Insights into Low-risk Gestational Trophoblastic Neoplasia from a Tertiary Care Institute in India: A Short Communication
Download
Abstract
Objective: To report clinical characteristics, treatment outcomes and chemotherapy-related toxicities in patients with low-risk GTN at tertiary care centre in India.
Material and methods: This retrospective observational study was conducted at the Department of Medical Oncology of Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi over 2 years. From December 2021 to December 2023, the medical records of all the patients diagnosed with GTN were retrospectively analyzed for clinical and treatment details. Low-risk GTN patients received methotrexate (MTX) with folinic acid (FA) rescue. The Chemotherapy regimen for Low-risk GTN resistant to first-line chemotherapy had received multiagent chemotherapy EMA-CO every 2 weeks.
Results: Of the 40 patients with low-risk GTN, only 35 women were available for evaluation as 5 were lost to follow-up during the treatment period. The study found that the majority of patients (71.4%) experienced a molar pregnancy before developing gestational trophoblastic neoplasia (GTN), with 91.4% developing GTN within the first 4 months. Of these, 32 patients achieved complete responses (91.4%), while 3 experienced treatment failure (8.5%). All three patients who failed primary MTX therapy were subsequently treated with multiagent chemotherapy and achieved complete remission (CR). Overall survival (OS) and cure rates for all patients with low-risk GTN were 100%.
Conclusion: The MTX regimen was remarkably effective in treating women with low-risk GTN, achieving a complete response (CR) rate of 91.4% without encountering severe adverse effects.
Introduction
Gestational trophoblastic neoplasia (GTN) encompasses a spectrum of disorders that arise from placental tissue [1]. Gestational Trophoblastic Neoplasms (GTN) refers to persistent or metastatic disease requiring chemotherapy. Fortunately, most women with GTN can be successfully treated while preserving their fertility. This category includes invasive mole (IM), choriocarcinoma (CCA), placental site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor (ETT) [2]. Notably, IM and CCA, which constitute the majority of cases, produce elevated human chorionic gonadotropin (hCG) levels, aiding in diagnosis and treatment monitoring.
Successful outcomes, even in advanced-stage disease, are attributable to the remarkable sensitivity of these neoplasms to chemotherapy and the utility of hCG as a diagnostic and monitoring tool [3]. GTN classification into low- and high-risk categories, based on FIGO (International Federation of Gynecology and Obstetrics) anatomic staging and WHO (World Health Organization) prognostic risk score, guides treatment decisions [4]. Low-risk GTN typically responds well to single-agent chemotherapy, such as methotrexate (MTX) or actinomycin D (Act D), achieving nearly 100% survival rates [5]. For patients with a prognostic score of 5-6 or a pathological diagnosis of choriocarcinoma, the risk of failure of first-line single-agent chemotherapy is significantly increased and combined chemotherapy is selected according to the regimen of patients with high prognostic score [6]. Overall, 85-90% of low-risk patients can be cured without multiagent chemotherapy or hysterectomy [7]. Although the disease is extremely sensitive to chemotherapy approximately 9-30% of the patient may develop resistance to first-line chemotherapy [8, 9]. The risk of resistance with a high FIGO score of 5-6 is fourteen times higher than a low FIGO score of 0-4 [10]. Despite advancements in diagnostics, uncertainties persist regarding risk factors predicting molar gestation, progression to malignancy, and response to single-agent chemotherapy. The data regarding the outcome of GTN from developing countries are scanty. Hence we report the clinical characteristics and outcomes of consecutive GTN
patients treated at our centre for 2 years.
Materials and Methods
This retrospective observational study was conducted at the Department of Medical Oncology of Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi over 2 years. From December 2021 to December 2023, the medical records of all the patients diagnosed with GTN were retrospectively analysed for clinical and treatment details after ethical clearance from the institutional review board. Clinical and histopathological criteria established the diagnosis of low-risk GTN.
Clinically GTN was diagnosed after molar or non-molar pregnancy when there was: (i) a plateau (< 15% drop) in the level of human chorionic gonadotropin (hCG) in four reading during 3 weeks, (ii) a 10% increase in hCG level for 3 readings during 2 weeks, or (iii) histologically confirmed choriocarcinoma [11].
Inclusion criteria include all those patients who were diagnosed with low-risk GTN based on clinical and/ or histopathological criteria. Exclusion criteria include High-risk GTN with FIGO score >6, patients who did not complete treatment and got lost to follow-up.
After the GTN diagnosis was made, an oncology staging workup was performed as per the hospital protocol. It includes history taking, physical examination, laboratory testing and imaging. About the imaging Chest X-ray and Ultrasound whole abdomen and pelvis were routinely performed. If they show findings of metastatic disease then cross-sectional imaging of Computed tomography (CT) or Magnetic resonance imaging (MRI) was performed.
Following the initial assessment, patients underwent categorization into low-risk disease group. This classification relied on the FIGO anatomic staging (Appendix A) and the WHO prognostic risk score (Appendix B). The WHO risk score, including age, antecedent pregnancy, interval to chemotherapy initiation, pretreatment serum Beta hCG level, tumor size, site and the number of metastases, and prior chemotherapy response helps in risk assessment. The low-risk disease was defined by either FIGO stage I GTN or stage II/III GTN with a WHO risk score below 7 [4]. Low-risk GTN patients received methotrexate (MTX) with folinic acid (FA) rescue. The methotrexate regimen includes 1.0–1.5 mg/kg IM every other day x 4 days, alternating with leucovorin 15 mg PO, every 2 weeks.
The Chemotherapy regimen for Low-risk GTN resistant to first-line chemotherapy was multiagent chemotherapy EMA-CO every 2 weeks (Figure 1).
Figure 1. Multiagent EMA-CO Regimen.
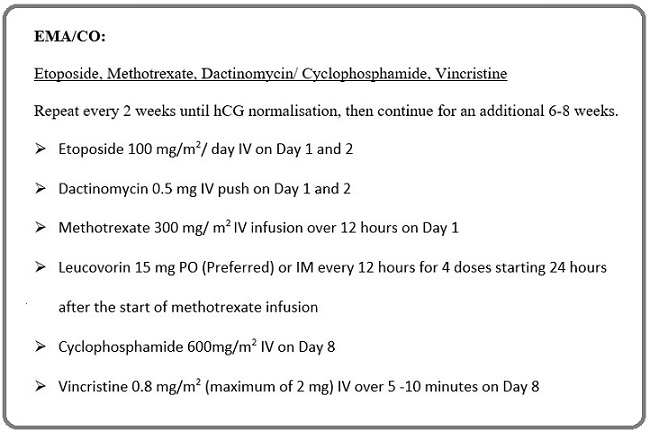
The chemotherapy is to be continued 6-8 weeks post-normalisation of Beta hCG levels. After completion of chemotherapy, all patients were evaluated every month for 1 year. All women of childbearing age were advised contraception during the follow-up period. Before each cycle of chemotherapy patient was advised to complete blood count, kidney function test and liver function test. Therapy was delayed for those having a WBC count < 3000/mm3, and platelet count < 1lakh/ mm3. A blood transfusion was done for Hb< 10g/dl along with chemotherapy.
Statistical analysis of the data was carried out and demographic data were determined using percentage, mean, and standard deviation. All statistical analyses were performed using the statistical package for the Social Science (SPSS) software, version 29.
Results
Of the 40 patients with low-risk GTN, only 35 women were available for evaluation as 5 were lost to follow-up during the treatment period.
Table 1 shows the patient’s clinical characteristics (n=35). The median age was 28 years (range, 18-44 years).
| Characteristics | Low-Risk GTN (WHO prognostic score ≤ 6) |
| (n= 35) | |
| Age at diagnosis (years) | |
| Median age | 28 |
| < 40 year, n (%) | 32 (91.4) |
| > 40 year, n (%) | 3 (8.6) |
| Antecedent pregnancy | |
| Mole, n (%) | 25 (71.4) |
| Abortion, n (%) | 10 (28.6) |
| Term, n (%) | 0 |
| Interval from antecedent pregnancy (in months) | |
| <4, n (%) | 32 (91.4) |
| 4-6, n (%) | 2 (5.7) |
| >6, n (%) | 1 (2.9) |
| Tumor size (in cm) | |
| < 3, n (%) | 20 (57.15) |
| 3-5, n (%) | 13 (37.15) |
| ≤5, n (%) | 2 (5.7) |
| Pre-treatment Beta hCG (mIU/dl) | |
| < 1000, n (%) | 2 (5.7) |
| 103- < 104, n (%) | 28 (80) |
| 104- < 105, n (%) | 5 (14.3) |
| >105, n (%) | 0 |
| FIGO Stage | |
| Stage I, n (%) | 33 (94.3) |
| Stage II, n (%) | 0 |
| Stage III, n (%) | 2 (5.7) |
| WHO prognostic score | |
| 0-2, n (%) | 17 (48.6) |
| 3-4, n (%) | 15 (42.8) |
| 5-6, n (%) | 3 (8.6) |
| Site of metastasis | |
| Lung | 2 (5.7) |
| Spleen, Kidney | 0 |
| GIT | 0 |
| Liver, brain | 0 |
Ninety-one percent patients were younger than 40 year of age. The study found that the majority of patients (71.4%) experienced a molar pregnancy before developing gestational trophoblastic neoplasia (GTN), with 91.4% developing GTN within the first 4 months. Most patients (80%) had human chorionic gonadotropin (hCG) levels between 1,000 and 10,000 mIU/dL, while 94.3% were at FIGO stage I. The WHO prognostic scores range from 0 to 2, 3-4 and 5-6 in 48.6%, 42.8% and 8.6% respectively. Only 35 patients opted for chemotherapy, with methotrexate (MTX) being the primary single-agent treatment. Patients who did not respond to methotrexate were subsequently administered multiagent EMA-CO chemotherapy. Only 5.7% of patients had extra pelvic spread to the lungs. No patient had spleen, kidney, liver, GIT and brain metastasis.
Table 2 displays treatment outcomes for patients who underwent primary single-agent chemotherapy with MTX (n = 35). Of these, 32 patients achieved complete responses (91.4%), while 3 experienced treatment failure (8.6%).
| Variables | Complete response | Failed response |
| Patients | 32 (91.4%) | 3 (8.6%) |
| Median WHO prognostic score | 3 | 5 |
| Median duration of disease (months) | 2 | 4 |
Analysis showed no statistically significant difference in median age between patients with complete and failed responses (P > 0.05, two-tailed Mann-Whitney U test). Patients who failed MTX treatment had a median WHO prognostic score of 5 (range, 5-6), compared to a score of 3 (range, 0–6) for those with complete responses. All three patients who failed primary MTX therapy were subsequently treated with multiagent chemotherapy and achieved complete remission (CR). Overall survival (OS) and cure rates for all patients with low-risk GTN were 100%. Regarding chemotherapy toxicity, no patients experienced MTX-related hepatic toxicity. Among the 35 patients who underwent primary MTX therapy, three developed grade-I oral mucositis, and four experienced grade-II bone marrow suppression. Conversely, among the subset of patients receiving primary methotrexate (n = 3) followed by sequential multiagent EMA-CO chemotherapy (n = 3), one patient encountered grade-II oral mucositis and another experienced grade-III bone marrow suppression.
Discussion
The prognosis of patients with low-risk GTN is very favourable; overall survival can approach 90-100% [8,11,12]. Similarly in our study, primary chemotherapy and sequential multi-agent chemotherapy in failed response were collectively associated with an OS rate of 100%. Low-risk GTN is extremely sensitive to chemotherapy but few patients had a high risk of treatment failure with single-agent chemotherapy and required an EMA-CO regimen. Additional studies are required to better characterise which sub-group has treatment failure [13].
Our hospital employs the 8-day IM MTX-FA regimen, which yielded a notably high complete response (CR) rate of 91.4% (n = 32/35) with minimal toxicity. MTX is widely utilized in clinical settings due to its therapeutic efficacy, well-tolerated nature, and cost-effectiveness. It can be administered with or without folinic acid (FA) to mitigate MTX-related toxicity. Various MTX regimens are used globally, including weekly intramuscular (IM) MTX, 5-day IM MTX, 5-day IV MTX, 8-day IM MTX-FA, and high-dose intermittent infusion IV MTX-FA [12]. However, there is no universal consensus on the optimal MTX regimen. In our study, we employed the 8-day IM MTX-FA regimen, which demonstrated high effectiveness in treating low-risk GTN among women, achieving a CR rate of 91.4% without significant adverse effects. Actinomycin D (Act D) is a commonly utilized chemotherapy for patients with low-risk GTN. It is typically employed as a second-line single-agent therapy in cases of MTX resistance or when MTX usage is contraindicated. For patients resistant to MTX, sequential Act D therapy demonstrates remarkably high CR rates, nearly approaching 100%. However, the clinical utility of Act D is often limited by its associated toxicity-related adverse events, including hair loss, loss of appetite, diarrhea, and particularly blister formation if extravasation occurs [5, 11].
Winter et al indicate that a significant proportion of patients exhibit resistance to first-line chemotherapy agents, particularly those with FIGO/WHO prognostic scores of 5–6 [14]. The Sheffield Trophoblastic Disease Centre in the United Kingdom reported an 81% resistance rate among patients with a FIGO/WHO score of 6, compared to 34% resistance in those with a lower score [15]. A Canadian study on low-risk GTN found a chemotherapy failure rate of 32% for patients with FIGO/ WHO scores of 0–4, escalating to 59% for scores of 5–6 [16]. Braga et al. suggest that around 60% of women with gestational trophoblastic neoplasia and a FIGO risk score of 5–6 achieve remission with single-agent therapy, while nearly all remaining patients achieve complete remission with subsequent multiagent chemotherapy [17]. Primary multiagent chemotherapy is recommended for patients with metastatic disease and choriocarcinoma or those identified by predictors such as metastatic disease, choriocarcinoma, and pre-treatment human chorionic gonadotropin concentration of more than 4,11,000 mIU/ ml [17]. In our study, three patients (8.6%) developed resistance to first-line chemotherapy, all of them had prognostic score of 5-6, out of which two patients (5.7%) also had lung metastasis. The complete response (CR) rates to sequential multiagent EMA-CO chemotherapy (n = 3) were 100% in our study.
A hysterectomy may be necessary for large or chemo-resistant uterine tumors and prolonged bleeding as a hysterectomy may reduce the tumor burden and decrease the number of chemotherapy courses. No patient in our study requires a hysterectomy for low-risk GTN [18, 19]. The toxicity profile was favourable and comparable with other studies as most of the patients in our study are young [20].
The limitations of our study include a small sample size, single-centre experience, and retrospective design. However, data on GTN in the literature is scarce and our study contributes to limited literature.
In conclusion, in our study, we conducted a retrospective analysis of patients with low-risk GTN treated at tertiary care centre using the 8-day IM MTX-FA protocol. Our findings revealed that this MTX regimen was remarkably effective in treating women with low- risk GTN, achieving a complete response (CR) rate in 91.4% of patients without encountering severe adverse effects. Furthermore, our analysis suggests that primary and sequential multiagent therapy demonstrate significant efficacy in managing low-risk GTN, particularly in cases with a prognostic score of 5-6 and lung metastasis.
Acknowledgements
Nil
Conflict of interest
Nil
References
- Gestational trophoblastic disease Sekharan PK . Obstet Gynecol India .2008;58(8):299e307.
- Diagnosis, classification and treatment of gestational trophoblastic neoplasia Biscaro A, Braga A, Berkowitz RS . Revista Brasileira De Ginecologia E Obstetricia: Revista Da Federacao Brasileira Das Sociedades De Ginecologia E Obstetricia.2015;37(1). CrossRef
- Evaluation of the results of chemotherapy in high-risk gestational trophoblastic tumors with multidrug EMA-CO regimen + granulocyte- colony-stimulating factor (G-CSF) support Ravi Byahut , Mithilesh Kumar . International Journal of Contemporary Medical Research .2018;5(1):1-4.
- Current management of gestational trophoblastic diseases Berkowitz RS , Goldstein DP . Gynecologic Oncology.2009;112(3). CrossRef
- The efficacy and safety of first-line single-agent chemotherapy regimens in low-risk gestational trophoblastic neoplasia: A network meta-analysis Li J, Li S, Yu H, Wang J, Xu C, Lu X. Gynecologic Oncology.2018;148(2). CrossRef
- Chinese Anti-Cancer Association Gynecological Oncology Committee. Guidelines for the diagnosis and treatment of gestational trophoblastic disease (2021 edition) China Oncol.2021;31(6):520-532.
- Effectiveness and toxicity of first-line methotrexate chemotherapy in low-risk postmolar gestational trophoblastic neoplasia: The New England Trophoblastic Disease Center experience Maestá I, Nitecki R, Horowitz NS , Goldstein DP , Freitas Segalla Moreira M, Elias KM , Berkowitz RS . Gynecologic Oncology.2018;148(1). CrossRef
- Low-risk persistent gestational trophoblastic disease: outcome after initial treatment with low-dose methotrexate and folinic acid from 1992 to 2000 McNeish I. A., Strickland S., Holden L., Rustin G. J. S., Foskett M., Seckl M. J., Newlands E. S.. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2002;20(7). CrossRef
- Relapse rate of patients with low-risk gestational trophoblastic tumor initially treated with single-agent chemotherapy Matsui H, Suzuka K, Yamazawa K, Tanaka N, Mitsuhashi A, Seki K, Sekiya S. Gynecologic Oncology.2005;96(3). CrossRef
- Resistance to single-agent chemotherapy and its risk factors in low-risk gestational trophoblastic neoplasms Mousavi AS , Zamani A, Khorasanizadeh F, Gilani MM , Zendehdel K. The Journal of Obstetrics and Gynaecology Research.2015;41(5). CrossRef
- Gestational trophoblastic neoplasia: treatment outcomes from a single institutional experience Al-Husaini H., Soudy H., Darwish A., Ahmed M., Eltigani A., Edesa W., Elhassan T., et al . Clinical & Translational Oncology: Official Publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico.2015;17(5). CrossRef
- First-line chemotherapy in low-risk gestational trophoblastic neoplasia Lawrie TA , Alazzam M, Tidy J, Hancock BW , Osborne R. The Cochrane Database of Systematic Reviews.2016;2016(6). CrossRef
- Treatment outcomes for 618 women with gestational trophoblastic tumours following a molar pregnancy at the Charing Cross Hospital, 2000-2009 Sita-Lumsden A., Short D., Lindsay I., Sebire N. J., Adjogatse D., Seckl M. J., Savage P. M.. British Journal of Cancer.2012;107(11). CrossRef
- Treatment of low-risk gestational trophoblastic neoplasia Winter MC . Best Practice & Research. Clinical Obstetrics & Gynaecology.2021;74. CrossRef
- Management and Outcomes of Patients with Stage I and IlIl Low-Risk Gestational Trophoblastic Neoplasia Treated in Sheffield, UK, from 1997-2006 Macdonald MC , Hancock BW , Winter MC , Coleman RE , Tidy JA . The Journal of Reproductive Medicine.2016;61(7-8).
- Single or two drug combination therapy as initial treatment for low risk, gestational trophoblastic neoplasia. A Canadian analysis Hoskins PJ , Le N, Kumar A, Pina A, Sabourin JN , Kim H, Osborne RJ . Gynecologic Oncology.2020;157(2). CrossRef
- Predictors for single-agent resistance in FIGO score 5 or 6 gestational trophoblastic neoplasia: a multicentre, retrospective, cohort study Braga A, Paiva G, Ghorani E, Freitas F, Velarde LGC , Kaur B, Unsworth N, et al . The Lancet. Oncology.2021;22(8). CrossRef
- Gestational trophoblastic disease Seckl MJ , Sebire NJ , Berkowitz RS . Lancet (London, England).2010;376(9742). CrossRef
- The role of operation in the current therapy of gestational trophoblastic disease Hammond C. B., Weed J. C., Currie J. L.. American Journal of Obstetrics and Gynecology.1980;136(7). CrossRef
- Clinicopathological features and outcomes of choriocarcinoma: A retrospective analysis from an Indian tertiary cancer center Ghosh J, Dey S, Mandal D, Ganguly S, Biswas B, Dabkara D, Ghosh A, et al . Cancer Research, Statistics, and Treatment.2021;4(3). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2025
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times