and high (5–6 points) risk for metastasis. Another risk model was calculated with parameters like age, tumor localization (limb vs. others) and mitotic count.
Pathologically, SFT is comprised of randomly arranged spindle shaped cells within a collagenous stroma with interspersed staghorn shape blood vessels. The disposition of cellular and stromal components in SFT is called “patternless pattern”. Histology may show spectrum from a paucicellular content with abundant stromal collagen to highly cellular tumors with paucistromal content. The mitotic count is usually low and is used to establish the recurrence risk. Other pathological include nuclear pleomorphism and necrosis.Final diagnosis of SFT can be reached with the help of immunostaining markers like CD34, bcl-2 and CD99. In our case report,the clues towards benign counterpart of SFT included paucicellularity with absence of mitosis and necrosis and staining positive for CD34 and STAT6 markers. The expression of CD34 is strong in more than 80% of SFT tumors, but its expression can be absent in aggressive SFTs [17, 18]. But rarely EMA, SMA, keratin, desmin and S100 may come positive [2, 19]. Discovery of nuclear expression of STAT6 protein in SFT is the most sensitive and specific marker for diagnosis of conventional and malignant SFT [20]. STAT6 has a sensitivity of 98–100% for SFT with strong nuclear STAT6 taken to be a surrogate marker for NAB2-STAT6 fusion gene [6, 21]. In our case report,finally, the diagnosis of SFT was established based on the expression of IHC for STAT6 and CD34.
Surgical resection is the mainstay treatment of localized SFT. The surgical approach is offered with the aim of achieving wide resection. Since SFT is a ubiquitous tumor, there are different technical aspects according to site of SFT. Thoracic SFTs are large,pedunculated and are commonly attached to visceral pleura. Thus, sleeve lung resection is the most frequently performed surgical resection. In SFT arising from parietal pleura or lungs, isolated parietal pleurectomy along with chest wall resections or pulmonary wedge resections, are frequently performed procedures [22]. Video-assisted thoracic surgery is feasible in patients with tumors size smaller than 10 cm [23]. Pre-operative embolization or a thoracoscopy ligation of vessels is performed in patients having large pedunculated tumors with large feeding vessels seen on angiography.Also in sessile SFT,intraoperative blood loss can be minimized effectively with embolization [24]. In our case report,the mass was encapsulated with some fixity to apical lobe of left lung ,so sleeve resection of lung was done along with excision of mass.
To attain better local control of disease,adjuvant therapy in the form of External beam radiotherapy is recommended in malignant SFT with various studies on retrospective analysis showed a clear trend toward better local control. The 5-year local control is found to be 60% vs. 90%, favoring the postoperative radiotherapy [25]. Chemotherapy has been reserved in the advanced or metastatic settings. Though the role of value of cytotoxic drugs in less aggressive SFTs is much more controversial. As our case is diagnosed with benign counterpart of SFT,no adjuvant therapy was needed and patient was kept on close follow up.
In conclusion, SFT is a rare thoracic tumor which can be recognized on usual histomorphology and immunophenotypic profile with mediastinal SFT being a very rare entity.Use of specific markers with CD34, STAT6 clinched the diagnosis in the index case. Surgical resection is the standard of care for these patients, with adjuvant therapy reserved only for malignant SFT.
Declaration
Study has been done with ethical standards
Funding
No funds, grants, or other support was received.
Conflict of Interest
None
Financial interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics Approval
Ethical approval was waived by the local Ethics Committee as it is part of routine care.
Informed Consent
Taken from the patient
References
- Extrapleural solitary fibrous tumor: A distinct entity from pleural solitary fibrous tumor. An update on clinical, molecular and diagnostic features Ronchi A, Cozzolino I, Zito Marino F, Accardo M, Montella M, Panarese I, Roccuzzo G, Toni G, Franco R, De Chiara A. Annals of Diagnostic Pathology.2018;34. CrossRef
- Mesenchymal tumours of the mediastinum—part I Bakker MA , Marx A, Mukai K, Ströbel P. Virchows Archiv.2015;467. CrossRef
- Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases England DM , Hochholzer L, McCarthy MJ . The American Journal of Surgical Pathology.1989;13(8). CrossRef
- Malignant giant solitary fibrous tumor of the mediastinum De Raet J, Sacré R, Hoorens A, Fletcher C, Lamote J. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer.2008;3(9). CrossRef
- Prediction of local and metastatic recurrence in solitary fibrous tumor: construction of a risk calculator in a multicenter cohort from the French Sarcoma Group (FSG) database Salas S, Resseguier N, Blay JY , Le Cesne A, Italiano A, Chevreau C, Rosset P, Isambert N, et al . Annals of Oncology: Official Journal of the European Society for Medical Oncology.2017;28(8). CrossRef
- Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model Demicco EG , Park MS , Araujo DM , Fox PS , Bassett RL , Pollock RE , Lazar AJ , Wang W. Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2012;25(9). CrossRef
- The evolving classification of soft tissue tumours - an update based on the new 2013 WHO classification Fletcher CDM . Histopathology.2014;64(1). CrossRef
- The 2020 WHO Classification: What’s New in Soft Tissue Tumor Pathology? Kallen ME , Hornick JL . The American Journal of Surgical Pathology.2021;45(1). CrossRef
- Tumor-induced hypoglycemia Le Roith D. The New England Journal of Medicine.1999;341(10). CrossRef
- Solitary fibrous tumors of the soft tissues: review of the imaging and clinical features with histopathologic correlation Wignall OJ , Moskovic EC , Thway K, Thomas JM . AJR. American journal of roentgenology.2010;195(1). CrossRef
- Solitary fibrous tumor of the pleura: Can computed tomography features help predict malignancy? A series of 56 patients with histopathological correlates Hélage S, Revel MP , Chabi ML , Audureau É, Ferretti G, Laurent F, Alifano M, Mansuet-Lupo A, Buy JN , Vadrot D. Diagnostic and Interventional Imaging.2016;97(3). CrossRef
- Imaging features of solitary fibrous tumors Ginat DT , Bokhari A, Bhatt S, Dogra Vi. AJR. American journal of roentgenology.2011;196(3). CrossRef
- The utility of 18F-FDG PET/CT in solitary fibrous tumors of the pleura Tazeler Z, Tan G, Aslan A, Tan S. Revista Espanola De Medicina Nuclear E Imagen Molecular.2016;35(3). CrossRef
- Resectable extra-pleural and extra-meningeal solitary fibrous tumours: A multi-centre prognostic study Pasquali S, Gronchi A, Strauss D, Bonvalot S, Jeys L, Stacchiotti S, et al . European Journal of Surgical Oncology: The Journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology.2016;42(7). CrossRef
- Risk factor analysis for the recurrence of resected solitary fibrous tumours of the pleura: a 33-year experience and proposal for a scoring system Tapias LF , Mino-Kenudson M, Lee H, Wright C, Gaissert HA , Wain JC , Mathisen DJ , Lanuti M. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery.2013;44(1). CrossRef
- A novel risk score to predict early and late recurrence in solitary fibrous tumour Georgiesh T, Boye K, Bjerkehagen B. Histopathology.2020;77(1). CrossRef
- Differential diagnosis of solitary fibrous tumors: A study of 454 soft tissue tumors indicating the diagnostic value of nuclear STAT6 relocation and ALDH1 expression combined with in situ proximity ligation assay Ouladan S, Trautmann M, Orouji E, Hartmann W, Huss S, Büttner R, Wardelmann E. International Journal of Oncology.2015;46(6). CrossRef
- Solitary fibrous tumour: significance of p53 and CD34 immunoreactivity in its malignant transformation Yokoi T, Tsuzuki T, Yatabe Y, Suzuki M, Kurumaya H, Koshikawa T, Kuhara H, et al . Histopathology.1998;32(5). CrossRef
- [Malignant solitary fibrous tumor of pleura with focal expression of cytokeratin] Leroy X, Copin MC , Petit S, Moukassa D, Gosselin B. Annales De Pathologie.2001;21(2).
- Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing Robinson DR , Wu Y, Kalyana-Sundaram S, Cao X, Lonigro RJ , Sung Y, Chen C, et al . Nature Genetics.2013;45(2). CrossRef
- STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors Yoshida A, Tsuta K, Ohno M, Yoshida M, Narita Y, Kawai A, Asamura H, Kushima R. The American Journal of Surgical Pathology.2014;38(4). CrossRef
- Solitary fibrous tumors of the pleura: results of surgical treatment and long-term prognosis Harrison-Phipps KM , Nichols FC , Schleck CD , Deschamps C, Cassivi SD , Schipper PH , Allen MS , Wigle DA , Pairolero PC . The Journal of Thoracic and Cardiovascular Surgery.2009;138(1). CrossRef
- Thoracic solitary fibrous tumors: an analysis of 70 patients who underwent surgical resection in a single institution Zhou C, Li W, Shao J, Zhao J. Journal of Cancer Research and Clinical Oncology.2020;146(5). CrossRef
- Unique Presentation and Management Approach of Pleural Solitary Fibrous Tumor Aridi T, Tawil A, Hashem M, Khoury J, Raad R, Youssef P. Case Reports in Surgery.2019;2019. CrossRef
- Extrameningeal solitary fibrous tumors—surgery alone or surgery plus perioperative radiotherapy: A retrospective study from the global solitary fibrous tumor initiative in collaboration with the Sarcoma Patients EuroNet Haas RL , Walraven I, Lecointe‐Artzner E, Houdt WJ , Strauss D, Schrage Y, Hayes AJ , Raut CP , et al . Cancer.2020;126(13). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2024
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times