Clinico-Epidemiological Profile and Treatment Outcome of Small Cell Lung Carcinoma: A Retrospective Study from a Tertiary Cancer Centre in Northeast India
Download
Abstract
Background: Small cell lung cancer (SCLC) accounts for 12-15% of all lung cancers and is associated with poor outcomes. This study aimed to understand the clinico-epidemiological profile and outcomes of SCLC in the North-East Indian population.
Methods: A retrospective analysis was performed on medical records of patients with SCLC who were diagnosed, treated, and followed up at the Department of Medical Oncology, Dr. B. Borooah Cancer Institute, during the period from January 2016 to December 2021.
Results: A total of 70 patients were evaluated, with a median age of 62 years, and 82.8% were male. The majority of patients (72.8%) were diagnosed with extensive-stage (ES) disease. Common symptoms included cough (61.4%), chest pain (45.7%), and breathing difficulty (44.2%). The most commonly used chemotherapy regimen was etoposide-platinum. Overall survival (OS) for all patients was 6.1 months. Three-year OS was significantly better in patients with limited-stage (LS) disease (16.4 months vs. 4.3 months in LS vs. ES disease, p<0.05). Median progression-free survival (mPFS) for all patients was 4 months. Survival rates at 6, 12, 24, and 36 months were 45.7%, 18.5%, 5.7%, and 1.4%, respectively. Median OS was significantly correlated with the number of metastatic sites (8.6 months with <3 organ involvement vs. 1.9 months with ≥3 organ involvement; p<0.0001). Patients who received combined modality treatment had better survival than those treated with chemotherapy alone (10.7 months in chemo-radiotherapy vs. 3 months in chemotherapy alone, p<0.001).
Conclusion: SCLC remains a highly aggressive disease with poor survival. Advanced-stage presentation with distant organ metastasis is the most important factor associated with poor survival in patients with SCLC1
Introduction
Lung cancer is a serious public health issue with high mortality and morbidity worldwide. Lung cancer is the second most common cancer (11.4%) after breast cancer (11.7%) diagnosed globally [1]. Small Cell Lung Cancer (SCLC) represents approximately 15% of all lung carcinomas [2, 3]. In contrast to the European countries, SCLC constitutes a smaller percentage of lung cancer cases in Asian countries. Small cell lung cancer (SCLC) is an aggressive cancer of neuroendocrine origin, which is strongly associated with cigarette smoking [4]. The aggressive nature of this variant is evidenced by the fact that the overall 2-year survival rate is 5.9% and the 5-year survival rate, a dismal 2.4% only. Patients typically present with a short duration of symptoms and frequently (60-65 %) present with metastatic disease [5]. SCLC is known for its rapid doubling time and potential for widespread metastases [6].
According to the proposal from Veterans Administration Lung Study Group (VALSG), SCLC staging can be categorized into two clinical subgroups: limited‐stage disease (LD) and extensive‐stage disease (ED) [7]. The therapeutic options in SCLC are dependent on the disease stage chosen. Approximately 30% of patients with SCLC are mostly diagnosed with LD [3]. However, a majority of patients (approximately 70%) with SCLC are diagnosed at extensive‐stage [8]. Various studies have shown very poor prognosis of both limited and extensive stage of SCLC. In Mohan et al., the median duration of survival of patients with limited and extensive disease was 15.3 months and 9.8 months, respectively [9].
Very little therapeutic clinical improvement has been achieved during the past 30 years, leading to SCLC being labelled as recalcitrant cancer [10]. Small cell lung cancer remains an aggressive disease, and most patients who are diagnosed with ED-SCLC eventually relapse despite an initial response to the chemotherapy [11].
The main reasons for poor outcome of SCLC are advanced stage at presentation, advanced age, poor performance status, co-morbidities, poor tolerance to therapy, systemic nature of the disease, aggressiveness of the disease, early relapses, and poor compliance. Very few literatures are available on this topic from this geographic region. This study was undertaken to generate more data on SCLC, and serve to form a tool for future references.
Materials and Methods
A retrospective observational descriptive single institutional study was done to study the clinico- epidemiological pattern and treatment outcome in patients with SCLC in North-East Indian population. Patients ≥18 years of age, histopathologically proven to have small cell lung carcinoma, registered, treated and followed up at Dr. B. Borooah Cancer Institute from January 2016 to December 2021 were included in this study. The study received the approval from the institutional ethics committee.
Data Collection And Follow Up
Data were collected retrospectively from hospital- based cancer registries, individual medical case notes, electronic patient records and pathology reports, including age, gender, Eastern co-operative oncology group (ECOG) performance status, history of smoking, history of tobacco and/or alcohol intake, history of any medical risk factors, symptom burden, stage, site and socio-economic background.
A detailed retrospective chart review was performed to document staging, treatment history, follow-up, and survival outcome. Stage was determined according to the Veterans Administration Lung Study Group (VALSG) staging [7]. Patients received chemotherapy, radiotherapy, either alone or in combinations. Details of the treatment received i.e., chemotherapy (CT)/ radiotherapy (RT)/ both, and the type of treatment noted. Standard criteria were used to assess radiological response to treatment as complete response (CR), partial response (PR), progressive disease (PD) and no response (NR).
Survival status was determined from the date of registration for each patient at BBCI. The overall survival (OS) was defined as the time from the date of registration to the date of death. Those patients whose data was not available in the records were contacted over phone. For the patients where survival information was not obtained, the interval between the date of diagnosis and the date of last follow up was used to calculate survival duration.
Statistical Analysis
Descriptive statistics was used to describe the numbers and percentages, bar and pie charts were used for graphical representation of the descriptive statistics. Patient and demographic features were summarized using median/ centiles, means and standard deviations. Kaplan Meier analysis was used for survival analysis and log rank test was used to see the survival difference among groups. Cox regression was used to evaluate hazard ratio with Boot strapping method. Chi square or Fisher exact test was used for evaluation between categorical variable.
Results
A total number of 70 patients were evaluated in the study. The clinical characteristics of all patients are summarized in Table 1.
| Characteristics (N=70) | Frequency (n) | Percentage (%) |
| Stage | ||
| Limited-stage | 14 | 20 |
| Extensive-stage | 51 | 72.8 |
| Not known | 5 | 7.14 |
| Sex | ||
| Male | 58 | 82.86 |
| Female | 12 | 17.14 |
| Age (in years) | ||
| Median | 62 | |
| ≥60 (n) | 46 | |
| <60 (n) | 24 | |
| Performance status (ECOG-PS) | ||
| 0 | 10 | 14.2 |
| 1 | 15 | 21.4 |
| 2 | 23 | 32.8 |
| 3 | 14 | 20 |
| 4 | 8 | 11.4 |
| Presentation | ||
| Cough | 43 | 61.43 |
| Breathing Difficulty | 31 | 44.29 |
| Fever | 13 | 18.57 |
| Generalized Weakness | 30 | 42.86 |
| Hemoptysis | 9 | 12.86 |
| Chest Pain | 32 | 45.71 |
| Anorexia | 22 | 31.43 |
| Backache | 21 | 30 |
| Neurological Deficit | 7 | 10 |
| SVCO | 8 | 11.43 |
| SIADH | 1 | 1.43 |
| Metastatic sites involvement | ||
| >3 sites | 25 | 49.02 |
| <3 sites | 26 | 50.98 |
| Metastatic sites | ||
| Bone | 31 | 44.29 |
| Liver | 36 | 51.43 |
| Pleural Effusion | 17 | 24.29 |
| Adrenal | 5 | 7.14 |
| Lymphadenopathy | 55 | 78.57 |
| Brain | 6 | 8.57 |
| Pericardial Effusion | 2 | 2.86 |
| Metastatic sites of disease (n=51) | ||
| <3 sites | 25 | 49.02 |
| ≥3 sites | 26 | 50.98 |
The most common age group affected was 60-69 years with the median age of 62 years. The majority of the patients were male (82.86%). Majority of the patients presented with symptoms of cough (61.4%), chest pain (45.7%), breathing difficulty (44.2%) and generalized weakness (42.8%). Among the patients diagnosed with SCLC, 72.8% were diagnosed with extensive-stage disease and 20% with limited-stage disease. The majority of the patients presented with performance status ECOG ≥2 (n=45/65). Majority of the patients presented with extensive involvement by the disease. The most common site of secondary involvement was seen to be lymph nodes (78.5%) followed by liver. Among the patients with extensive-stage disease, 50.9% patients were found to have involvement of ≥3 sites. Details of the treatment received were shown in Table 2.
| Treatment | N=70 | % |
| Intent of therapy | ||
| Curative | 51 | 20 |
| Palliative | 14 | 72.85 |
| Treatment not received | 5 | 7.15 |
| Modalities of treatment | ||
| Chemo only (Palliative) | 14 | 21.54 |
| Chemo + RT (Palliative) | 19 | 29.23 |
| Chemo + RT (Radical ) | 14 | 21.54 |
| Best supportive care only (BSC) | 12 | 18.46 |
| RT only (Palliative) | 6 | 9.23 |
| Lines of chemotherapy (n=65) | ||
| 1st-line | 47 | 72.3 |
| 2nd-line | 9 | 13.8 |
| 3rd-line | 2 | 3.07 |
| Chemotherapy cycles | ||
| 1st-line (n=47) | ||
| Carboplatin-etoposide | 27 | |
| Cisplatin-etoposide | 20 | |
| 2nd-line (n=9) | ||
| Irinotecan-cisplatin | 1 | |
| Single-agent irinotecan | 5 | |
| Capecitabine-temozolamide | 3 | |
| 3rd-line (n=2) | ||
| Irinotecan-cisplatin | 1 | |
| Capecitabine | 1 | |
| Average nos. of chemotherapy received in 1st-line (n=47) | ||
| >4 cycles | 26 | 55.3 |
| ≤4 cycles | 21 | 44.6 |
| Radiotherapy (RT) received (n=65) | ||
| Palliative RT | 15 | 23.08 |
| Radical CT-RT | 14 | 21.54 |
| PCI | 7 | 10.77 |
| WBRT | 6 | 9.23 |
| Consolidative RT | 10 | 15.38 |
Among all the patients who received therapy (65/70), treatment was started with palliative intent in 51 (72.8%) patients, whereas 14 (20%) patients were offered curative intent treatment considering their better chance of long-term survival. It was seen that most of the patients (33/65) received both chemotherapy and radiotherapy. Among them 19/33 patients received palliative radiotherapy and 14/33 patients received definitive radiotherapy. While 14/65 received palliative chemotherapy alone and 6/65 received palliative radiotherapy alone, 12/65 of the patients received only best supportive care (BSC). Forty- seven (47/65) patients received 1st-line chemotherapy, out of which nine-patients received up to 2nd-line chemotherapy and two-patients received up to 3rd-line of chemotherapy on subsequent progression of the disease. Etoposide-platinum regimen was the most commonly used chemotherapy regimen. Twenty-seven (n=27/47) patients received carboplatin-etoposide and 20 (n=20/47) patients received cisplatin-etoposide in the 1st-line setting. On progression of the disease, five-patients (n=5/9) received single agent irinotecan, three patients (n=3/9) received capecitabine-temozolamide and one-patient received irinotecan-cisplatin in 2nd-line. In 3rd-line, one patient each received irinotecan-cisplatin and single-agent capecitabine. Among the patients receiving chemotherapy in the 1st-line (n=47), 55.3% received ≥4 cycles of chemotherapy, while 44.6% received less ≤4 cycles of chemotherapy. The average numbers of chemotherapy cycles received was 4-5.
As shown in Table 3, the best response observed was PR which was seen in 21 patients (n=21/47), followed by SD in 11 (n=11/47) patients.
| N | % | |
| Response rates (n=47) | ||
| CR | Jul-47 | 14.89 |
| PR | 21/47 | 44.68 |
| SD | Nov-47 | 23.4 |
| PD | Aug-47 | 17.02 |
| Survival Rates (%) (n=70) | ||
| At 6 months | 32/70 | 45.71 |
| At 12 months | 13/70 | 18.57 |
| At 24 months | 4//70 | 5.71 |
| At 36 months | Jan-70 | 1.43 |
| Median OS (mOS) | ||
| All patients | 6.1 | |
| LS | 16.4 | < 0.05 |
| ES | 4.3 | |
| Median PFS (mPFS) | ||
| All patients | 4 | |
| LS | 6.4 | < 0.05 |
| ES | 2.7 |
With a median follow-up of 43 months, the overall survival (OS) for all the patients was 6.1 months. Three-year OS for patients was significantly better in patients with LS disease (16.4 months vs. 4.3 months in patients with LS disease vs. ES disease, p<0.05). The median progression free survival (mPFS) for all patients was 4 months. The mPFS for patients with LS disease was found to be significantly better in comparison to patients with ES disease (6.4 months vs. 2.7 months, p<0.05). For the overall population, percentage of patient surviving at 6-, 12-, 24-, and 36-months were 45.7%, 18.5%, 5.7% and 1.4% respectively (percentage of patient surviving at 6, 12, 24, and 36 months for the LS were 85.7 %, 57.1%, 28.5% and 7.1% respectively). In case of ES disease, 39.2% and 9.8% patients survived up to 6- and 12-months respectively, with no patient survived beyond 24 months.
On univariate analysis done as shown in Table 4 (Figure1), it was observed that, the mOS was significantly correlated with the stages of the disease (16.4 months in LS vs. 4.3 months in ES disease; p<0.05) and number of distant involved sites (8.6 months with <3 organ involvement vs. 1.9 month with ≥3 organ involvement; p<0.0001).
| Variables | Number of patients (n/N) | mOS (months) | P value | |
| Age (years) | <60 | 24/70 | 6.1 | 0.815 |
| ≥60 | 46/70 | 6.4 | ||
| Gender | M | 58/70 | 7.4 | 0.942 |
| F | 12/70 | 3.3 | ||
| Performance status (ECOG-PS) | ≥2 | 45/70 | 6.7 | 0.89 |
| <2 | 25/70 | 8.4 | ||
| Stage of disease | LS | 14/65 | 16.4 | <0.05 |
| ES | 51/65 | 4.3 | ||
| Metastatic sites of involvement | <3 | 25/51 | 8.6 | <0.0001 |
| ≥3 | 26/51 | 1.9 | ||
| Liver metastasis | Yes | 36/70 | 2.4 | 0.11 |
| No | 34/70 | 10.6 | ||
| Bone metastasis | Yes | 31/70 | 6.1 | 0.728 |
| No | 39/70 | 6.6 | ||
| Brain metastasis | Yes | 6/70 | 6.8 | 0.28 |
| No | 64/70 | 6.1 | ||
| Adrenal metastasis | Yes | 5/70 | 5.7 | 0.71 |
| No | 65/70 | 6.2 | ||
| SVCO | Yes | 8/70 | 6.5 | 0.43 |
| No | 62/70 | 6.1 | ||
| Pleural effusion | Yes | 17/70 | 4.9 | 0.28 |
| No | 53/70 | 6.1 | ||
| Lymphadenopathy | Yes | 55/70 | 5.7 | 0.75 |
| No | 15/70 | 10.9 |
Figure 1. Disease Stage Wise Distribution of OS.
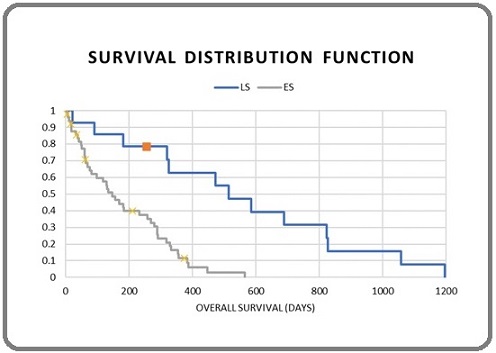
No significant correlation of survival was seen with baseline parameters like, gender and performance status. It has also been observed that patients with <3 metastatic sites survived much longer than patients with ≥3 metastatic sites (Figure 2).
Figure 2. Overall Survival and Metastatic Sites.
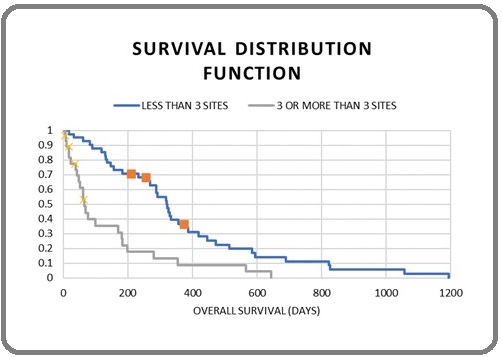
Though no significant correlation was found between OS with secondary site of involvement, it was observed that, the patients without liver metastasis lived longer than those with no liver metastasis (10.6 months vs. 2.4 months, p=0.11).
As described in Table 5, on analyzing the data for correlation of OS with modalities of treatment, it is seen that the patients who received chemo-radiotherapy survived better than best supportive care alone (10.7 months in chemo-radiotherapy vs. 0.9 months in BSC, P<0.0001).
| Variables | Different modalities of treatment | mOS (months) | p-value |
| Chemo-radiotherapy vs. pall CT alone | 10.7 vs 3.0 | 0.001 | |
| Chemo-radiotherapy vs. BSC | 10.7 vs 0.95 | <0.0001 | |
| (Pall CT + pall RT) vs. (radical CTRT) | 4.3 vs 13.3 | 0.001 | |
| (Pall CT + Consolidative RT) vs. pall CT | 11.85 vs 3.03 | 0.004 | |
| (Pall CT + Consolidative RT) vs. BSC | 11.85 vs 0.95 | <0.0001 | |
| Pall CT vs. radical CTRT | 3.03 vs 13.3 | <0.0001 | |
| Best supportive care vs. radical CTRT | 0.95 vs 13.3 | <0.0001 | |
| No. of chemo cycles (in 1st-line) | ≤4 vs. >4 | 2.8 vs 11.05 | 0.00001 |
The survival of the patients who received combined modality of treatment was better than those patients treated with chemotherapy alone (10.7 months in chemo-radiotherapy vs. 3 months in chemotherapy alone, p<0.001) (Figure 3).
Figure 3. Overall Survival and Modalities of Treatment (all patients).
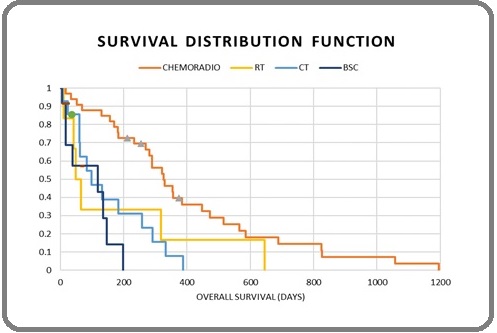
Compared to the palliative intent chemo-radiotherapy, palliative chemotherapy alone and palliative radiotherapy alone, those patients receiving definitive chemo-radiotherapy showed significantly better overall survival. (4.3 months in palliative chemotherapy and palliative radiotherapy vs. 13.3 months in definitive chemo-radiotherapy p=0.001; 3.03 months in palliative chemotherapy alone vs. 13.3 months in definitive chemo-radiotherapy, p<0.0001; 1.9 months in palliative radiotherapy alone vs. 13.3 months in definitive chemo-radiotherapy, p=0.011 respectively). Survival rate in patients who received ≥4 cycles of chemotherapy regimen in 1st-line was better than those who received <4 cycles of chemotherapy (11 months in >4 cycles vs. 2.8 months in ≤4 cycles, p=0.00001).
Discussion
SCLC is a very aggressive disease with tendency of early dissemination and poor prognosis. The median age of the patients in our study was 62 years which is similar to the studies done by Huang et al., [12] O’Sullivan et al., [13] Tendler et al. [14]. Male to female ratio of patients is 4.8:1. Cough was the main symptom (61.4%), the majority of the patients presented with ECOG-PS ≥2 (69.2%).
It was observed that 72.8% cases presented in extensive stage while 20% patients presented with limited- stage disease. This is comparable to the studies done by Abdullah et al., [15] Ramaswami et al., [16] O’Sullivan et al., [13] Toh et al., [4] Kuo et al. [17]. The most common site of secondary involvement was seen to be lymph node (78.5%), followed by liver (51.4%) and bone (44.2%) [15]. When the data was analyzed for disease burden in the patients with ES disease, it was found that 49.02% patients presented with <3 distant organ involvement and 50.9%
patients presented with ≥3 distant organ involvement.
Majority of the patients (72.8%) presented with advanced disease and this may be due to ignorance of the disease, lack of awareness of the general population, aggressiveness and tendency of early dissemination of the disease, poor socio-economic status. Covid-19 related restrictions during the pandemic period is another reason for the delay in seeking medical care.
With a median follow-up of 43 months, the OS for all the patients was 6.1 months. Three-year OS for patients was significantly better in patients with LS disease (16.4 months in LS disease vs. 4.3 months in ES disease, p<0.05). This survival outcome is comparable to the studies done by Mohan et al., [9] Kuo YH et al., [17] Abdullah et al., [15] O’Sullivan et al., [13] Tendler et al. [14]. The median PFS for all patients was 4 months. The mPFS for patients with LS disease was found to be significantly better in comparison to patients with ES disease (6.4 months vs. 2.7 months, p<0.05). Similar survival outcome was seen in Tendler et al. [14].
However, other baseline parameters like age, gender, performance status did not correlate significantly with survival in our patients. The prognostic value of these parameters has been advocated by some, but not other studies [4, 15, 16].
The OS rate at 6-, 12-, 24- and 36-months were 45.7%, 18.5%, 5.7% and 1.4% respectively. The overall survival rate at 6-, 12-, 24- and 36-months for patients with LS disease were 85.71%, 57.14%, 28.57% and 7.14% respectively. This is comparable to the previous studies done by Abdullah et al.. [15] With ES disease, none of the patients survived beyond 24 months, whereas, the survival rates at 6-, and 12-monthes were 39.2% and 9.8% respectively.
The most common chemo-toxicity observed was nausea and vomiting which was found in 38.3% patients followed by diarrhea and grade II mucositis in 21.2% of patients each. Grade II and III cytopenia was observed in 17% and 12.7% patients respectively. Febrile neutropenia, grade III hyponatremia, tachyarrhythmia and neutropenic enterocolitis was seen in 4.2%, 2.6%, 2.1% and 2.1% patients respectively.
The patients who received chemo-radiotherapy survived better than those patients who received best supportive care (BSC) alone (10.7 months in chemo-radiotherapy vs. 0.9 months in BSC, p<0.0001). The survival of the patients who received combined modality of treatment was better than those patients treated with chemotherapy alone (10.7 months in chemo-radiotherapy vs. 3 months in chemotherapy alone, p<0.001). Compared to the palliative intent chemo-radiotherapy, palliative chemotherapy alone and palliative radiotherapy alone, those patients receiving definitive chemo-radiotherapy showed significantly better overall survival. (4.3 months in palliative chemotherapy and palliative radiotherapy vs. 13.3 months in definitive chemo-radiotherapy p=0.001; 3.03 months in palliative chemotherapy alone vs. 13.3 months in definitive chemo-radiotherapy, p<0.0001; 1.9 months in palliative radiotherapy alone vs. 13.3 months in definitive chemo-radiotherapy, p=0.011 respectively). Similar to some of these findings were also observed by Toh et al. [4] and Kuo YH et al. [17].
Survival rate in patients who received ≥4 cycles of chemotherapy regimen in 1st-line was better than those who received <4 cycles of chemotherapy (11 months in>4 cycles vs. 2.8 months in ≤4 cycles, p=0.00001). Similar findings were also seen by Huang et al. [12].
No correlation of overall survival was found with metastatic sites of involvement apart from liver metastasis, which is similar to the findings in Huang et al. [12]. Those without liver metastasis were found to live longer than those with liver metastasis (median overall survival 10.6 months vs. 2.4 months) [15]. It was observed that, those patients who had more distant organ involvement had a significantly inferior survival than those who had less organ involvement, indicating that the metastasis to distant organs might be a stronger predictor for survival [7] [15]. No significant relation was detected between SVCO and prognosis as observed by Abdullah et al. [15].
In conclusion, SCLC remains a highly aggressive disease with a poor prognosis. Advanced stage of presentation with metastasis to distant organ is the most important factors associated with poor survival amongst the patients with SCLC. Many gaps in our characterization of SCLC remain and clinical progress lags behind that seen in NSCLCs. Although several novel therapeutic targets are being actively pursued in clinical research, several gaps exist in our understanding of the disease, which contribute to the modest effect that current treatments have had on patient survival.
Acknowledgments
The authors are grateful to the resident doctors and the staff at the Department of Medical Oncology for their assistance and cooperation during data collection for the study.
Financial Support and Sponsorship
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries2020 Journal Citation Reports (Clarivate Analytics): 1/243 (Oncology) Online ISSN:1542-4863 Hyuna Sung PhD , et al . .
- Mouse models of chemically-induced lung carcinogenesis Vikis HG , Rymaszewski AL , Tichelaar JW . Frontiers in Bioscience (Elite Edition).2013;5(3). CrossRef
- New insights into small-cell lung cancer development and therapy Wang Y, Zou S, Zhao Z, Liu P, Ke C, Xu S. Cell Biology International.2020;44(8). CrossRef
- Survival of small-cell lung cancer and its determinants of outcome in Singapore Toh C, Hee S, Lim W, Leong S, Fong K, Yap S, Hsu AAL , et al . Annals of the Academy of Medicine, Singapore.2007;36(3).
- Small Cell Lung Cancer Bernhardt EB , Jalal SI . Cancer Treat Res.2016;170:301-322. CrossRef
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray F, Ferlay J, Soerjomataram I, Siegel RL , Torre LA , Jemal A. CA: a cancer journal for clinicians.2018;68(6). CrossRef
- Staging and imaging of small cell lung cancer Kalemkerian GP . Cancer Imaging: The Official Publication of the International Cancer Imaging Society.2012;11(1). CrossRef
- Small-cell lung cancer Jackman DM , Johnson BE . Lancet (London, England).2005;366(9494). CrossRef
- Survival in small cell lung cancer in India: prognostic utility of clinical features, laboratory parameters and response to treatment Mohan A., Goyal A., Singh P., Singh S., Pathak A. K., Bhutani M., Pandey R. M., Guleria R.. Indian Journal of Cancer.2006;43(2). CrossRef
- Small-cell lung cancer: what we know, what we need to know and the path forward GazdarAF , Bunn PA , Minna JD . Nature Reviews. Cancer.2017;17(12). CrossRef
- Management of Small Cell Lung Cancer: Progress and Updates Altan M, Chiang AC . Cancer Journal (Sudbury, Mass.).2015;21(5). CrossRef
- Survival and pretreatment prognostic factors for extensive-stage small cell lung cancer: A comprehensive analysis of 358 patients Huang L, Hu X, Wang Y, Li J, Wang H, Liu P, Xu J, et al . Thoracic Cancer.2021;12(13). CrossRef
- Real-World Treatment Patterns, Clinical Outcomes, and Health Care Resource Utilization in Extensive-Stage Small Cell Lung Cancer in Canada O'Sullivan DE , Cheung WY , Syed IA , Moldaver D, Shanahan MK , Bebb DG , Sit C, Brenner DR , Boyne DJ . Current Oncology (Toronto, Ont.).2021;28(4). CrossRef
- Treatment patterns and survival outcomes for small-cell lung cancer patients - a Swedish single center cohort study Tendler S, Zhan Y, Pettersson A, Lewensohn R, Viktorsson K, Fang F, De Petris L. Acta Oncologica (Stockholm, Sweden).2020;59(4). CrossRef
- Factors Affecting Survival in Small Cell Lung Cancer Sakin A, Turgut E, Aybek M, Usta A, Koşan Ö, Çelik K, Ozturk S, Koçer M. European Journal of General Medicine.2018;13. CrossRef
- Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL , Piccirillo J. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2006;24(28). CrossRef
- Survival of patients with small cell lung carcinoma in Taiwan Kuo Y, Lin Z, Yang Y, Shao Y, Shau W, Kuo RNC , Yang JC , Lai M. Oncology.2012;82(1). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2024
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times