Unraveling Rarity: Decoding an Unusual Ovarian Mass in a Young Woman with Menstrual Irregularities
Download
Abstract
Sclerosing Stromal Tumor (SST) is a relatively rare sex cord stromal tumor with only a hand full of cases reported in the literature till now. Due to its unique benign nature with nil recurrence rate, it is essential to diagnose this entity and differentiate it from the non-neoplastic and malignant lesions of the ovary. Here, we report a case of SST in a young female who presented with irregular menstruation and abdominal distension. A combined approach involving clinical suspiciousness, radiological, histopathological and immunohistochemistry findings helped us to arrive the diagnosis of this extremely rare entity, which aided in the effective management of the patient.
Introduction
Ovarian sex cord-stromal tumors are rare and accounts for only 8% of all primary ovarian neoplasm [1]. SSTis an uncommon benign subtype accounting for only 2% of sex cord-stromal tumors [2]. Sclerosing stromal tumor is a rare benign sex-cord stromal tumor of the ovary that affects primarily young females under the age of 30. The distinct clinical and pathological feature of this ovarian tumor helps it to be differentiated from other stromal tumors. Well circumscription, unilaterality and nil recurrence rate are considered to be its unique characteristic features [3]. The distinctive histological features of this tumor are the presence of network of thin walled vessels, sclerosis, heterogeneity of the cellular areas and ill defined cellular pseudolobules separated by a densely hyalinised or markedly edematous stroma.
Case report
A 26 year old gravid 3, para 2, live 2 woman presented in the outpatient department of obstetrics and gynecology with abdominal distension, pelvic pain and irregular menstruation for 3 months. On clinical examination a large abdomino-pelvic mass was palpable. Ultrasonography showed a large heterogeneous, predominantly solid pelvic mass with some cystic foci measuring 6 X 7 cm. Computerised tomography showed a large well defined homogeneous solid lesion not separately defined from left ovary. No calcifications were seen. Radiological diagnosis was suggestive of germ cell tumor with a high possibility of being dysgerminoma. Serum CA-125 level was not elevated. The patient was hospitalized and was planned for left salpingo-ophorectomy. All hematological investigations and serum hormonal assays were normal. The gross examination of the resected specimen showed an encapsulated 6 X 7 cm ovarian mass and stretched fallopian tube on its surface. The outer surface was smooth and intact. Cut section was pearly white solid tumor, fleshy to rubbery in consistency. No hemorrhage or necrosis was observed (Figure 1 A).
Figure 1. A - Gross picture of ovarian tumor showing Pearly White glistening cut surface. B- Hypercellular and hypocellular areas with prominent vascularity (H and E ×100). C- The hypocellular areas show fibrous stroma, that often undergoes hyaline degeneration, edema, and myxoid changes (H and E ×100). D- Tumor cells are of two populations. One population is of spindle cells. The other population of cells is round with clear cytoplasm (H and E ×400). E- Tumor cells revealing diffuse immunoreactivity to inhibin (IHC for inhibin 400x). F- Tumor cells revealing non immunoreactivity to CD10 (IHC for CD10 400x).
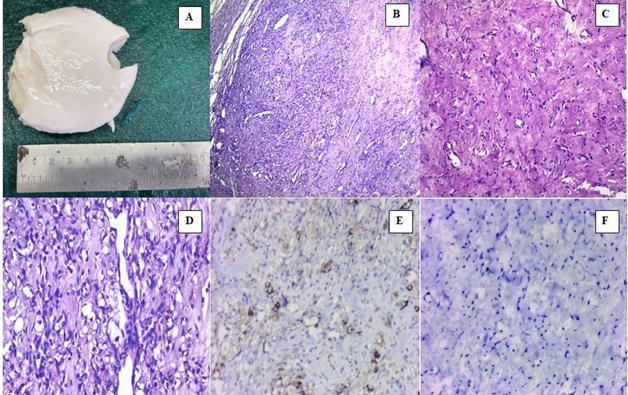
Microscopic examination showed a tumor with lobular pattern of growth having prominent interlobular fibrosis. Alternate hyper and hypocellular areas were seen with prominent hemangiopericytoma like vascular distribution. The lobules were composed of spindle-shaped cells along with round clear and signet ring like cells (Figure 1 B, C and D). On applying immunohistochemistry, the tumor cells showed a strong cytoplasmic positivity for smooth muscle actin and inhibin. However, the neoplastic cells were negative for CD 10 (Figure 1 E and F). Based on the histopathological and immunohistochemical profile a final diagnosis of sclerosing stromal tumor of ovary was made.
Disscussion
Sex cord stromal tumors of the ovaries are exceptional ovarian neoplasms accounting for approximately 5–8% of ovarian tumors.1 This group of ovarian tumors includes granulosa cell tumors, fibroma, thecoma, steroid cell tumors, Sertoli–Leydig cells tumors and sclerosing stromal tumors. Sclerosing stromal tumors accounts for approximately 6 to 8% of sex cord stromal tumors [3]. Chalvardijan and Scully were the first to coin and describe this tumor in 1973 [4]. These tumors are benign and rare, and their unique feature is that they can be distinguished from other stromal tumors only by histopathology and radiology.
Much debate on the origin of these tumors with many hypothesis has been postulated. Ismail et al. put out the theory that the morphology of SSTs may be caused by an endocrine milieu and that they may arise from pre-existing ovarian fibromas [5]. Damajanov et al. hypothesised that SSTs are formed from the pluripotent immature stromal cells of the ovarian cortex based on ultrastructural characteristics [6]. The perifollicular myoid stromal cells from the theca externa, which are actin positive and unique to muscles, are also thought to be the source of SSTs. Currently, the majority of researchers think that SST develops from undifferentiated mesenchymal cells in the ovarian cortex that have the ability to differentiate into smooth muscle [7]. This perspective is backed by ultrastructural electron microscopy analysis and immunohistochemistry.
Evidences in literature have proved that SSTs are more common in the second or third decade of life in contrast to other stromal tumors which occurs in fifth or sixth decade. The youngest patient reported is a 7-month-old infant presenting with vaginal bleeding due to hyperestrogenism caused by SST [8]. Earlier SSTs were considered to be a non functional benign ovarian tumor but it was only in 1975 it was affirmed that SSTs can secrete steroid hormones. Peripheral conversion of these steroid hormones can lead to increase in patients estrogen levels causing irregular menstruation, amenorrhea, and infertility which could be the presenting complaints of some. Steroid hormone-related symptoms which includes virilization indicate the possibility of steroid hormone release from the SST. Masculinization due to increased androgen levels has been a rare event associated with SSTs with only 8 cases reported till date [9]. Reversal of the hormone level on removal of the tumor has been well documented. In our case the patient had complaints of menorrhagia which indicates that the tumor could be functional but however hormonal assays were not done on the patient to prove it. On ultrasonography, an SST presents as a mass with solid and cystic components that have irregularly thickened septa and heterogeneous echogenicity. On MRI, it presents as a large mass with hyperintense cystic components, and on T2-weighted MRI, as a heterogeneous solid mass with intermediate to high signal intensity.
The aforementioned imaging findings will be seen in all borderline and malignant ovarian neoplasms. More reliably SSTs show an early peripheral enhancement on contrast-enhanced computed tomography images. Pronounced vascularity in the peripheral and central intercystic space using colour Doppler can also be used to make a preoperative presemptive diagnosis. However Park et al in his series have stated that none of the above imaging findings were able to clearly ratify SST from other ovarian malignancies. This could be justified in our case also where both ultrasound and CT imaging studies suggested it to be a germ cell tumor.
Sclerosing stromal tumors demonstrate a characteristic constellation of histologic findings which includes a pseudolobular growth pattern with variably cellular areas, edema, prominent vascularity in a hemangiopericytoma- like in appearance, and stromal cells admixed with luteinized cells. Luteinized stromal cells are recognized by their oval and round shape and originally eosinophilic to clear, lipid containing cytoplasm. Luteinized cells with abundant of lipid filled cytoplasm with peripherally pushed nucleus gives an appearance of signet ring cells. The presence of such cells can lead to misdiagnosis of krukenburg tumor [9]. However bilaterality, elevated tumor markers and gastrointestinal symptoms will help to rule out the diagnosis. Massive ovarian edema also enters the differential of this tumor but can be differentiated by preserved ovarian tissue within edematous stroma and absence of heterogeneity. The edema in SSTs has a zonal distribution in contrast to that seen in massive edema or an edematous fibroma [9]. Other sex cord stromal tumors like thecoma and fibroma can resemble SSTs however homogenous appearance and lack of vascularity goes more in favour of the former. Microcystic stromal tumor is another benign ovarian tumor which closely resembles SSTs. The separation of tumor cells by collagenous stroma or thick hyaline bands makes microcystic stromal tumor a histopathological mimic of SSTs. However, microcystic tumors lack lutein cells and the characteristic vasculature of SSTs, while displaying prominent cystic changes.
Sclerosing stromal tumor cells show strong positivity for vimentin, SMA, and inhibin, which strongly suggest their stromal origin. Other markers, such as calretinin or desmin, can be positive or negative, whereas epithelial markers and S-100 are negative [7]. However, other authors have stated that inhibin and calretinin are the most useful markers for distinguishing stromal tumors. CD 10 is a marker of endometrial stromal sarcoma. Its utility in sex cord stromal tumors has been widely studied. Oliva et al have documented that 85% of SSTs show moderate intensity of CD10 expression. However in our case the tumor cells were negative for CD 10 expression while it showed a strong positivity for inhibin [10]. Park et al have documented strong nuclear positivity of TFE 3 in SSTs in his study which was supported by many authors however the pathophysiology of its expression needs to be evaluated [11].
The expression of vascular permeability factor/ vascular endothelial growth factor (VPF/VEGF) in the luteinized theca like cells can be correlated to the characteristic vasculature and edema that we see as a hallmark feature of all SSTs. Both reverse transcription- polymerase chain reaction and immunohistochemistry were used for demonstration of their expression. Molecular studies using fluorescence in situ hybridization analysis have shown the presence of chromosome 12 trisomy in SSTs as well as in other ovarian stromal tumors [12]. Kim et al have documented that recurrent fusion of FHL2-GLI2 is likely to be the oncogenic driver of SSTs [13].
In conclusion, sclerosing stromal tumors are rare, benign ovarian neoplasm of young females. Clinical examination and radiological investigations can lead to a presumptive diagnosis of SSTs, however histopathological examination is necessary for a definitive diagnosis. Its classical histomorphological features helps to delinate SSTs from other sex cord stromal tumors. Sclerosing stromal tumors should be considered in young females with unilateral, solid/cystic, complex ovarian mass. Favourable prognosis and nil recurrence reported till date in literature should be taken into account in planning the treatment and restrict the surgery to enucleation or unilateral oophorectomy.
Acknowledgments
Statement of Transparency and Principals:
• Author declares no conflict of interest
• Study was approved by Research Ethic Committee of author affiliated Institute.
• Study’s data is available upon a reasonable request.
• All authors have contributed to implementation of this research.
References
- Sclerosing stromal tumor of the ovary Atram M, Anshu , Sharma S, Gangane N. Obstetrics & Gynecology Science.2014;57(5). CrossRef
- Sclerosing stromal tumor of the ovary: a case series and review of literature Mittermair C, Cunha TM , Urbas R, Koch H, Forstner R. BJR case reports.2022;8(2). CrossRef
- Sclerosing Stromal Tumor: A Rare Ovarian Neoplasm Bairwa S, Satarkar RN , Kalhan S, Garg S, Sangwaiya A, Singh P. Iranian Journal of Pathology.2017;12(4).
- Sclerosing stromal tumors of the ovary Chalvardjian A., Scully R. E.. Cancer.1973;31(3). CrossRef
- Bilateral virilizing sclerosing stromal tumours of the ovary in a pregnant woman with Gorlin's syndrome: implications for pathogenesis of ovarian stromal neoplasms Ismail S. M., Walker S. M.. Histopathology.1990;17(2). CrossRef
- Sclerosing stromal tumor of the ovary: A hormonal and ultrastructural analysis Damajanov I., Drobnjak P., Grizelj V., Longhino N.. Obstetrics and Gynecology.1975;45(6). CrossRef
- Sclerosing stromal tumor of ovary: a case report Khanna M, Khanna A, Manjari M. Case Reports in Pathology.2012;2012. CrossRef
- A rare cause of vaginal bleeding in a 7-month-old female infant Duke DS , Yoo EY , Newton C, Schwartz MZ . Journal of Pediatric Surgery.2008;43(3). CrossRef
- Sclerosing stromal tumor of the ovary with masculinization, Meig's syndrome and CA125 elevation in an adolescent girl: A case report Chen Q, Chen Y, Tang H, Shen Y, Tan X. World Journal of Clinical Cases.2020;8(24). CrossRef
- CD10 expression in pure stromal and sex cord-stromal tumors of the ovary: an immunohistochemical analysis of 101 cases Oliva E, Garcia-Miralles N, Vu Q, Young RH . International Journal of Gynecological Pathology: Official Journal of the International Society of Gynecological Pathologists.2007;26(4). CrossRef
- Clinicopathological Characteristics of Ovarian Sclerosing Stromal Tumor with an Emphasis on TFE3 Overexpression Park CK , Kim H. Anticancer Research.2017;37(10). CrossRef
- Sclerosing stromal tumors of the ovary: a clinicopathologic, immunohistochemical and cytogenetic analysis of three cases Kostopoulou E., Moulla A., Giakoustidis D., Leontsini M.. European Journal of Gynaecological Oncology.2004;25(2).
- Identification of recurrent FHL2-GLI2 oncogenic fusion in sclerosing stromal tumors of the ovary Kim SH , Da Cruz Paula A, Basili T, Dopeso H, Bi R, Pareja F, Silva EM , et al . Nature Communications.2020;11(1). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2024
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times