Review on Histopathological Techniques in Cervical Cancer Screening
Download
Abstract
Histopathological techniques remain the basis for cervical cancer care by making diagnosis and model treatment options to improve patient outcomes. It involves the examination of cervical tissue samples to establish a diagnosis, grade precancerous lesions, and stage invasive cancer that guide treatment options. We examined published reports and provided updated information on histopathological techniques in cervical cancer screening using the PRISMA guidelines for review. Histopathology examination is used to confirm precancerous lesions and stage invasive cancer. Accumulated data obtained also informs healthcare providers about the severity of the lesions, allowing decisions for personalized treatment plans and prognosis. While cytology screening is an important tool for cervical cancer screening, histopathological techniques are indispensable to confirm the diagnosis and determine appropriate treatments. Therefore, it is essential to continue streamlining the techniques and scoring patterns to guarantee for their reliability in various contexts.
Introduction
Cancer of the cervix is one of the leading causes of cancer-related death among women worldwide. In 2018, approximately 570,000 women were diagnosed with cervical cancer in the world which claims the lives of more than 311,000 women each year, particularly in developing countries [1]. Cancer of the cervix begins on the surface of the cervix, the entrance to the uterus from the vagina. According to the World Health Organization (WHO), almost all cervical cancer cases (99%) are linked to infection with high-risk human papillomaviruses (HPV), an extremely common virus transmitted through sexual contact and the highest regional incidence and mortality rates are seen in Africa and Asia [2]. First sexual intercourse at a younger age and multiple sexual partners have been shown to exert strong effects on the risk. Also smoking, prolonged use of oral contraceptives, low socioeconomic status, sexually transmitted infections, low immune status, and factors related to poverty are associated with cervical cancer [3]. This study was done to increase available knowledge on the role of histopathology laboratory in cervical cancer screening which determines treatments of precancerous and cancerous cells by classifying the microscopic patterns of cell organization in tissue sections from biopsy and surgical specimens.
Anatomy of the Cervix
The cervix is located inside the pelvic cavity, about 3 to 6 inches in the vaginal canal. It begins at the base of the uterus and extends downward onto the top part of the vagina. The phrase “cervix” comes from the Latin phrase meaning “neck”. The cervix is shaped roughly like a cylinder or tube and connects important body parts. It connects to the uterus to the vagina. The cervix is wider in the middle and narrows at both ends, where it opens into the uterus and vagina [4]. The anatomical cervix consists of the internal Os, the opening that leads to the uterus, and the endocervical canal, which is a tunnel that extends from the internal Os to the ectocervix which is the part of the cervix that bulges onto the top of the vagina [4]. The location in which the endocervical canal overlaps with the ectocervix is known as the transformation zone (TZ). The TZ is the part of the cervix where cell changes happen most. It’s the most common site for abnormal cells to grow in the cervix, indicating conditions like cervical dysplasia or cervical cancer. A substantial amount of dense fibromuscular tissue is located throughout the cervix. The cervix is lined by two major types of cells; glandular cells lining the innermost portion of the cervix, the endocervical canal, and squamous cells which lines the vagina, the ectocervix, and the outermost portion of the cervix. The TZ is the meeting point of these many cell types, where cell modifications occur regularly [5].
Cytology Screening
The goal of screening for cervical cancer is to find precancerous cervical cell changes when treatment can prevent cervical cancer from developing. Sometimes, cancer is found during cervical screening. Cervical cancer found at an early stage is usually easier to treat. By the time symptoms appear, cervical cancer may have begun to spread, making treatment more difficult [2].
Cervical cancer can be screened for in three primary ways:
1) The Pap test, also called a Pap smear or cervical cytology collects cervical cells so they can be checked for changes caused by HPV that may if left untreated turn into cervical cancer. Both cervical cancer and precancerous cells can be detected by it. A Pap test also sometimes finds conditions that are not cancer, such as infection or inflammation
2) The human papillomavirus (HPV) test checks cells for infection with high-risk HPV types that can cause cervical cancer
3) The HPV/Pap test uses an HPV test and Pap test together to check for both high-risk HPV and cervical cell lesions [2].
Papanicolaou test
Papanicolaou test is the cornerstone for cervical cancer screening. The technique, also known as the Pap test or the Pap smear was developed in the 1940s by Georgios Papanikolaou. It involves exfoliating cells from the transformation zone of the cervix to enable microscopic examination of the cells for precancerous and cancerous lesions [6]. The primary aim of the cervical smear test is to prevent the development of invasive cancer of the cervix by detecting precancerous lesions known as cervical intraepithelial neoplasia (CIN) in the uterine cervix, which is usually asymptomatic and only found by screening. A pelvic examination is performed in addition to the Pap smear. In women older than age 30, the Pap test may be combined with a test for human papillomavirus (HPV). In the technique known as liquid-based cytology, these collected cells are released into a vial of liquid preservative that is then used in the cytology lab to produce a slide for microscopic evaluation of the cells. The older, traditional Pap technique (conventional cytology) involves the direct transfer of the cervical cells to a microscope slide for evaluation [6].
Cervical smear
Ideally, cervical screening should be scheduled when the patient is not menstruating and advised to avoid vaginal intercourse, douching, use of tampons, and use of medicinal vaginal cream or contraceptive cream for 24 - 48 hrs. before cervical screening [6, 7]. For a proper pap smear, the patient should be in the dorsal lithotomy posture and supine. When the speculum is inserted, the patient’s coccyx needs to be at the edge of the examination table to allow for sufficient vision of the cervix. Collection of the Papanicolaou test involves sampling the cervix at the transformation zone using a spatula or brush [8]. The transformation zone is where the ectocervix and endocervix meet and dysplasia is most likely to be identified. The methods for collecting cervical cells and examining them for dysplastic alterations have improved since the screening test was first introduced. In the conventional Pap test, the cervical sample is transferred to a slide, and a chemical fixative is subsequently applied. While many institutions continue to employ this process, many more now use the more recent liquid approaches, such as Sure Path and Thin Prep. The cervical sample used in these tests is submitted to a lab for additional processing after being suspended in a liquid medium. Samples are plated as thin layers on slides after undergoing density gradient sedimentation (SurePath) or filtration procedures (ThinPrep). Samples are transferred to a cytopathologist for a cytologic study after cell collection is finished. The results can be used to triage abnormalities that may require further diagnostic testing, including colposcopy and biopsy of suspicious lesions. Staining is typically done using the Papanicolaou (Pap) staining method, which involves multiple steps including nuclear staining and counter-staining steps to enhance the visualization of cell structures [8].
Microscopic examination
Pap test results show whether cervical cells are normal or abnormal. A Pap test may also come back as unsatisfactory [2]. Normal Pap test results show no abnormal cervical cells were found. A normal test result may also be called a negative test result or negative for intraepithelial lesion or malignancy.
In unsatisfactory Pap test results, women who receive unsatisfactory Pap test results will almost certainly be asked to retake the test two to four months later. It’s possible that there weren’t enough cells in the lab sample, or that the cells were clumped together or hidden by mucus or blood. Abnormal Pap test results may also be called positive test results. Some of the cells of the cervix look different from the normal cells. An abnormal test result doesn’t mean it’s cancer and will recommend monitoring, more testing, or treatment [2].
Furthermore, Papanicolaou test results are routinely reported according to the Bethesda system. This was introduced in 1988 and revised in 2001 and 2008, with the hope of standardizing pathology reports and improving their usefulness. The most recent update to the system was done in 2014 as described [8].
HPV testing
The HPV test and Pap test are done the same way.
The HPV test looks for cervical infection by high-risk types of HPV that are more likely to cause precancers and cancers of the cervix. The test can be done by itself or at the same time as the Pap test (called a co-test) (with the same swab or a second swab), to determine the risk of developing cervical cancer [2]. When cytology and HPV tests are used together called co-testing, sensitivity approaches 100 %, and the 5-year risk of cervical intraepithelial neoplasia grade 3 or higher (CIN3+) after a negative co-test is far below that with negative cytology alone, supporting longer testing intervals. The American Cancer Society, The American Society of Colposcopy and Cervical Pathology (ASCCP), and the American Society of Clinical Pathology recommend co-testing as a preferred screening strategy, although the appropriateness of this recommendation has been questioned [9]. The American Cancer Society recommends a primary HPV test, which is an HPV test that is done by itself for screening as the preferred way to screen for cervical cancers or precancers in individuals 25 to 65 years with a cervix [10]. HPV test results show whether high-risk HPV types were found in cervical cells. An HPV test will come back as a negative test result or a positive test result. In a negative HPV test result, high-risk HPV is not found and the next test would be in 5 years while a positive HPV test result would recommend treatments [2].
Normal cytology
Diagnostic cytology of the cervix can be made strong if normal cytology is known thoroughly. The cervical lining comprises three layers of squamous cells, the basal, intermediate, and superficial cells. Knowing the dimensions of these cells, especially the intermediate cells, helps to diagnose the squamous intraepithelial lesions accurately. Furthermore, recognizing the para-basal cells in the menopausal smears, either singly or as syncytial aggregates, is important to avoid over-diagnosis of squamous intraepithelial lesions [5].
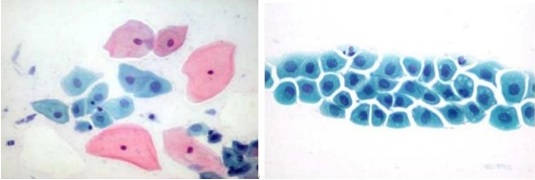
Superficial squamous cells are polygonal in shape with translucent, eosinophilic Thin cytoplasm that may contain brownish keratohyalin granules. Their nuclei are pyknotic, centrally located, and measure 16 to 20 µm2 in area. These cells are seen Singly and in loose clusters (Figure 1).
Figure 1. Illustrating Normal Squamous Cell in a Cervical Smear [11].
• Intermediate squamous cells are oval or polygonal in shape with translucent, Eosinophilic, or basophilic cytoplasm that usually shows folding. Their nuclei are vesicular with fine chromatin and measure about 35 µm2 in area. On occasion, the superficial and intermediate squamous cells are spindle-shaped and display a long cytoplasmic extension or “tail” that is a rare collection of different types of intracytoplasmic filaments called Herxheimer’s spirals.
• Parabasal squamous cells are rarely seen in a smear from a premenopausal woman unless she is in the post-partum period. They are usually seen in post-menopausal atrophy. They are seen singly and are oval with Opaque basophilic cytoplasm. They have centrally located vesicular nuclei with fine Chromatin which measure about 50 µm2 in area.
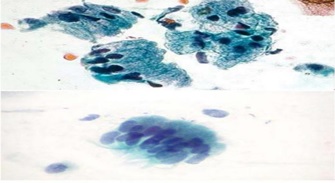
• Endocervical glandular cells are columnar with pale, abundant, Mucinous cytoplasm and basally located vesicular nuclei displaying a granular Chromatin and micronucleoli. A few are ciliated. In conventional Pap smears, Endocervical cells are present singly or in monolayered sheets with characteristic Honeycomb and picket-fence arrangements. Naked nuclei within mucus are a Common finding, but they are not regarded as evidence of Pap smear adequacy. Endocervical glandular cells commonly present singly in liquid-based preparations (Figure 2).
Figure 2. Illustrating Normal Mucus-secreting Endocervical Smear [11].
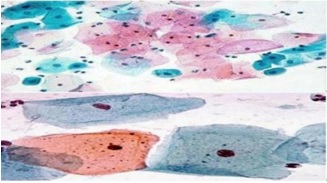
• Metaplastic squamous cells arise from the reserve cells of endocervical columnar Epithelium. They exfoliate singly or in pavement-like sheets and are polygonal or oval. Their cytoplasm varies with the level of cell maturation and in Immature cells it may be thin and vacuolated with cytoplasmic extensions. It is Waxy, basophilic, or eosinophilic in mature cells. Their vesicular nuclei have granular Chromatin and have an area of about 50 µm2 (Figure 3).
Figure 3. Illustrating Metaplastic Squamous Cells Admixed with Superficial and Intermediate Cells [11]..
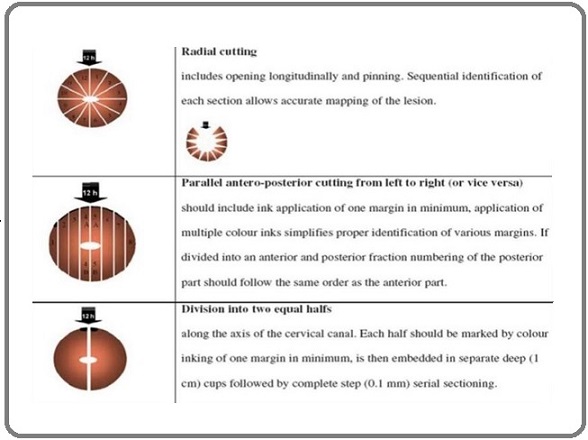
A 4% neutral solution of formaldehyde is ideal for routine histopathological diagnostic procedures. Cervical biopsy must be carefully oriented and embedded according to routine procedures. The precise orientation of a cervical biopsy is essential for evaluating the entire squamocolumnar junction where most precancerous and carcinomatous lesions originate. Routine cervical biopsy staining should include hematoxylin-eosin and a connective tissue stain, for instance, van Gieson’s. PAS or alcian blue reaction may help detect glycogen or mucopolysaccharides in squamous or glandular epithelial cells to evaluate the degree of cellular maturation [12] (Figure 4).
Figure 4. Illustrating Techniques for Sectioning a Conus for Orientation [11].
Precancerous Changes
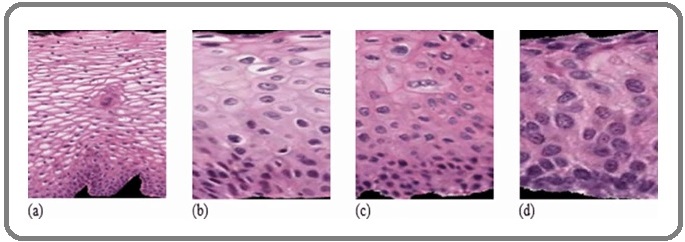
Precancerous lesions of the cervix are abnormal cellular changes located around the cervix that start in the cells on the surface of the cervix near the SCJ (Transformation zone area). According to the World Health Organization (WHO), this change can exist at any of three stages: cervical intraepithelial neoplasia stage 1 (CIN1), stage 2 (CIN2), or stage 3 (CIN3) [13]. These conditions are not yet cancer. However, CIN2 or CIN3, together known as cervical intraepithelial neoplasia stage 2 plus (CIN2+), may lead to cervical cancer if left untreated and indicate lesions penetrating the lower, middle, or upper third of the epithelium, respectively. Of the three stages, CIN2 and CIN3 are high-grade cancer precursors that have a higher probability of progression to invasive cancer [14]. In cytological examination, the presence of koilocytes which are atypical cells with a perinuclear cavitation or halo in the cytoplasm raises suspicions of underlying CIN. In addition, dysplastic cells display an increase in the nuclear to cytoplasmic-ratio. In histology, the changes that indicate intraepithelial neoplasia include enlarged nuclei, increased nuclear-cytoplasmic ratio, increased hyperchromasia, increased nuclear polymorphism, and increased anisokaryosis. Furthermore, the quantity and aberrant configurations of mitotic figures increase along with the severity of CIN. Hence, the percentage of dysplastic squamous epithelium defines the lesions. About one-third of the epithelium’s thickness exhibits dysplastic alterations in low-grade CIN, also known as CIN-1. While CIN-3 can demonstrate full-thickness involvement, CIN-2 only affects
one-half to two-thirds of the thickness. Similarly, carcinoma in situ is diagnosed when dysplasia is seen throughout the epithelium and resembles cervical cancer but has not invaded the basement membrane [8].
When cervical lesions are not identified and treated promptly, they could develop into cervical cancer. Microscopic examination showed that about 80% to 90% of cervical cancers are squamous cell cancers while the remaining 10% to 20% arise from mucous-producing cervical gland cells [14] (Figure 5).
Figure 5. CIN Grading Pattern Showed (a) Normal, (b) CIN1, (c) CIN2, (d) CIN3 [15].
Invasive Cervical Cancer
The National Cancer Institute (NCI) defines invasive cervical cancer as cancer that has spread from the cervix’s surface to tissues deeper in the cervix or other body parts [16]. The current system of staging for cervical cancer is based on the International Federation of Gynecology and Obstetrics (FIGO) classification, a system with 4 stages used to identify the clinical stage of invasive cervical cancer. Chest radiography, excretory urography, cystoscopy, and proctoscopy may be used as supplementary tests in this staging system, which is a clinical approach based on findings from clinical assessment or examination of patients under anesthesia [17]. FIGO staging was based mainly on clinical examination in consideration of the prevalence of cervical cancer in low-income populations with limited access to advanced technology. In 2018, this practice was revised by the FIGO Gynecologic Oncology Committee to allow imaging and pathological findings, where available, to assign the stage. The revised staging is shown in Table 1 as presented at the FIGO XXII World Congress of Gynecology and Obstetrics [18].
| Stage | Description |
| I | The carcinoma is strictly confined to the cervix (extension to the uterine corpus should be disregarded) |
| IA | Invasive carcinoma that can be diagnosed only by microscopy, with a maximum depth of invasion< 5mma |
| IA1 | Measured stromal invasion <3mm in depth |
| IA2 | Measured stromal invasion ≥3mm and <5mm in depth |
| IB | Invasion carcinoma with measured deepest invasion ≥5mm (greater than stage IA), lesion limited to the cervix uteri with size measured by maximum tumor diameterb |
| IB1 | Invasion carcinoma ≥5mm depth of stromal invasion, and <2cm in greatest dimension |
| IB2 | Invasive carcinoma ≥2cm and <4cm in greater dimension |
| IB3 | Invasive carcinoma ≥4cm in greatest dimension |
| II | The carcinoma invades beyond the uterus but has not extended onto the lower third of the vagina or the pelvic wall |
| IIA | Involvement limited to the upper two-thirds of the vagina without parametrial involvement |
| IIA1 | Invasive carcinoma <4cm in greatest dimension |
| IIA2 | Invasive carcinoma ≥4cm in greatest dimension |
| IIB | With parametrial involvement but not up to the pelvic wall |
| III | The carcinoma involves the lower third of the vagina and/or extends to the pelvic wall and/ or causes hydronephrosis or nonfunctioning kidney and/ or involves pelvic and/ or para-aortic lymph nodes |
| IIIA | The carcinoma involves the lower third of the vagina, with no extension to the pelvic wall |
| IIIB | Extension to the pelvic wall and/ or hydronephrosis or nonfunctioning kidney (unless known to be due to another cause) |
| IIIC | Involvement of pelvic and/ or para-aortic lymph nodes (including micrometastases)c, irrespective of tumor size and extent (with r and p notations)d |
| IIIC1 | Pelvic lymph node metastasis only |
| IIIC2 | Para-aortic lymph node metastasis |
| IV | The carcinoma has extended beyond the true pelvis or has involved (biopsy-proven) the mucosa of the bladder or rectum. ( A bullous edema, as such, does not permit a case to be allotted to stage IV) |
| IVA | Spread to adjacent pelvic organs |
| IVB | Spread to distant organs |
It is important to know that imaging and pathology can be used at the same time where available to supplement the clinical findings concerning the tumor size and extent in all stages. The pathological findings supersede imaging and clinical findings. Besides, the involvement of vascular and lymphatic spaces should not change the pathological staging, the lateral extent of the lesion is no longer considered. Moreover, isolated tumor cells do not change the stage simply their presence should be recorded.
In addition, adding the notation of r (imaging) and p (pathology) indicates the findings that are used to allocate the case to Stage IIIC. For example, if imaging indicates pelvic lymph node metastasis, the stage allocation would be Stage IIIC1r; if confirmed by pathological findings, it would be Stage IIIC1p. The type of imaging modality or pathology technique used should always be documented. When in doubt, the lower staging should be assigned.
The role of histopathological techniques
Confirms cytological finding
Histopathology investigation is crucial in diagnosing cervical cancer by confirming a suspected abnormal condition, that is dysplasia based on cytology examinations [19]. Cytology screening is indispensable to confirm the diagnosis and determine the appropriate treatment [19].
Grade cervical lesions
Histopathology determines the grade of cervical intraepithelial neoplasia (CIN) and cervical cancer (CC) by classifying into a diagnosis the patterns of microscopic organization of cells in tissue sections from biopsy or surgical specimens [20].
Stage invasive cancer
Histopathology is used to stage invasive cervical cancer based on the size of the tumor and the spread of the disease into the vagina, parametrium, urinary bladder, rectum, and distant organs. The diagnosis of invasive cervical cancer may be suggested by abnormal physical findings on speculum and vaginal examination and may be confirmed by histological examination of tissue specimens [21].
Determining treatment
The histopathological techniques is used to establish diagnosis and determine the chemotherapeutic course of precancerous and cancerous lesions using the microscopic organization of cells in tissue sections from the biopsy or surgical specimens.Conclusion
Histopathological techniques examine cervical tissues and confirm cytological findings, grade precancerous lesions, and stage invasive cancer which guide treatment options. The knowledge acquired would further informs the healthcare providers about the severity of the lesions, allowing for personalized treatment plans and prognoses to improve patient outcomes. Therefore, it is necessary to confirm the cytology lesions and determine the appropriate treatment.
Acknowledgments
Statement of Transparency and Principals:
• Author declares no conflict of interest
• Study was approved by Research Ethic Committee of author affiliated Institute.
• Study’s data is available upon a reasonable request.
• All authors have contributed to implementation of this research.
References
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries Sung H, Ferlay J, Siegel RL , Laversanne M, Soerjomataram I, Jemal A, Bray F. CA: a cancer journal for clinicians.2021;71(3). CrossRef
- National Cancer Institute. 2023. Infectious agent: HPV and Cancer. https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer .
- Knowledge, attitude and practice of cervical cancer screening and associated factors amongst female students at Wollega University, western Ethiopia Tilahun T, Tulu T, Dechasa W. BMC research notes.2019;12(1). CrossRef
- Cleveland Clinic. Cervix. https://my.clevelandclinic.org/health/body/23279-cervix. 2022.
- Pap Smear Collection and Preparation: Key Points Kamal M. CytoJournal.2022;19. CrossRef
- Pap smear. Medscape. https://emedicine.medscape.com/article/1947979-overview. Karjane NW . 2018.
- Cervical cancer: Epidemiology, risk factors and screening Zhang S, Xu H, Zhang L, Qiao Y. Chinese Journal of Cancer Research = Chung-Kuo Yen Cheng Yen Chiu.2020;32(6). CrossRef
- Cervical Screening: Overview, Human Papillomavirus, Papanicolaou Test. Medscape. https://emedicine.medscape.com/article/1618870-overview. Karjane NW . 2021.
- Cost-Effectiveness of Primary HPV Testing, Cytology and Co-testing as Cervical Cancer Screening for Women Above Age 30 Years Jin XW , Lipold L, Foucher J, Sikon A, Brainard J, Belinson J, Schramm S, et al . Journal of General Internal Medicine.2016;31(11). CrossRef
- American Cancer Society. Diagnosing HPV. https://www.cancer.org/cancer/risk-prevention/hpv/hpv-and-hpv-testing.html 2023.
- European guidelines for quality assurance in cervical histopathology Bulten J, Horvat R, Jordan J, Herbert A, Wiener H, Arbyn M. Acta Oncologica (Stockholm, Sweden).2011;50(5). CrossRef
- Gisela Dallenbach-Hellweg Magnus von Knebel Doeberitz Trunk MJ (n.d.) . .
- Precancerous lesions of the cervix and its determinants among Ethiopian women: Systematic review and meta-analysis Tsehay B, Afework M. PloS One.2020;15(10). CrossRef
- Precancerous Lesions of the Cervix and Associated Factors among Women of East Gojjam, Northwest Ethiopia, 2020 Getinet M, Taye M, Ayinalem A, Gitie M. Cancer Management and Research.2021;13. CrossRef
- A Hybrid Deep Learning and Handcrafted Feature Approach for Cervical Cancer Digital Histology Image Classification Almubarak H, Stanley R, Guo P, Long L., Antani S, Thoma G, Zuna R, Frazier S, Stoecker W. International Journal of Healthcare Information Systems and Informatics.2019;14. CrossRef
- Invasive cervical cancer: Symptoms, diagnosis, and treatment. Medical new Today. https://www.medicalnewstoday.com/articles/invasive-cervical-cancer Haye V. 2023.
- Diagnosis, staging, and surveillance of cervical carcinoma Kaur H, Silverman PM , Iyer RB , Verschraegen CF , Eifel PJ , Charnsangavej C. AJR. American journal of roentgenology.2003;180(6). CrossRef
- Imaging and Staging of Cervical Cancer Devine C, Viswanathan C, Faria S, Marcal L, Sagebiel TL . Seminars in ultrasound, CT, and MR.2019;40(4). CrossRef
- New technologies for cervical cancer screening Brown AJ , Trimble CL . Best Practice & Research. Clinical Obstetrics & Gynaecology.2012;26(2). CrossRef
- Colposcopy and treatment of cervical precancerous. International Agency for Research on Cancer. https://www.ncbi.nlm.nih.gov/books Sankaranarayanan R, Prendiville W. 2017.
- Patient education: Follow-up of low-grade abnormal Pap tests (Beyond the Basics). Up To Date. https://www.uptodate.com/contents/follow-up-of-low-grade-abnormal-pap-tests-beyond-the-basics/print Goodman A. 2022.
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2024
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times