Addressing Constipation in Head and Neck Cancer Patients Undergoing Radiotherapy: A Clinical Audit to Improve Management
Download
Abstract
Background and objective: Constipation is a prevalent and often overlooked symptom in patients undergoing radiotherapy (RT) for head and neck cancer, leading to patient distress and potential treatment non-compliance. This clinical audit aimed to assess the burden of constipation and evaluate the effectiveness of a guideline-based management approach.
Methods: This standard-based clinical audit was conducted in the Department of Radiation Oncology from July 2021 to January 2022, involving patients with constipation during RT for head and neck cancer. The intervention implemented followed the European Society for Medical Oncology (ESMO) 2018 flowchart for constipation management, divided into four key checkpoints: history taking, laxative documentation, symptom relief assessment, and maintenance therapy prescription. A standard of 65% adherence to these checkpoints was set.
Results: A total of 48 patients were evaluated retrospectively and 44 prospectively. Constipation was reported by 32 (66.6%) patients in the initial assessment and 26 (60%) patients in the re-assessment. Prior to intervention, none of the ESMO flowchart checkpoints were consistently addressed, while after implementation, adherence significantly increased to 92% for history taking and laxative documentation, and 77% for symptom relief assessment and maintenance therapy prescription. The mean number of days for complete symptom relief decreased from 7 to 2 days, and the time to reporting constipation reduced from 5 to 3 days. The number of active reviews until symptom resolution increased from 0 to 2 times.
Conclusion: Empirical management of constipation during RT for head and neck cancers is associated with poor outcomes. Implementing guideline-based treatment approaches, as outlined by the ESMO flowchart, resulted in significantly improved symptom control and patient care. These findings highlight the importance of a structured and proactive approach to managing constipation in this patient population.
Introduction
Constipation is a common symptom in patients with cancer. Literature shows that constipation occurs in 23-65% of advanced cancer [1, 2]. In clinical practice more so in patients undergoing Radiotherapy (RT) for head and neck cancer, this symptom is not only very common, but many a times left unattended. Most patients are on opioid analgesics, concurrent chemotherapy, anti-emetics and decreased oral intake lacking dietary fiber which induce constipation in a multifaceted manner. A typical RT protocol for head and neck cancer lasts for 6-7 weeks with each fraction of RT is delivered every day for five days a week. Unrelieved constipation causes a vicious cycle of pain, discomfort, poor oral intake, poor compliance to prescribed medications and pain leading to treatment interruption. Uninterrupted RT treatment is recommended as it has a bearing on the ultimate survival outcomes [3-5]. Hence compliance to planned treatment is threatened by improper management of constipation.
At our Institution, though constipation was recognized in patients undergoing RT for head and neck cancers, due to anatomical non-proximity of the symptom with the RT treatment site (generally head and neck region) and poor insight into symptom management, many were treated empirically with poor results. We carried out this audit with the intent to know the symptom burden and to manage constipation appropriately, which would improve our clinical practice for a better patient care in patients undergoing RT for head and neck cancer.
Materials and Methods
This audit was conducted in the department of Radiation Oncology from July 2021 to January 2022. Protocol or guideline-based treatment for the management of constipation were not used prior to the audit. The European Society for Medical Oncology (ESMO) guidelines for management of constipation 2018 – the flow chart, was used as audit criteria, in this standard based audit [6]. The ESMO Clinical Practice Guideline (CPG) is directed towards adult cancer patients experiencing constipation because of their cancer diagnosis or treatment. The procedures followed were in accordance with the ethical framework of revised Helsinki Declaration of 1975. This audit was presented as abstract at the 30th Annual International Conference of the Indian Association of Palliative Care 2023.
Inclusion criteria
All head and neck cancer patients receiving curative intent radiotherapy to the head and neck region with or without concurrent chemotherapy treated under a single consultant who experienced at least one episode of constipation during RT.
Data collection
Along with the demographic, tumour, and treatment related characteristics, the medical case records of these patients were evaluated for the complaint of constipation during RT. In patients with constipation, for ease of quantification, a note was made whether the four pertinent checkpoint questions from the ESMO flow chart (Figure 1) were documented or not –
Figure 1. ESMO Flowchart for Management of Constipation*. *Reproduced from ESMO guidelines 2018 [6].
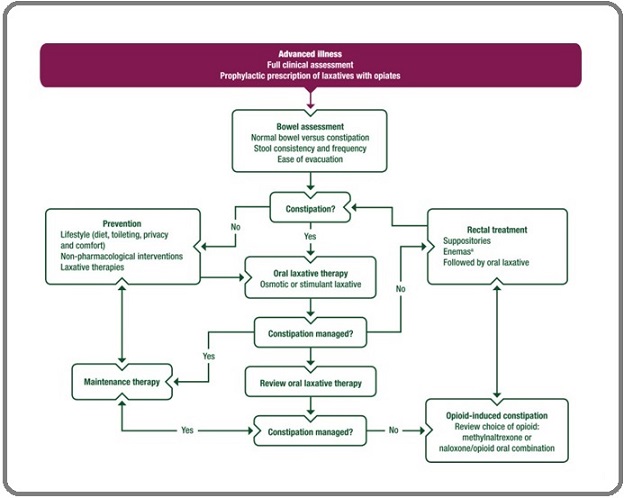
1. Is the history of constipation taken with respect to – stool consistency, frequency, ease of evacuation?
2. Are the type of laxative used and concurrent use of any other constipating drugs documented? 3. Was the patient reviewed for symptom relief? 4. Was the patient put on maintenance therapy?
Initial assessment
A retrospective audit of the case records of the patients treated between 1st July 2021 to 30th September 2021, along with demographic, tumour, and treatment related parameters with complains of constipation was noted. If constipation was documented, a note was made whether the four pertinent questions were dealt with or not.
Intervention
After collecting the baseline data from the initial assessment, in the month of October 2022 intervention was planned which constituted of priming of the team consisting of Radiation Oncologist, nurse and junior doctor to promptly elicit history of constipation during RT. Once symptom was identified, the team had to follow the audit criteria and address all the 4 check points.
Audit criteria – The ESMO guidelines 2018 for the management of constipation - the flow chart with 4 check points.
Setting the standard – the standard was set by us, to follow the flow chart in at least 66% of the patients. Though ideally it must be followed in all patients (100%), as this was our first audit, the aim was to follow in at least two-thirds (66%) of the symptomatic patients.
Re-assessment
After one month of priming period, data with respect to same 4 pertinent checkpoint questions and other demographic characteristics were collected in patients complaining of constipation in a prospective manner from 1st November to 31st January 2022.
Analysis and statistics
Frequency, percentages, and measures of central tendencies were used to compare the results of the initial assessment and re-assessment audits.
Results
A total of 48 patients in the initial assessment and 44 patients in the re-assessment were evaluated. The mean age in both the audit was 54 years, 32 (66.6%) patients in the initial assessment and 26 (60%) patients in the re-assessment reported constipation during RT. Other demographic and RT related characteristics are as depicted in Table 1.
| Characteristics | Initial assessment (48) (%) | Re assessment (44) (%) |
| Age | 35-78 years (mean - 54) | 23-78 years (mean - 54) |
| Gender | ||
| Male: Female | 37/11 | 35/9 |
| Intent of Treatment | ||
| Definitive RT | 16 (33.3) | 19 (43) |
| Post op RT | 32 (66.6) | 25 (5) |
| Radiotherapy Dose | ||
| Median | 66Gy | 66Gy |
| Range | 60-70Gy | 60-70Gy |
| Concurrent Chemotherapy | ||
| Yes | 26 (54) | 21 (48) |
| Cisplatin/Carboplatin | 23/3 | 19/2 |
| No | 22 (46) | 23 (52) |
| Primary Cancer Site | ||
| Oral Cavity | 33 (68.75) | 32 (73) |
| Laryngo-pharynx | 15 (31.25) | 12 (27) |
| Constipation | ||
| Yes | 32 (66.6) | 26 (60) |
| No | 16 (33.3) | 18 (40) |
| In Constipated Patients (number = 32/26) | ||
| Chemo received/not received | 11/21 | 10/16 |
| Oral cavity/Laryngopharynx | 20/12 | 18/8 |
Comparing the pre intervention and post intervention, the 4 checkpoints of the ESMO guidelines were never addressed (0%) as compared to 92%, 92%, 77% and 77% respectively. The mean number of days for complete symptom relief were 7 v/s 2 days respectively. Mean number of days to report constipation was 5 days v/s 3 days respectively. Local examination (per-rectal examination) rate was 0 v/s 12 (46%). The mean number of active reviews till complete symptom relief was 0 v/s 2 times as shown in Table 2.
| Checkpoints from flow chart | Initial assessment (Number-32) | Re-assessment (Number-26) |
| 1.Comprehensive history taken? (Stool consistency, frequency, ease of evacuation?) | 0 (0 %) | 24 (92%) |
| 2. Documentation of the type of laxative used and concurrent use of any other constipating drug? | 0 (0 %) | 24 (92%) # |
| 3. Active review for symptom relief? | 0 (0 %) | 20 (77%)* |
| 4. Put on maintenance therapy? | 0 (0 %) | 20 (77%) |
| Mean duration to report constipation | 5 days (range 2-7) | 3 days (range 2-5) |
| No. of days for complete symptom relief | Range - 1-10 Days | Range 1-6 Days |
| Mean – 7 days | Mean – 2 days | |
| No.of Reviews till complete relief | Not documented | Mean – 2 (active review) |
| Type of laxatives used | Oral syrups and tablets, saline enemas. | Oral syrups, tablets, rectal suppositories, adjuvant local applications, saline enemas – wherever found appropriate |
Discussion
Symptom management is one of the cornerstones of medicine. American Society of Clinical Oncology (ASCO) CPG [7], for integration of Palliative care into standard oncology care, in their 3rd recommendation propose that patients with advanced cancer should receive symptom, distress, and functional status management (e.g. pain, dyspnoea, fatigue, sleep disturbance, mood, nausea, or constipation).
Failure to achieve good symptom relief increases the patient’s distress, leads to poor compliance to planned treatment, thus jeopardizing the treatment outcomes and compromises quality of life. Though there are literature regarding the incidence and management of constipation in advanced/ metastatic cancers [8] or in opioid use [9, 10] there is dearth of data when it comes to constipation occurring during curative cancer therapy.
In our audit, nearly two-thirds (60-66%) of the patients on radiotherapy for Head and neck cancer reported constipation, which is same as the incidence of constipation reported in advanced cancer [2]. All the four checkpoints of the ESMO flowchart [6] were never addressed earlier, while they were better addressed in the re-assessment group. These results were well beyond the set standards of 66%, meaning more than two-thirds of the patients with complaints of constipation had prompt relief of symptom. The mean number of days to report constipation was also reduced from 5 days to 3 days, as the patients and caregivers were primed in an active manner during routine weekly reviews while on RT. With the active intervention, constipation was relieved early (mean 2 v/s 7 days), active reviews improved (0 v/s 2) and a wide range of laxative combinations based on the history, concurrent medications and clinical examination were explored in the re-assessment group as against the empirical use of laxatives earlier.
There exists no literature till date regarding incidence and management of constipation during RT for head and neck cancer, which made us wonder whether this is unique to our region or is it a real-world issue which is under reported. Even literature suggests that underestimation of symptom intensity by health care providers increased the risk of inadequate treatment (p < 0.001) [11]. This audit helped us to identify, quantify and manage the symptom of constipation appropriately, during RT and to bring about change in approach towards symptom management leading to better patient care. Though a clinical audit, to our knowledge, this is the only study which has tried to address constipation occurring during standard curative treatment. Though symptoms directly related to treatment (in our case RT), like oral mucositis, dysphagia, xerostomia, or dermatitis are given prime importance, the symptom of constipation is just one of the many symptoms which is not directly related to RT but impacts the quality of life, leading to poor compliance to curative treatment. Literature on importance of symptom care in clinical practice is the need of the hour for a better patient care.
Constipation during RT for head and neck cancers require active intervention. Management of constipation using existing guidelines help manage the symptom better leading to early symptom relief and better treatment compliance.
References
- Prevalence of symptoms among patients with advanced cancer: an international collaborative study. Symptom Prevalence Group Vainio A, Auvinen A. Journal of Pain and Symptom Management.1996;12(1). CrossRef
- A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease Solano JP , Gomes B, Higginson IR . Journal of Pain and Symptom Management.2006;31(1). CrossRef
- Time factor in postoperative radiotherapy: A multivariate locoregional control analysis in 868 patients Suwinski R, Sowa A, Rutkowski T, Wydmanski J, Tarnawski R, Maciejewski B. International Journal of Radiation Oncology*Biology*Physics.2003;56(2). CrossRef
- Treatment delays in oral cavity squamous cell carcinoma and association with survival Fujiwara RJT , Judson BL , Yarbrough WG , Husain Z, Mehra S. Head & Neck.2017;39(4). CrossRef
- Postoperative radiotherapy in squamous cell carcinoma of the oral cavity: the importance of the overall treatment time Langendijk J. A., Jong M. A., Leemans C. R., Bree R., Smeele L. E., Doornaert P., Slotman B. J.. International Journal of Radiation Oncology, Biology, Physics.2003;57(3). CrossRef
- Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines Larkin P. J., Cherny N. I., La Carpia D., Guglielmo M., Ostgathe C., Scotté F., Ripamonti C. I.. Annals of Oncology: Official Journal of the European Society for Medical Oncology.2018;29(Suppl 4). CrossRef
- Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update Ferrell BR , Temel JS , Temin S, Alesi ER , Balboni TA , Basch EM , Firn JI , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2017;35(1). CrossRef
- The management of constipation in palliative care: clinical practice recommendations Larkin P. J., Sykes N. P., Centeno C., Ellershaw J. E., Elsner F., Eugene B., Gootjes J. R. G., et al . Palliative Medicine.2008;22(7). CrossRef
- Incidence of constipation associated with long-acting opioid therapy: a comparative study Staats PS , Markowitz J, Schein J. Southern Medical Journal.2004;97(2). CrossRef
- Drugs for Treating Opioid-Induced Constipation: A Mixed Treatment Comparison Network Meta-analysis of Randomized Controlled Clinical Trials Sridharan K, Sivaramakrishnan G. Journal of Pain and Symptom Management.2018;55(2). CrossRef
- Inadequate symptom control in advanced cancer patients across Europe Laugsand EA , Jakobsen G, Kaasa S, Klepstad P. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer.2011;19(12). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2024
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times