Exploring Multiple Primary Malignancies: An Institutional Experience with Dual Malignancy Cases
Download
Abstract
Objective: To determine the incidence, clinical characteristics, and survival outcomes of patients with multiple primary malignancies at a tertiary care cancer centre.
Methods: A retrospective analysis of 28 patients (11 males, 17 females) with histologically confirmed multiple primary malignancies was conducted from 2020 to 2022. Data on demographic characteristics, tumour sites, histopathology, staging, and survival were collected. Kaplan-Meier method was used for survival analysis with log-rank tests for group comparisons.
Results: The incidence of multiple primary malignancies was 0.73%. Metachronous tumours (67.9%) were more common than synchronous tumours (32.1%). Breast cancer was the most frequent primary (32.1%) and secondary (28.6%) malignancy. Mean survival time significantly differed between males (198.0 months, 95% CI: 76.8-122.4) and females (125.2 months, 95% CI: 88.5-162.0, p=0.093). Advanced stage presentation was common, with 42% of primary and 25% of secondary tumours diagnosed at stage III. The event-free survival was higher for metachronous than synchronous malignancies (log-rank p<0.05). Among patients, 28.6% had a positive family history, and 17.9% reported substance addiction.
Conclusion: Multiple primary malignancies show distinct patterns in incidence, tumour site distribution, and survival outcomes, with metachronous presentations and breast cancer predominance being key characteristics in our population.
Introduction
Multiple primary malignancies (MPM) are not uncommon, with improved survival rates and advanced diagnostic tools that allow for the detection of synchronous occult tumours that were previously overlooked [1-4]. Inherited predisposition may also play a role in developing some MPMs, as seen in the association between hereditary nonpolyposis colorectal cancer and an increased risk of developing ovarian cancer or cancer of the endometrium and small intestine [5]. Epidemiological studies on MPMs can provide important information on the risk factors for each type of tumour [6].
A literature review of 1,104,269 cancer patients revealed that the prevalence of MPM ranges from 0.73% to 11.7%, with the incidence increasing with age [7]. MPMs can be divided into two categories depending on the interval between tumour diagnosis and synchronous cancers occurring simultaneously or within six months after the first malignancy. Multiple metachronous malignancies are secondary cancers that develop after more than six months [6].
While the triad of primary tumours presenting asynchronously is a proven entity, as in MEN syndrome, linked with specific genetic alterations, the synchronous occurrence of carcinomas of different histology in one person is an uncommon event sparsely found in the reported literature [8]. Bililroth first reported this dual malignancy in 1889.
The MPM-positive diagnosis was based on Warren and Gates’s criteria proposed in 1932 [6]. Typically, a patient with a first cancer who develops a second cancer will do so over time. The presentation of a patient with concurrent primary tumours of distinct histology in separate organ systems is unusual [7, 9].
Multiple primary cancers were first reported approximately 100 years ago, and their frequency of occurrence has gradually increased [10]. In the Indian literature, scant data are available regarding multiple primaries, with most being case reports, such as 5% of women with endometrial cancer, 10% of women with ovarian cancer [11], and 3.4% of patients with gastric cancer, with the most common primary cancer being colorectal cancer (20.4%) [12]. Samadder et al. found synchronous and metachronous CRC 0.71% and 1.60% of synchronous and metachronous colorectal cancer (CRC), respectively [13].
It is necessary to distinguish between multiple synchronous and metachronous primary malignancies. The diagnostic criteria proposed by Ulbright and Roth were revised by Scully [14]. The current study aimed to explore cases of dual malignancy in patients with Multiple primary malignancies.
Materials and Methods
1. Study Design
This retrospective observational study was conducted at Healthcare Global (HCG) Hospital, Bangalore, a tertiary care cancer centre, analyzing data from January 2020 to December 2022. Permission for data collection was obtained from the head of the institution, and the study protocol was approved by the Institutional Ethics Committee (IEC) of HCG Hospital.
2. Study Population
2.1 Sample Selection
The study population comprised 28 patients with histologically confirmed multiple primary malignancies. Patient records were retrieved from the hospital’s electronic medical record system using ICD-10 coding for multiple malignancies.
2.2 Selection Criteria
Inclusion criteria encompassed: 1) patients with at least two neoplastic locations confirmed through histopathological examination, 2) different histopathologies observed in the two locations/sites, and 3) complete medical records available for review. Patients were excluded if they had 1) incomplete histopathological confirmation of each tumour, 2) suspected metastasis rather than primary malignancy, or 3) insufficient follow- up data for survival analysis.
3. Data Collection
3.1 Clinical Parameters
Data has been independently extracted using a standardized form and encompassed the following parameters: 1) demographic data (age, gender, family history, substance use), 2) clinical characteristics (date of diagnosis, tumour site, histopathology), 3) disease characteristics (TNM staging, grade), 4) treatment details (surgery, chemotherapy, radiotherapy), and 5) follow-up information (response, recurrence, survival status).
4. Statistical Analysis
4.1 Descriptive Statistics
Categorical variables were expressed as frequencies and percentages. Continuous variables were presented as mean ± standard deviation or median with interquartile range based on distribution normality assessed using the Shapiro-Wilk test.
4.2 Survival Analysis
Overall survival (OS) was calculated from the first primary malignancy diagnosis to death from any cause or last follow-up. Event-free survival (EFS) was measured from diagnosis until disease progression, death, or last follow-up, whichever occurred first. Patients alive at their last follow-up were censored at that date.
4.3 Statistical Methods
Survival probabilities were estimated using the Kaplan-Meier method, with differences between groups compared using log-rank tests. Gender-based survival comparisons were performed using chi-square tests. Statistical significance was set at p<0.05. All analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY).
5. Ethical Considerations
The study was conducted in accordance with the Declaration of Helsinki. The IEC waived the requirement for individual patient consent due to the study’s retrospective nature. Patient confidentiality was maintained by using coded identifiers and restricting data access to research team members.
Results
Patient characteristics: Between 2020 and 2022, we collected data from 28 patients (11 male and 17 female) with double primary tumours. The mean age at diagnosis of the first and second primary tumours was 57 years, and the median was 57. The most common age group for primary and secondary malignancies was 41-60 (46.6%). Double malignancy was less common in patients aged > 80 years than in age. Our study included patients with a minimum age of 27 years and a maximum of 96 years, with a standard deviation of 13.29 (Table 1).
| Characteristic | Frequency | Percent |
| Age | ||
| 21-40 years | 4 | 14.30 |
| 41-60 years | 13 | 46.40 |
| 61-80 years | 10 | 35.70 |
| >80 years | 1 | 3.60 |
| Family History | ||
| No | 20 | 71.40 |
| Yes | 8 | 28.60 |
| Habits | ||
| No | 23 | 82.10 |
| Yes | 5 | 17.90 |
Values are expressed in frequency and percentage
In the current study, the incidence of double malignancy was higher in females (17 female patients (60%) and 11 male patients (39%] ). Eight of the 28 patients (28%) had a family history of double malignancy. Only five out of 28 patients (17%) were found to be addicted to tobacco or other substances. The remaining 23 (82 %) patients were not addicted to any substance.
Site Distribution: Double malignancy was most common in the age group of 41-60 years (46.4%) and least common in the age group of >80 years (3.6%). In males, gastrointestinal (GI) malignancy (17.9%) was the most common, followed by myeloma (7.1%). In females, breast malignancy (32%) was the most common, followed by cervical cancer (7.1%) and ovarian carcinoma (3.6%). The second most common cancer in males was leukaemia (14.3%), followed by head and neck carcinoma (10.7%) and prostate carcinoma (7.1%) (Table 2).
| Primary Site 1 | N | % |
| Breast | 9 | 32.1 |
| Cervix | 2 | 7.1 |
| Gastrointestinal Tract | 5 | 17.9 |
| Hepatobiliary | 1 | 3.6 |
| Head and Neck | 2 | 7.1 |
| Leukaemia | 1 | 3.6 |
| Lymphoma | 2 | 7.1 |
| Myeloma | 2 | 7.1 |
| Ovary | 1 | 3.6 |
| Prostate | 2 | 7.1 |
| Thyroid | 1 | 3.6 |
Values are expressed in frequency and percentage.
The most common site of the second primary tumour was the breast (28.6 %), followed by leukaemia and GI (14.3% and 10.7 %, respectively). Hepatobiliary and head and neck tumours also have a considerable percentage of second primary tumours, with 7.1% each, while the remaining sites have a frequency of less than 5 % (Table 3).
| Primary Site 2 | N | % |
| Breast | 8 | 28.6 |
| Cervix | 1 | 3.6 |
| Gastro Intestinal Tract | 3 | 10.7 |
| Hema | 1 | 3.6 |
| Hepatobiliary | 2 | 7.1 |
| Head and Neck | 3 | 10.7 |
| Leukaemia | 4 | 14.3 |
| Lung | 2 | 7.1 |
| Lymphoma | 1 | 3.6 |
| Prostate | 2 | 7.1 |
| Sarcoma | 1 | 3.6 |
Values are expressed in frequency and percentage.
Breast cancer is the most frequently observed tumour pair in the first and second malignancies (14.2%). Among females, the most common second malignancy was breast cancer (28.6%), followed by GI malignancy (10.7%). The number of primary tumours present at stages III and IV was 42% and 10%, respectively. The number of secondary tumours present at stages III and IV was 25% and 21%, respectively. Among these patients, 28.6% had a positive family history, and 71.4% had no significant family history. The proportion of patients with chronic addiction was 17.9%.
Distribution types
According to our study, the incidence of metachronous tumours is higher than that of synchronous tumours. Metachronous malignancy accounted for 67.9% of the cases, while synchronous malignancy accounted for 32.1%.
The study found that the EFS was higher for metachronous malignancy, and the metachronous censor was higher than the synchronous censor. The overall survival was higher for males than females, but the small sample size may limit the generalization of this finding (Figure 1).
Figure 1. The Survival Analysis Results Comparing Overall and Event-free Survival (EFS) between Metachronous and Synchronous Malignancies.
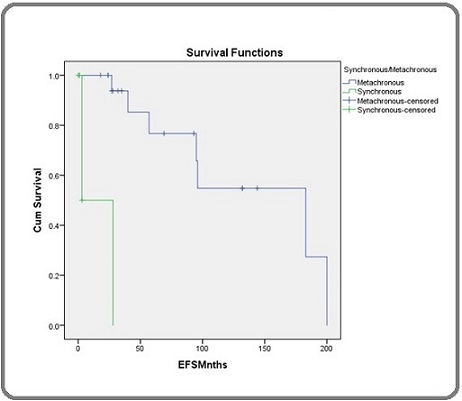
The mean survival time for females was estimated at 125.21 months with a standard error of 18.75 months. The 95% confidence interval for females ranged from 88.47 to 161.96 months, indicating some variability in survival times. The p-value of 0.093 suggests that the difference in survival times between males and females is not statistically significant. Therefore, while there is an observed difference in mean survival times between the genders, this finding does not reach statistical significance at the conventional p-value threshold of 0.05, implying that gender may not strongly impact survival outcomes in this cohort. Further studies with larger sample sizes may be needed to explore this trend more conclusively (Table 4).
| Mean Survival Time | |||||||
| Sex | 95% Confidence Interval | ||||||
| Estimate | Std. Error | Lower Bound | Upper Bound | df | Chi-Square | p-value | |
| Female | 125.214 | 18.745 | 88.473 | 161.955 | 1 | 2.818 | 0.093 |
| Male | 198 | 1.08 | 76.8 | 122.4 |
Figure 2 shows that the survival rates for females and males with hairy cell leukaemia are similar in the first 5 years.
Figure 2. The Survival Functions of a Group of People with Hairy Cell Leukaemia (HCL).
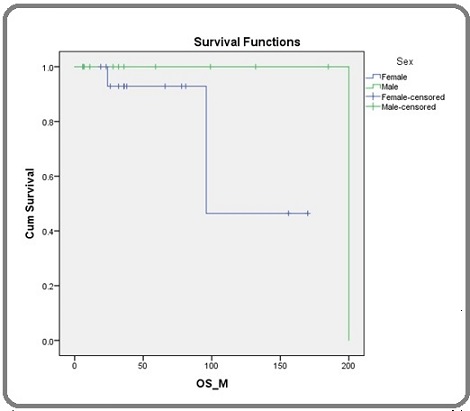
However, the survival rate for females starts to decline after 10 years, while the survival rate for males remains relatively stable. This suggests that females with HCL may be more likely to experience late-stage complications of the disease.
Discussion
The incidence of double malignancy observed in this study was less than 1%, which is relatively low compared to the reported overall incidence of Multiple Primary Malignancies (MPM) in cancer patients, which ranges from 0.7% to 18% [5, 15]. Discrepancies in these incidence rates across various studies can be attributed to differences in study durations and the inclusion of autopsy series in some investigations [5, 15].
The data indicated that most patients diagnosed with double malignancy at HCG Hospital were female, highlighting a potential gender disparity (female > male). Moreover, our findings revealed that the age group most commonly affected by double malignancy ranged from 40 to 60 years. In our cohort, metachronous malignancies were more frequently observed than synchronous ones. Notably, a higher occurrence of breast-breast pairs was evident in our study. Additionally, we observed no significant disparity between primary and secondary tumour sites occurring at the same anatomical location. Notably, most of the second primary cases were diagnosed at an advanced stage, underscoring the critical need for vigilant monitoring and early detection in these cases. Results revealed a noteworthy pattern where the development of second primary malignancies often occurred after a complete response to the initial malignancy. Conversely, only a minority of cases experienced secondary primary malignancies during treatment or alongside progressive disease of the primary malignancy.
The etiological mechanisms underpinning the emergence of multiple primary malignancies remain a subject of ongoing research. While comprehensive elucidation is pending, common factors implicated in their development include genetic susceptibility, the immune status of patients, and exposure to carcinogens, including chemotherapy and radiotherapy, commonly employed in cancer treatment. Specifically, previous exposure to radiation or chemotherapy has been recognized as contributing to the emergence of second primary malignancies. Predominantly, the secondary cancers observed encompass skin, breast, acute leukaemia, colorectal, lung, and stomach cancers. For instance, the risk of secondary cancer development following treatment for Hodgkin’s disease has been quantified at 10% at 20 years and 26% at 30 years [16], while patients receiving a doxorubicin-based regimen for breast cancer face a 3.8% risk at 10 years versus 7% at 15 years [17].
One established causal factor for double malignancy is genomic instability. However, the predominant drivers of secondary malignancies remain the carcinogenic effects of radiotherapy and chemotherapy. Patients with a familial history of hereditary cancer and those previously treated for cancer with genetic susceptibility bear an elevated risk of multiple primary malignancies. The treatment modality employed for the first malignancy, which inflicts damage to specific DNA regions resulting in chromosome loss or rearrangement, has been identified as a causal factor in developing second malignancies [18].
Contemporary technologies have facilitated the analysis of genetic alterations, encompassing point mutations, loss of heterozygosity, and genetic instability. Microsatellite instability (MSI) has become more frequently associated with multiple primary malignancies than sporadic cancers [19]. Interestingly, the prevalence of MSI tumours appeared similar among patients with synchronous and metachronous colorectal tumours.
Particularly striking is the well-documented cumulative lifetime risk of 36% for patients with Squamous Cell Carcinoma of the head and neck to develop a second primary malignancy over 20 years [21]. This heightened risk is predominantly attributed to carcinogenic exposure from common risk factors such as tobacco chewing, smoking, and alcohol consumption [20, 21]. Consequently, it is imperative to incorporate preventive strategies, advising patients with head and neck cancer to cease alcohol and tobacco use, adopt a healthful diet, and engage in regular physical activity. There is insufficient evidence to endorse chemo-preventive agents such as beta-carotenoids and antioxidants for averting second primary malignancies [22].
The possibility of multiple primary malignancies should invariably be contemplated during pretreatment evaluation. PET screening procedures demonstrate utility in the early detection of secondary tumours. While limited evidence suggests that screening may enhance outcomes among patients at risk of developing second malignancies, comprehensive research is warranted to establish optimal screening modalities and strategies to mitigate mortality associated with second primary malignancies across diverse tumour sites [23].
In conclusion, second primary malignancies (SPMs) are increasingly detected due to improved diagnostic techniques and cancer management. Early detection through strong clinical suspicion and careful evaluation is crucial. Operable synchronous SPMs can often be resected in a single stage, while regular follow-ups are essential for detecting metachronous SPMs. Patient education on preventive measures and screening is vital.
This study’s limitations include potential selection bias and a small sample size. Future research should focus on larger, multicenter prospective studies, investigating molecular and genetic pathways, developing predictive risk models, and creating tailored screening protocols. Collaborative healthcare efforts and enhanced patient education will improve outcomes in multiple primary malignancies.
Acknowledgments
Approval by the scientific committee: “The study was approved by the Institutional Ethics Committee of Healthcare Global (HCG) Hospital, Bangalore.”
Conflict of Interest
“None declared.”
Funding
No specific funding was received for this study
References
- Second primary neoplasms (SPN) in cancer patients Vaslamatzis M, Alevizopoulos N, Petraki C, et al . Proc ASCO.2003;22:3581.
- Synchronous and metachronous second (ST) and third (TT) primary tumours (PT) in the patient population Morgenfeld EL , Tognelli F, Gil Deza E, et al . Proc ASCO.2003;22:3152.
- Epidemiological analysis of site relationships of synchronous and metachronous multiple primary cancers in the National Cancer Center, Japan, 1962-1996 Kaneko S., Yamaguchi N.. Japanese Journal of Clinical Oncology.1999;29(2). CrossRef
- Multiple primary neoplasms at a single institution: differences between synchronous and metachronous neoplasms Aydiner A., Karadeniz A., Uygun K., Tas S., Tas F., Disci R., Topuz E.. American Journal of Clinical Oncology.2000;23(4). CrossRef
- Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors Travis LB , Demark Wahnefried W, Allan JM , Wood ME , Ng AK . Nature Reviews. Clinical Oncology.2013;10(5). CrossRef
- Multiple primary malignant tumours: A survey of the literature and statistical study Warren S GO . Am J Cancer.1932;16:1385-414.
- Five cases report of solid tumor synchronously with hematologic malignancy Cui Y, Liu T, Zhou Y, Ji Y, Hou Y, Jin W, Feng Y. Cancer Research and Treatment.2012;44(1). CrossRef
- A rare case of a synchronous anaplastic carcinoma thyroid with ductal carcinoma breast Ghosh S, Rao PBA , Sarkar S, Kotne S, Turlapati S. P. V., Mishra A. Case Reports in Oncological Medicine.2014;2014. CrossRef
- Report of two cases of quintuple primary malignancies and review of the literature Cercato MC , Colella E, Ferraresi V, Diodoro MG , Tonachella R. Anticancer Research.2008;28(5B).
- Multiple primary malignancies involving lung cancer Li F, Zhong W, Niu F, Zhao N, Yang J, Yan H, Wu Y. BMC cancer.2015;15. CrossRef
- Simultaneously detected endometrial and ovarian carcinomas--a prospective clinicopathologic study of 74 cases: a gynecologic oncology group study Zaino R., Whitney C., Brady M. F., DeGeest K., Burger R. A., Buller R. E.. Gynecologic Oncology.2001;83(2). CrossRef
- Synchronous and metachronous cancers in patients with gastric cancer Eom BW , Lee H, Yoo M, Cho JJ , Kim WH , Yang H, Lee KU . Journal of Surgical Oncology.2008;98(2). CrossRef
- Epidemiology and familial risk of synchronous and metachronous colorectal cancer: a population-based study in Utah Samadder NJ , Curtin K, Wong J, Tuohy TMF , Mineau GP , Smith KR , Pimentel R, et al . Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association.2014;12(12). CrossRef
- Metastatic and independent cancers of the endometrium and ovary: a clinicopathologic study of 34 cases Ulbright T. M., Roth L. M.. Human Pathology.1985;16(1). CrossRef
- Second primary malignant neoplasms: a clinicopathological analysis from a cancer centre in India Hulikal N, Ray S, Thomas J, Fernandes DJ . Asian Pacific journal of cancer prevention: APJCP.2012;13(12). CrossRef
- High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: report from the Late Effects Study Group Bhatia S, Yasui Y, Robison LL , Birch JL , Bogue MK , Diller L, DeLaat C, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2003;21(23). CrossRef
- Cardiovascular death and second non-breast cancer malignancy after postmastectomy radiation and doxorubicin-based chemotherapy Woodward WA , Strom EA , McNeese MD , Perkins GH , Outlaw EL , Hortobagyi GN , Buzdar AU , Buchholz TA . International Journal of Radiation Oncology, Biology, Physics.2003;57(2). CrossRef
- Leukaemia-specific chromosome damage detected by comet with fluorescence in situ hybridization (comet-FISH) Escobar PA , Smith MT , Vasishta A, Hubbard AE , Zhang L. Mutagenesis.2007;22(5). CrossRef
- Frequent replication errors at microsatellite loci in tumors of patients with multiple primary cancers Horii A., Han H. J., Shimada M., Yanagisawa A., Kato Y., Ohta H., Yasui W., Tahara E., Nakamura Y.. Cancer Research.1994;54(13).
- Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer Morris LGT , Sikora AG , Patel SG , Hayes RB , Ganly I. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2011;29(6). CrossRef
- Anatomic sites at elevated risk of second primary cancer after an index head and neck cancer Morris LGT , Sikora AG , Hayes RB , Patel SG , Ganly I. Cancer causes & control: CCC.2011;22(5). CrossRef
- The impact of smoking status, disease stage, and index tumor site on second primary tumor incidence and tumor recurrence in the head and neck retinoid chemoprevention trial Khuri F. R., Kim E. S., Lee J. J., Winn R. J., Benner S. E., Lippman S. M., Fu K. K., et al . Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology.2001;10(8). CrossRef
- Identifying and screening patients at risk of second cancers Vogel VG . Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology.2006;15(11). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2024
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times