Pneumocystis jirovecii Pneumonia Infection in Breast Cancer Receiving Dose-dense Chemotherapy: A Case Report and Literature Review
Download
Abstract
We herein report 3 cases of breast cancer who developed Pneumocystis Jirovecii Pneumonia during dose-dense chemotherapy. The patients developed fever, fatigue, and cough on the 4th to 5th cycle of chemotherapy. Chest computed tomography (CT) scan showed ground-glass opacities, later on confirmed PCP as bronchoalveolar lavage (BAL) was performed. They were treated with Trimethoprim-Sulfamethoxazole (TMP-SMX) for 3 weeks, with clinical improvement. The first case was able to complete subsequent cycles of chemotherapy with TMP-SMX prophylaxis. The second case omitted the remaining cycles of chemotherapy and proceeded with adjuvant hormonal and radiotherapy. The third case also discontinued subsequent cycles and proceeded with surgery. All 3 cases didn’t developed complications nor recurrence of infection on their subsequent treatments. Clinicians must be vigilant in recognizing those at risk for PCP especially among cancer patients undergoing dose-dense chemotherapy. Moreover, as the infection is life-threatening, early treatment must be initiated to prevent serious complications. Prophylactic antibiotic must be considered if there is a high-risk for recurrence of infection, thus preventing delay in surgery or adjuvant therapies.
Introduction
Breast Cancer incidence has risen in the past decades, with annual increase of 0.5%, contributed by the early localized diagnosis and hormone receptor-positive disease [1]. With the advent of neoadjuvant chemotherapy, more tumors are down-staged with the view of doing breast-conserving surgery [2]. It is also being used to achieve a pathologic complete response, which has shown to be a positive surrogate prognostic factor of survival after treatment [3].
Dose Dense Chemotherapy is a recommended strategy in the neoadjuvant/adjuvant setting, by the process of shortening dosing interval and increasing the dosing intensity. Clinical outcomes improve in terms of Disease-Free Survival (DFS) and Overall Survival (OS) with the said regimen [4]. However, concomitant infection during this treatment may occur owing to its higher dose and shorter interval. In one randomized trial, 11.5% of patients who had dose-dense chemotherapy developed infections and 89.8% had leukopenia/neutropenia during the treatment [5].
Pneumocystis Jirovecii Pneumonia (PCP) is an opportunistic infection often affects those with impaired immunity such as Human Immunodeficiency Virus (HIV) Infection. However, HIV-negative patients are also affected such as those with hematologic and solid malignancies, autoimmune disorders, and organ transplantation. They typically colonize in the lungs, sometimes asymptomatic, activate an inflammatory response, ultimately causing lung damage, which has the potential to worsen other comorbid pulmonary conditions [6]. The pathogen populates when there is low CD4 count (<200cells/uL) typically in HIV-positive patients [7]. Waks et al previously reported a case of 19 early-stage breast cancer patients who developed PJP with neoadjuvant dose-dense chemotherapy [8]. They developed the infection after 3rd to 4th cycle of dose-dense Adriamycin (A) and cyclophosphamide (C) regimen, with an overall incidence of 0.6%. Several case reports and series were also published with the same timing of chemotherapy and PJP development [9-11]. Of note, the typical findings of these patients are the presence of bilateral diffuse infiltrations and ground-glass opacities, and positive of PCR of P. jirovecii DNA.
Herein, we report 3 cases of Pneumocystis Pneumonia (PCP) patients undergoing dose-dense chemotherapy for early-staged breast cancer.
Case 1
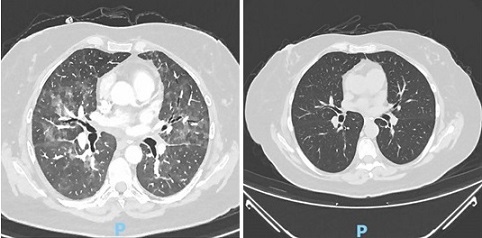
A 57-year old woman diagnosed with stage IA (cT1N0M0) left breast cancer, ER-positive, HER2-negative, ki-67 of 80% received a neoadjuvant chemotherapy with dose-dense (dd) epirubucin and cyclophosphamide (EC) regimen. Chemotherapy was administered every 2 weeks for a total of 4 cycles. After the 1st cycle, she developed fever, cough, and worsening asthma condition. CT scan of the chest was done revealed no significant changes. A diagnosis of tracheobronchitis was given and treated with moxifloxacin. Subsequent chemotherapy cycles were continued. After the 4th cycle of ddEC, she was admitted at our institution due to fever and partial respiratory failure. Laboratory results showed low hemoglobin, normal leukocyte count, normal procalcitonin, and elevated C-reactive protein and LDH. Liver function and renal tests were non-significant. A chest CT scan reported bilateral and diffuse parenchymal changes which are characterized by a ground-glass infiltrate, with coalescent areas of oligoemia, leading to a mosaic perfusion pattern and interstitial, interlobular thickening more on the lower lobes (Figure 1).
Figure 1. Chest CT Scan of Case 1 on Admission and after the Treatment for PJP, Showing Characteristic of Bilateral Diffuse Parenchymal Changes which are Ground-glass Infiltrates with Coalescent of Oligemia(a) and Resolution of Ground-glass Densifications. No copyright attribution.
A ventilatory support was also done.
Based on these findings, a PCP was suspected. Bronchioalveolar lavage (BAL) was performed revealing positive for pneumocystis jirovecii DNA. Trimethoprim/ sulfamethoxazole (SMX+TMP) was initiated with systemic corticosteroids for the respiratory failure. After 3 weeks of treatment, her symptoms significantly improved and labs were normal. Her repeat chest CT scan also showed global resolution of ground-glass densification.
A decision in MDT to interrupt neoadjuvant chemotherapy and proceed with the surgery was made. She subsequently underwent tumorectomy with reconstruction. The histopathaology showed ypT0ypN0, RCB-0 (pCR). Adjuvant radiotherapy was given and she’s continuously following-up in the clinic.
Case 2
A 59-year old woman, diagnosed with stage IIIA right breast cancer, ER- and PR-positive, HER2-negative, ki-67 of 20%, underwent upfront breast conserving surgery with sentinel lymph node biopsy (SLNB). She’s planned to receive adjuvant chemotherapy with 4 cycles of ddEC every 2 weeks followed by 4 cycles of dose-dense paclitaxel for 4 cycles every 3 weeks, adjuvant radiotherapy and hormonal therapy.
On the 4th dose of ddEC, she developed fever, rhinorrhea, myalgia, and extreme fatigue. Laboratories were done showing an absolute neutrophil count (ANC of 300cells/mm3. She was started on moxifloxacin tablet. 6 days after, her fever persisted and was admitted at our institution with stable vital signs and no cardiorespiratory distress signs. Laboratories showed hgb = 9.3mg/dl, WBC = 0.10 x 109/L, platelets = 123 x 109/L, elevated C-reactive protein, LDH, and procalcitonin, with normal live rand kidney function tests. She was started on piperacillin+tazobactam with vancomycin for the febrile neutropenia. An initial chest CT scan was done showing normal findings. 6 days after, her fever persisted, prompting to repeat chest CT scan showing diffuse mosaic pattern in both lungs (Figure 2).
Figure 2. Chest CT Scan on Admission and after the Treatment for PJP, Characterized as Diffuse Mosaic Pattern in both Lungs (a) and Full Resolution of the Mosaic Pattern (b). No Copyright Attribution.
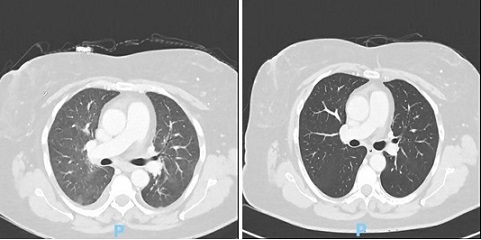
Bronchoalveolar lavage was performed with a result of positive for Pneumocystis jirovecii DNA. She was started with Trimethoprim + Sulfamethoxazole 800/160mg 5x a day, with clinical improvement and eventually discharged. Repeat chest CT scan after 3 weeks showing full resolution of the previously described mosaic pattern. The decision of the team was to omit the subsequent chemotherapy of paclitaxel and proceeded with the adjuvant radiation and hormonal therapy.
Case 3
A 49-year old woman diagnosed with triple-negative invasive breast carcinoma, cT1cN0M0, ki-67: 80%, underwent neoadjuvant dose-dense (dd) epirubicin-cyclophosphamide (EC) every 2 weeks for 4 cycles followed by dose-dense 4 cycles of paclitaxel and total steroids of 96mg per cycle in the paclitaxel. After the 5th cycle of chemotherapy, she developed fever of 38 oC with accompanying chills and low back pain. Due to persistence of symptoms and worsening fever and now with dry cough, she was admitted at our hospital. The vital signs were as follow: BP 110/65, HR 88bpm, o2 saturation 100% at room air. Physical exam showed no cyanosis no adventitious breath sounds. Laboratories revealed normal leukocyte count, slightly low hemoglobin and platelets, and high C-reactive protein, LDH, and procalcitonin. She was started on moxifloxacin. 4 days after the start of antibiotic, her fever worsen. A chest CT scan was done (Figure 3) showing multiple areas of ground-glass densification creating a mosaic pattern with thickening of the interlobular septa. BAL was positive for pneumocystis jirovecii DNA.
Figure 3. Chest CT Scan on Admission and after the Treatment for PJP, Described as Multiple Areas of Ground-glass Densification Creating a Mosaic Pattern with Thickening of Interlobular Septa (a), and Complete Resolution of Ground-glass and Attenuation Gradient. No Copyright Attribution .
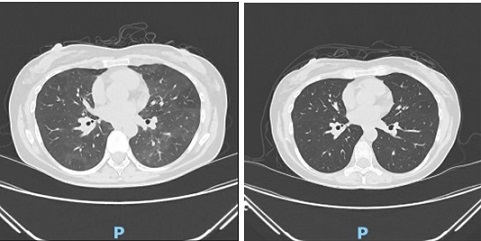
Moxifloxacin was shifted to Sulfamethoxazole + Trimethoprim (SMX+TMP) 800/160mg 5x a day. however, she developed skin rashes. Antibiotic was reduced to 3x a day and started on methylprednisolone 32mg/day. 2 weeks after the treatment, there was resolution of symptoms and normalization of blood tests. A repeat chest CT scan showed complete resolutions of ground-glass and attenuation gradient. She continued to complete her neoadjuvant chemotherapy with prophylactic SMX+TMP and proceeded with the surgery as scheduled. Table 1 shows the laboratory results of the 3 the cases with the comparisons.
| WBC | Neutrophils | CRP | Procalcitonin | LDH | |
| (cells/m3) | (cells/m3) | ||||
| Reference Range | 4.0-10.0 x109 / L | 1.90-7.00 x109 / L | <0.60 mg/dL | <0.1ng/mL | 120-246 UI/L |
| Case 1 | 10.4 | 9.01 | 0.81 | <0.06 | 323 |
| Case 2 | 7.8 | 6.47 | 9.84 | 0.11 | 433 |
| Case 3 | 14.4 | 12.64 | 5.99 | <0.06 | 310 |
(WBC, White Blood Cell; CRP, C-Reactive Protein; LDH, Lactate Dehydrogenase). No copyright attribution.
Case 1 and 3 have elevated WBC and neutrophils. All cases have normal C-reactive protein.
Case 2 has elevated procalcitonin, and all the 3 cases have elevated LDH results.
Discussion
Pneumocystis Pneumonia is a serious infection caused by the pathogen Pneumocystis jirovecii that often affects the immunocompromised including cancer patients. This the first case series in Portugal that involved breast cancer patients developing PCP during dose-dense chemotherapy.
Pneumocystis Jirovecii is believed to be a fungal pathogen first identified by Chagas in 1909. It was previously coined as Pneumocystis carinii, however because it was isolated from different host species, it was believed that PCP is not a zoonotic disease [12]. The organism usually colonize the lungs causing the pneumonia in those with low immune system, as was seen in our cases. Because of the immunosuppressed state of the patients, early detection of infections is essential in the care of the cancer patients. Other types of solid tumors may also affected by the infection. The most common is lung cancer followed by breast, pancreatic, rectal, thyroid, and other malignancies [13]. Different factors contribute to the development of infection such as dose and timing of chemotherapy, steroids intensity that is used as antiemetic, and pre-existing lung condition (e.g. ILD) [8]. There are common patients’ characteristics in our case that was also seen in the previous studies. Similar to the case series by Shiiba et al, all of the cases developed PCP after the 3rd to 4th cycle of chemotherapy which was also seen in our study. The steroid dose was also identical to that of our patients, which averaged to a total of 96mg of dexamethasone for the 4 cycles. Although the they are beneficial during cancer treatment to prevent chemotherapy-induced allergy and as anti-emetics, they may also pose a risk for infection. Caution must be taken when using steroids.
Dose-dense chemotherapy is defined as delivering the drugs by escalating the doseper-cycle or reducing the interval between cycles [14]. In a clinical trial by Citron et al, dose density improves the clinical outcome significantly, and sequential dosing is as effective as concurrent [4]. Although Waks et al reported the actual incidence of PCP in dose-dense chemotherapy is seemed to be low (0.6%), in another case series in Japan showed 3.6% incidence rate [9]. The actual incidence in our institution was not determined in this study.
The common symptoms of PCP include dyspnea, nonproductive cough, and fever. They may also have tachycardia and tachypnea. Lung findings may also be normal. It may be difficult to differentiate patients from the typical bacterial pneumonia, as they may present similarly. Non-response to empirical antibiotics may suspect PCP especially in the background of immunocompromised state. (1,3)-Beta D-Glucan (BG) is a cell wall constituent of Pneumocystis jirovecii [15]. Various assays has been developed, with 96% sensitivity rate being demonstrated in both HIV-positive and negative patients [16]. However, other fungal infection if present may decrease the sensitivity. The advantage of this test is that it is non-invasive and the result is rapid. It was not done in our study since B-D-glucan assay is not available in our institution. Serum Lactate Dehydrogenase (LDH) has also been found to be elevated in patients with PCP. In one study, the sensitivity of LDH differs between HIV-positive and HIV negative patients, with sensitivity of 63% and 100%, respectively [17]. Cancer patients may also have an elevated LDH due to increased metabolism. It is difficult to conclude that LDH can be used as a serum indicator of PCP since they are more correlated with oxygenation and BAL neutrophils [18]. Therefore, LDH may be more of a reflection of lung inflammation that is not specific for PCP. Other tests include KL-6 and S-adenosylmethionine, but they have a low sensitivity and cannot be part of the recommendation at this time. Lymphopenia is an established risk factor predisposing to PCP, although the exact value cut-off of Absolute Lymphocyte Count (ALC) is unknown [19]. They are less obvious in non-HIV patients.
The typical findings of PCP in chest CT scan is the presence of ground-glass opacity. They occur more frequent in the non-HIV immunocompromised patients as compared to HIV-positive that usually present cystic lesions [20]. The difference in the radiographic characteristics is due to inflammatory response difference between them. HIV-positive patients tends to have long-standing, low-intensity inflammation hence the formation of cysts as compared to more pronounced, rapid lung destruction in non-HIV patients as was seen in our cases. Although ground-glass opacities can also be seen in other cases such as alveolar hemorrhage, pulmonary edema, or COVID-19 infection, they were ruled out based on the patients’ clinical history. The current gold standard in the diagnosis of PCP is thru the bronchoalveolar lavage (BAL) culture isolation [21]. In a meta-analysis done, the accuracy of polymerase chain reaction (PCR) was 97% sensitive and 94% specific in identifying Pneumocystis jirovecii from the sample [22]. This was the test the confirmed PCP in our cases.
Treatment for presumed PCP should not be compromised while pending for diagnostic measures especially if with strong clinical suspicions. Mild cases can be treated with oral medications. In our cases they presented with respiratory compromise hence treatment with IV antibiotics and hospital admission are warranted. Due to its efficacy, the first-line treatment for PCP regardless of HIV status is Trimethoprim+Sulfamethoxazole (TMP-SMX) given for 21 days [23]. The treatment response in immunocompromised host often requires 7-10 days before clinical improvement can be seen. Adjunctive corticosteroids can be given and proven to be effective in those with respiratory failure. There is a benefit in giving prophylactic antibiotic for PCP in cancer patients. A large meta-analysis conducted has shown to reduced all-cause mortality for patients receiving chemotherapy [24]. Data on the duration of prophylaxis are still insufficient and warrants further studies. The current recommendation is to give prophylactic antibiotic in high-risk to develop infection and febrile neutropenia at the start of chemotherapy. Green et al stated in a meta-analysis stated that PCP prophylaxis is warranted when the risk for infection is >3.5%, thus reducing occurrence by 91% [25].
In conclusion, PCP infection among breast cancer patients undergoing dose-dense chemotherapy are underrepresented subset of patients. Early recognition of at-risk patients is warranted especially in those with high risk factors such as use and intensity of corticosteroids, lymphopenia, and pre-existing lung conditions. This pool of data may contribute to the future development of risk calculator for the prevention of PCP pneumonia.
Acknowledgments
The authors would like to acknowledge the patients for their permission to write this case series, the oncology, radiology, and pulmonary department of Champalimaud Foundation for their contribution in the cases.
Disclosures
The authors have no conflict of interests, financial support, nor direct human involvement in the writing of this work.
Ethical Approval
This case series was conducted in accordance with the Declaration of Helsinki. Protection of patients’ privacy was performed during the collection and evaluation of all the data.
References
- Breast Cancer Statistics, 2022 Giaquinto AN , Sung H, Miller KD , Kramer JL , Newman LA , Minihan A, Jemal A, Siegel RL . CA: a cancer journal for clinicians.2022;72(6). CrossRef
- Neoadjuvant chemotherapy in breast cancer Charfare H, Limongelli S, Purushotham AD . The British Journal of Surgery.2005;92(1). CrossRef
- Evaluation of the Efficacy of Neoadjuvant Chemotherapy for Breast Cancer Wang H, Mao X. Drug Design, Development and Therapy.2020;14. CrossRef
- Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741 Citron ML , Berry DA , Cirrincione C, Hudis C, Winer EP , Gradishar WJ , Davidson NE , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2003;21(8). CrossRef
- Effect of Tailored Dose-Dense Chemotherapy vs Standard 3-Weekly Adjuvant Chemotherapy on Recurrence-Free Survival Among Women With High-Risk Early Breast Cancer: A Randomized Clinical Trial Foukakis T, Minckwitz G, Bengtsson N, Brandberg Y, Wallberg B, Fornander T, et al . JAMA.2016;316(18). CrossRef
- Pneumocystis jirovecii Pneumonia in the Non-HIV-Infected Population Avino LJ , Naylor SM , Roecker AM . The Annals of Pharmacotherapy.2016;50(8). CrossRef
- Prescription of Pneumocystis Jiroveci Pneumonia Prophylaxis in HIV-Infected Patients Lin X, Garg S, Mattson CL , Luo Q, Skarbinski J. Journal of the International Association of Providers of AIDS Care.2016;15(6). CrossRef
- Pneumocystis jiroveci pneumonia (PCP) in patients receiving neoadjuvant and adjuvant anthracycline-based chemotherapy for breast cancer: incidence and risk factors Waks AG , Tolaney SM , Galar A, Arnaout A, Porter JB , Marty FM , Winer EP , Hammond SP , Baden LR . Breast Cancer Research and Treatment.2015;154(2). CrossRef
- Pneumocystis jirovecii Pneumonia in Three Patients With Breast Cancer Receiving Neoadjuvant Dose-Dense Chemotherapy Shiiba R, Himeji D, Matsumoto R, Tanaka G, Otomo N. Cureus.2022;14(2). CrossRef
- Pneumocystis jirovecii in a patient on dose-dense chemotherapy for early breast cancer Khoo C, Gilchrist J, Williamson JP , Paul , Kefford R. Respirology Case Reports.2019;7(7). CrossRef
- [Concurrent COVID-19 and Pneumocystis Jirovecii Pneumonia in a Patient with Breast Cancer Receiving Adjuvant Dose-Dense Chemotherapy] Fujii K, Chihara Y, Mishima C, Yamamoto M. Gan to Kagaku Ryoho. Cancer & Chemotherapy.2022;49(13).
- Pneumocystis jiroveci Pneumonia: A Review of Management in Human Immunodeficiency Virus (HIV) and Non-HIV Immunocompromised Patients Ibrahim A, Chattaraj A, Iqbal Q, Anjum A, Rehman MEU , Aijaz Z, Nasir F, et al . Avicenna Journal of Medicine.2023;13(1). CrossRef
- Clinical characteristics and risk factors associated with Pneumocystis jirovecii infection in patients with solid tumors: study of thirteen-year medical records of a large cancer center Takeda K, Harada S, Hayama B, Hoashi K, Enokida T, Sasaki T, Okamoto K, Nakano K, Ohkushi D. BMC cancer.2021;21(1). CrossRef
- Dose-Dense Chemotherapy: Principles, Clinical Results and Future Perspectives Citron ML . Breast Care (Basel, Switzerland).2008;3(4). CrossRef
- Diagnosing Pneumocystis jirovecii pneumonia: A review of current methods and novel approaches Bateman M, Oladele R, Kolls JK . Medical Mycology.2020;58(8). CrossRef
- Diagnostic accuracy of serum 1,3-β-D-glucan for pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis Onishi A, Sugiyama D, Kogata Y, Saegusa J, Sugimoto T, Kawano S, Morinobu A, Nishimura K, Kumagai S. Journal of Clinical Microbiology.2012;50(1). CrossRef
- Accuracy of serum LDH elevation for the diagnosis of Pneumocystis jiroveci pneumonia Vogel MN , Weissgerber P, Goeppert B, Hetzel J, Vatlach M, Claussen CD , Horger M. Swiss Medical Weekly.2011;141. CrossRef
- Serum indicators for the diagnosis of pneumocystis pneumonia Tasaka S, Hasegawa N, Kobayashi S, Yamada W, Nishimura T, Takeuchi T, Ishizaka A. Chest.2007;131(4). CrossRef
- Acute Pneumocystis jirovecii Pneumonia Due to Absolute Lymphopenia Hilker E, Patil SM , Wilhite R, Holliday Z. Cureus.2022;14(3). CrossRef
- Radiological features of Pneumocystis jirovecii Pneumonia in immunocompromised patients with and without AIDS Hardak E, Brook O, Yigla M. Lung.2010;188(2). CrossRef
- Utility of flexible bronchoscopy with polymerase chain reaction in the diagnosis and management of pulmonary infiltrates in allogeneic HSCT patients Tang F, Zhao X, Xu L, Zhang X, Chen Y, Mo X, Liu K, Huang X. Clinical Transplantation.2018;32(1). CrossRef
- Use of real-time polymerase chain reaction for the diagnosis of Pneumocystis pneumonia in immunocompromised patients: a meta-analysis Summah H, Zhu Y, Falagas ME , Vouloumanou EK , Qu J. Chinese Medical Journal.2013;126(10).
- An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients Limper AH , Knox KS , Sarosi GA , Ampel NM , Bennett JE , Catanzaro A, Davies SF , et al . American Journal of Respiratory and Critical Care Medicine.2011;183(1). CrossRef
- Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy Gafter-Gvili A, Fraser A, Paul M, Vidal L, Lawrie TA , Wetering MD , Kremer LCM , Leibovici L. The Cochrane Database of Systematic Reviews.2012;1(1). CrossRef
- Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials Green H, Paul M, Vidal L, Leibovici L. Mayo Clinic Proceedings.2007;82(9). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2024
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times