Effects of Betanin Isolate Administration on the Lung Histopathological Image of Wistar Rats (Rattus norvegicus) Exposed to 40 ppm Formaldehyde
Download
Abstract
Purpose: This research investigates the effects of betanin isolate derived from beetroot on the lung histopathological image of Wistar rats exposed to 40 ppm formaldehyde, which is a known carcinogen. This research aims to evaluate the potential of betanin isolate to prevent lung cancer caused by formaldehyde exposure.
Patients and methods: This research uses Wistar rats (Rattus norvegicus) as sample, which are divided into six groups, control group, positive control group, negative control group, and three treatment groups with different doses of betanin isolate. The research adopts a true experiment with post-test only design. The rats were exposed to 40 ppm formaldehyde for 12 weeks, except for the control group. The lung histopathological image of the rats was examined and graded for dysplasia. The data were analyzed using Mann-Whitney U test to compare the degree of dysplasia among different groups.
Results: The control group is significantly different from all other groups, except treatment group 3. The negative control and control group has significant difference, which means the treatment of formaldehyde in this research is successful in inducing dysplasia. There is also seen a significant difference between treatment group 1 and treatment group 3 which indicates that the protective effect of betanin is dose dependent. There are some tissues with no dysplasia in treatment group 3 that shows the protective effect of betanin in higher dose.
Conclusion: This research shows that betanin isolate has a dose-dependent protective effect, with higher dose being more effective. However, the highest dose tested is not enough to prevent dysplasia completely.
Introduction
Formaldehyde is a chemically reactive substance characterized by its colorless nature, pungent odor, and significant toxicity. As the most basic aldehyde, denoted as H-CHO, formaldehyde is produced through the catalytic oxidation of methanol. Notably, it exhibits a high solubility in water. Due to its notable water solubility and reactivity, formaldehyde is anticipated to primarily manifest its toxic effects at the point of entry. This reactivity in target tissues gives rise to local irritation, acute and chronic toxicity, as well as the presence of genotoxic and cytotoxic properties. It is noteworthy that the metabolism of histamine and methylalanine also possesses the capability to generate formaldehyde as a secondary product in their respective metabolic pathways [1].
The development of cancer is a multifaceted process involving distinct stages. Initiation occurs when carcinogens prompt genetic alterations that confer neoplastic capabilities upon transformed cells. Promotion then follows, marked by the encouragement of clonal proliferation within these initiated transformed cells. Ultimately, the progression phase ensues, culminating in malignant behavior typified by invasion and its associated outcomes [2]. Formaldehyde is one of known carcinogenic substance that can cause neoplasm progression [3].
Genotoxicity is believed to hold a significant role in the carcinogenic potential of formaldehyde within human nasal tissues. Furthermore, the process of cellular replication, triggered in response to the cytotoxic effects induced by formaldehyde, may serve to promote the carcinogenic response [4]. Exposure to formaldehyde has been observed to perturb the expression levels of microRNAs (miRNAs) within cultured lung cells. Investigating the potential effects of formaldehyde on miRNAs is crucial due to their role in the regulation of gene expression. MiRNAs exert their influence by binding to mRNA, which results in the rapid decay of the message, translational repression of mRNA signals, and the induction of cleavage of newly translated polypeptides [5]. Upon exposure to formaldehyde, lung epithelial cells exhibited decreased expression of 89 out of 534 measured miRNAs using human miRNA microarrays. Importantly, all of the modulated miRNAs displayed down-regulation. This pattern of miRNA down-regulation has been observed not only in rat lung cells exposed to cigarette smoke but also in various tumor cell types, including lung cancer, breast cancer, and leukemia [6].
Betanin (betanidin 5-O-β-d-glucoside) is one of the most abundant type of betacyanin and is commonly used as a colorant for foods, drugs, and cosmetics. Betacyanin is a type of betalains, which general are a heterocyclic water soluble nitrogen coumpound. Betalains can be found in beetroot (Beta vulgaris L.), especially in the tuberous part. Betanin exhibits substantial potential as an effective antioxidant within the realm of the food industry, whether harnessed in the form of extracts or as a powdered substance. Furthermore, it finds application as a natural pigment. Notably, the antioxidative attributes of Betanin have been empirically validated in human macromolecules, including low-density lipoproteins, cellular membranes, and intact cells, particularly within biological lipid matrices [7]. At the cellular level, the chemopreventive properties of beetroot are associated with various atypical mechanisms. These mechanisms include anti-inflammatory, antioxidant, proapoptotic, antiproliferative, and free radical-scavenging actions, which have been subjects of prior investigations. Prior research has demonstrated notable elevations in the expression of proapoptotic proteins such as BAX, caspase 9, and caspase 3, along with factors like cytochrome and reactive oxygen species (ROS). Simultaneously, there have been reductions in the expression of antiapoptotic protein BCL2 and poly (ADP-ribose) polymerase (PARP), contributing to DNA damage and ultimately culminating in apoptosis [8]. As such, betanines has the potential to counter the carcinogenic effect of formaldehyde on lung tissues. As of now, there has been no research comparing lung tissue induced with formaldehyde without given betanin and the one given with betanin. This research aims to fill this research gap and provide data on how betanin protective mechanism results in lung tissue affected by carcinogenic substance such as formaldehyde.
Materials and Methods
Materials
This research is a true experiment research with post- test only study design. The main sample of this experiment is Wistar rat (Rattus norvegicus). This experiment uses 10% formaldehyde as a carcinogenic inducer, which was a dilution of the 37% stock solution. The formaldehyde solution was diluted by taking 27.02 mL and placed in a 100 mL volumetric flask. Distilled water was added until the limit mark and vortexed the solution to make it homogeneous.
Treatment procedure
Wister rats are grouped into 6 groups. The control group receives no formaldehyde and no betanin isolate. The negative control group receives formaldehyde only. The positive control group (given formaldehyde and vitamin C 9 mg/200 grams of rat BW. Wistar rats in negative control, positive control, and all treatment groups is being induced with 40 ppm formaldehyde. Formaldehyde was induced using a micropipette on cotton wool and then inhaled by rats of treatment group 1 (given formaldehyde induction of 40 ppm and isolate betanin 20 µg/200 grams of rat BW), treatment group 2 (given formaldehyde induction of 40 ppm and isolate betanin 40 µg/200 grams of rat BW), and treatment group 3 (given formaldehyde induction of 40 ppm and isolate betanin 80 µg/200 grams of rat BW). The treatment was carried out for 12 weeks. This process was carried out according to laboratory standards using PPE. The treatment was carried out for 6 hours every day, and overall it was carried out for 16 weeks.
Data analysis
The data obtained will be ordinal data. These two unpaired ordinal data will then be subjected to statistical tests to determine the significance of differences in the degree of dysplasia in each tissue. The statistical test used in accordance with the type of data is Mann-Whitney U.
Results
Histopathological Result of All Groups
The followings are the histopathological results of all groups which includes the control group (given no formaldehyde and no betanin isolate), negative control group (given formaldehyde only), positive control group (given formaldehyde and vitamin c 9 mg/200 grams of rat BW), treatment group 1 (given formaldehyde induction of 40 ppm and isolate betanin 20 µg/200 grams of rat BW), treatment group 2 (given formaldehyde induction of 40 ppm and isolate betanin 40 µg/200 grams of rat BW), and treatment group 3 (given formaldehyde induction of 40 ppm and isolate betanin 80 µg/200 grams of rat BW) (Table 1).
| Group | Specimen | Histopathological Grading |
| Control Group | Rat 1 | No Dysplasia |
| Rat 2 | No Dysplasia | |
| Rat 3 | No Dysplasia | |
| Rat 4 | No Dysplasia | |
| Rat 5 | No Dysplasia | |
| Positive Control Group | Rat 1 | Grade 3 Dysplasia |
| Rat 2 | Grade 2 Dysplasia | |
| Rat 3 | Grade 3 Dysplasia | |
| Rat 4 | Grade 3 Dysplasia | |
| Rat 5 | Grade 2 Dysplasia | |
| Negative Control Group | Rat 1 | No Dysplasia |
| Rat 2 | Grade 2 Dysplasia | |
| Rat 3 | Grade 2 Dysplasia | |
| Rat 4 | Grade 1 Dysplasia | |
| Rat 5 | Grade 2 Dysplasia | |
| Treatment 1 | Rat 1 | Grade 1 Dysplasia |
| Rat 2 | Grade 2 Dysplasia | |
| Rat 3 | Grade 3 Dysplasia | |
| Rat 4 | Grade 3 Dysplasia | |
| Rat 5 | Grade 3 Dysplasia | |
| Treatment 2 | Rat 1 | Grade 3 Dysplasia |
| Rat 2 | Grade 3 Dysplasia | |
| Rat 3 | Grade 1 Dysplasia | |
| Rat 4 | Grade 1 Dysplasia | |
| Rat 5 | Grade 1 Dysplasia | |
| Treatment 3 | Rat 1 | Grade 2 Dysplasia |
| Rat 2 | No Dysplasia | |
| Rat 3 | Grade 1 Dysplasia | |
| Rat 4 | Grade 1 Dysplasia | |
| Rat 5 | No Dysplasia |
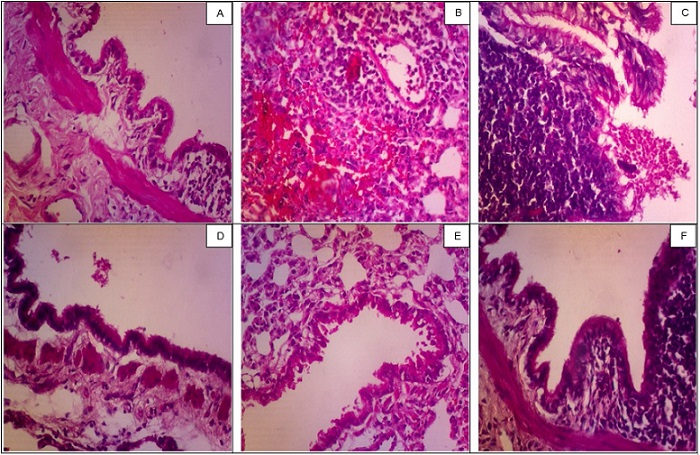
Representative images of lung histopathology in each group can be seen in Figure 1.
Figure 1.Histopathological Appearance of the Lung. A, Rat 5 in control group (Normal). B, Rat 1 in positive control group (Hyperplasia). C, Rat 4 in negative control group (Lymph node enlargement). D, Rat 3 in treatment 1 group (Normal). E: Rat 1 in treatment 2 group (Hyperplasia). Rat 1 in treatment 3 group (Normal).
Analysis of the Histopathological Difference Between Each Group
The analysis finding of this study is shown on (Table 2).
| Group Comparison | Significance | |
| Control Group | Positive Control Group | .005* |
| Negative Control Group | .017* | |
| Treatment 1 | .005* | |
| Treatment 2 | .005* | |
| Treatment 3 | 0.053 | |
| Positive Control Group | Control Group | .005* |
| Negative Control Group | .032* | |
| Treatment 1 | 0.811 | |
| Treatment 2 | 0.212 | |
| Treatment 3 | .022* | |
| Negative Control Group | Control Group | .017* |
| Positive Control Group | .032* | |
| Treatment 1 | 0.1 | |
| Treatment 2 | 0.661 | |
| Treatment 3 | 0.268 | |
| Treatment 1 | Control Group | .005* |
| Positive Control Group | 0.811 | |
| Negative Control Group | 0.1 | |
| Treatment 2 | 0.356 | |
| Treatment 3 | .031* | |
| Treatment 2 | Control Group | .005* |
| Positive Control Group | 0.212 | |
| Negative Control Group | 0.661 | |
| Treatment 1 | 0.356 | |
| Treatment 3 | 0.145 | |
| Treatment 3 | Control Group | 0.053 |
| Positive Control Group | .022* | |
| Negative Control Group | 0.268 | |
| Treatment 1 | .031* | |
| Treatment 2 | 0.145 |
This analysis uses Mann-Whitney U non- parametric testing to determine the significance of difference between two ordinal data. The table shows there are significant difference between control group and negative control group, control group and treatment group 1, control group and treatment group 2, positive control group and control group, positive control group and negative control group, positive control group and treatment group 3, and between treatment group 1 and treatment group 3.
Discussion
Formaldehyde is a known carcinogenic substance, especially for nasopharyngeal cancer. The carcinogenic effects of formaldehyde result from complex mechanisms that likely encompass multiple pathways. Exposure to formaldehyde is linked to critical events in carcinogenesis, including DNA reactivity, gene mutations, chromosomal breakage, aneuploidy, epigenetic alterations (involving the binding to lysine residues of histones), depletion of glutathione, oxidative stress, and the promotion of cellular proliferation through cytotoxicity. These mechanisms collectively contribute to the carcinogenic potential of formaldehyde, although a comprehensive understanding of the process is still evolving [9]. This can be seen in the result of this research, as the control group which were given with no formaldehyde and no betanin isolate has a significant difference with the negative control group which were given 40 ppm dose of formaldehyde. Negative control group are shown to have developed grade 1 and grade 2 dysplasia, opposed to the control group with no dysplasia. This shows that the formaldehyde treatment on this research is adequate in inducing neoplasm progression in Wistar rats lung tissue, which is fundamental for the main treatments.
None of the treatment group has significant difference with the negative control group, which mean the induction of betanin up to 80 µg/200 grams of rat body weight does not significantly protect Wistar rat’s lung tissue from formaldehyde carcinogenicity. It is worth noting that there is a significant difference between treatment group 1 and treatment group 3, which implies that the dosage difference between 20 µg/200 grams of rat BW and 80 µg/200 grams of rat BW does make a difference in the histopathological grading result of Wistar rats induced with 40 ppm of formaldehyde. The treatment group 3 also shows a non-significant difference with control group, which differs from treatment group 1 and treatment group 2 that has significant difference with control group. This could mean on 80 µg/200 grams of rat BW dose, the histopathological difference with control group (healthy Wistar rats not induced with formaldehyde) becomes insignificant. It can also be seen that some rats develop in treatment group 1 and treatment group 2. This means the effect of betanin isolate on Wistar rats lung tissue induced with 40 ppm formaldehyde does offer protection against neoplasm development and it is dosage-related. The induction of betanin isolate with 80 µg/200 grams of rat BW dose successfully protected several rat samples from developing dysplasia. The absence of a significant difference between treatment group 3 and positive control does not imply that treatment group 3 has no effect on neoplasm progression. Rather, it suggests that treatment control, which is known to inhibit neoplasm growth. However, the effect of treatment group 3 is not strong enough to produce a statistically significant difference from positive control, which may be due to the small sample size, the high variability of the outcome, or the low potency of the treatment. These effects can be attributed to betanin’s potent antioxidant and free radical scavenging capabilities. Their scavenging efficacy rivals that of butylated hydroxytoluene, a commonly used synthetic antioxidant. as galvinoxyl, hydroxyl, and superoxide [10]. When human liver hepatoma cells cultured in vitro were exposed to exogenous H O , incubation with betalains led to a reduction in DNA damage [11]. This may counteract formaldehyde’s ability to induce DNA damage in cells.
The consumption of betanin in drinking water has been demonstrated to inhibit esophageal carcinogenesis in rats exposed to carcinogens [12]. This inhibition is associated with a decrease in inflammation and angiogenesis while promoting apoptosis. Additionally, betanins in drinking water have shown the ability to counteract lung carcinogenesis in mice exposed to carcinogens. This effect is characterized by reduced angiogenesis and an increased rate of apoptosis, mediated by the activation of caspase-3, -7, -9, and PARP [13].
In conclusion, This experiment investigates the effects of betanin isolate administration on the lung histopathological image of Wistar rats exposed to 40 ppm formaldehyde. The positive and negative control group shows an induction of dysplasia in the lung tissue of rats, which is a sign of neoplasm progression and carcinogenicity. There is a significant difference between treatment group 1 that receives 20 µg/200 grams of rat BW and treatment group 2 that receives 80 µg/200 grams. This implies that the protective effect of betanin isolate is dose-dependent and more potent in higher dose. There are tissues without dysplasia on treatment group 3, but there is no difference between treatment group 3 and positive control group which means that the protective effect on 80 µg/200 grams dose does protect the lung tissue to some extend but not strong enough to significantly differentiate the population from the positive control.
Acknowledgments
We would like to thank Sudi Indra Jaya for his guidance and support during the research process. We also thank the Faculty of Medicine, Mataram University, the Faculty of Medicine, Gajah Mada University, and the Faculty of Medicine, Hasanudidin University.
Disclosure
The author reports no conflicts of interest in this work.
References
- Formaldehyde toxicity reports from in vitro and in vivo studies: a review and updated data Bernardini L, Barbosa E, Charão MF , Brucker N. Drug and Chemical Toxicology.2022;45(3). CrossRef
- Underwood’s Pathology : a clinical approach. Sixth Edit Cross SS . Elsevier.2013.
- Formaldehyde exposure and leukemia risk: a comprehensive review and network-based toxicogenomic approach Kang DS , Kim HS , Jung J, Lee CM , Ahn Y, Seo YR . Genes and Environment.2021;43. CrossRef
- Distribution of DNA adducts caused by inhaled formaldehyde is consistent with induction of nasal carcinoma but not leukemia Lu K, Collins LB , Ru H, Bermudez E, Swenberg JA . Toxicological Sciences: An Official Journal of the Society of Toxicology.2010;116(2). CrossRef
- Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Filipowicz W, Bhattacharyya SN , Sonenberg N. Nature Reviews. Genetics.2008;9(2). CrossRef
- Formaldehyde carcinogenicity research: 30 years and counting for mode of action, epidemiology, and cancer risk assessment Swenberg JA , Moeller BC , Lu K, Rager JE , Fry RC , Starr TB . Toxicologic Pathology.2013;41(2). CrossRef
- Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments Vieira Teixeira da Silva D, Dos Santos Baião D, Oliveira Silva F, Alves G, Perrone D, Mere Del Aguila E, M Flosi Paschoalin V. Molecules (Basel, Switzerland).2019;24(3). CrossRef
- Red Beet Biotechnology. Food and Farmaceutical Applications Kapadia GJ , Subba RG . Springer. New York, EUA.2013.
- Final report on carcinogens background document for formaldehyde National Toxicology Program . Report on Carcinogens Background Document for [substance Name].2010;(10-5981).
- Plant Betalains: Safety, Antioxidant Activity, Clinical Efficacy, and Bioavailability Khan MI . Compr Rev Food Sci Food Saf [Internet].2016;15(2):316-330. Available from: https://ift.onlinelibrary.wiley.com/doi/10.1111/1541-4337.12185.
- Betanin--a food colorant with biological activity Esatbeyoglu T, Wagner AE , Schini-Kerth VB , Rimbach G. Molecular Nutrition & Food Research.2015;59(1). CrossRef
- Drinking water with red beetroot food color antagonizes esophageal carcinogenesis in N-nitrosomethylbenzylamine-treated rats Lechner JF , Wang L, Rocha CM , Larue B, Henry C, McIntyre CM , Riedl KM , Schwartz SJ , Stoner GD . Journal of Medicinal Food.2010;13(3). CrossRef
- Cytotoxic effect of the red beetroot (Beta vulgaris L.) extract compared to doxorubicin (Adriamycin) in the human prostate (PC-3) and breast (MCF-7) cancer cell lines Kapadia GJ , Azuine MA , Rao GS , Arai T, Iida A, Tokuda H. Anti-Cancer Agents in Medicinal Chemistry.2011;11(3). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2024
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times