Role of IMP3 Expression in Colorectal Carcinoma: An Immunohistochemical Study
Download
Abstract
Purpose: Colorectal carcinoma (CRC) ranks as the third most frequently diagnosed cancer globally and the second most common cause of cancer deaths. CRC is a major public health issue that is associated with high incidence and mortality rates. Insulin-like growth factor II m-RNA-binding protein 3 (IMP3) is an oncofetal RNA-binding protein that plays a key role in the carcinogenesis and progression of various neoplasms. Its overexpression is correlated with aggressive behavior and unfavorable prognosis of many cancers, so it can serve as a prognostic biomarker. This study aimed to evaluate the immunohistochemical (IHC) expression of IMP3 in CRC in relation to the available clinicopathological data to clarify its prognostic significance.
Methods: Seventy specimens of CRC were included in this study. Immunohistochemistry (IHC) was used to evaluate IMP3 expression in CRC. A statistical analysis of the results was conducted to correlate IMP3 expression with the clinicopathological findings.
Results: IMP3 expression was detected in 48 (68.6%) of the studied CRC cases. Statistically significant correlations were detected between IMP3 expression in relation to tumor grade (p=0.003), T stage (p<0.001), N stage (p=0.001), and lymphovascular invasion (LVI) (p=0.006).
Conclusion: IMP3 upregulation in CRC is associated with an unfavorable prognosis; its overexpression is correlated with tumor progression and spread, so it could be tried as a therapeutic modality for CRC to reduce the tumor progression and improve the clinical outcome.
Introduction
Colorectal carcinoma (CRC) is one of the most prevalent malignant neoplasms worldwide. It is associated with high incidence and mortality rates, constituting the third most frequent cancer and the second most common cause of cancer deaths [1, 2]. In Egypt, CRC ranks the 7th most common cancer constituting 3.9% of all cancers [1, 3]. The majority of CRC cases are sporadic (70%), while familial and hereditary CRC account for 25% and 5–10% of CRC cases, respectively [4]. Histologically, more than 90% of CRC cases are adenocarcinomas. Mucinous, signet ring, medullary, micropapillary, and cribriform variants are additional histologic subtypes [5]. The high morbidity and mortality rates associated with CRC are attributed to distant metastases. Over 50% of cases are associated with liver metastasis, which is the leading cause of death in most cases [6]. The invasive properties and metastatic potential of CRC cells are the main indicators of the outcome. Factors implicated in molecular mechanisms of CRC progression and metastasis are distinct candidates for novel prognostic biomarkers [7]. Insulin-like growth factor II m-RNA-binding protein
3 (IMP3), a member of the IMP family, has an important role in mRNA stabilization as well as cell growth and migration during embryogenesis. Three members of the IMP family of proteins are currently recognized: IMP1, IMP2, and IMP3 [8]. IMP3 is an oncofetal protein that has been detected in early human embryogenesis and cancer tissues. It is secreted from immature tissues and the placenta, but it isn’t expressed by normal adult tissues [9]. IMP3 has attracted great attention as a cancer-related protein. According to previous studies, IMP3 promotes the survival, proliferation, adhesion, and invasiveness of tumor cells. Additionally, it has been noted that IMP3 expression is correlated with tumor angiogenesis [10]. Recent studies have reported that IMP3 plays a significant role as a cancer-specific gene involved in different aggressive and advanced cancers, so it may serve as a useful prognostic marker for these tumors. Its overexpression has been observed in various human cancers, including lung cancer [11], laryngeal carcinoma [12], breast cancer [13], and pancreatic cancer [14].
Previous reports demonstrated that IMP3 expression in CRC has a valuable prognostic role; it has been reported that upregulated IMP3 expression in CRC promotes tumor cell proliferation, adhesion, and invasion, so it is an indicator of poor prognosis [15]. However, the level of IMP3 expression and its clinicopathological significance in CRC need further elucidation. This study aimed to evaluate the expression of IMP3 in CRC and to correlate its expression with some available clinicopathological parameters, such as patient’s age, sex, degree of differentiation, tumor stage, lymph node metastasis (LNM), lymphovascular invasion (LVI), and perineural invasion to assess its prognostic significance.
Materials and Methods
Clinical data and specimens collection
A total of seventy patients who were diagnosed as CRC patients between September 2022 and September 2023 were enrolled in the current cross-sectional study. CRC was diagnosed by histopathological examination of specimens obtained by lower endoscopy and endoscopic-guided colonic punch biopsies, which were done at the Department of Tropical Medicine and and the Department of General Surgery at Sohag University Hospital for various indications. Patients then underwent colectomy at the Department of General Surgery, and the specimens were sent to the Pathology Laboratory. Hematoxylin and eosin (H&E)-stained sections were evaluated to confirm the diagnosis, histological subtype, and tumor grade. The depth of invasion, LVI, perineural invasion, and presence of regional LNM were also assessed. The studied cases were staged based on the Tumor-Node-Metastasis (TNM) staging system of the 8th edition of the American Joint Committee on Cancer (AJCC), the most widely used tool for predicting the prognosis of CRC patients [16]. Clinical details were collected from patients’ records.
Inclusion and exclusion criteria
Specimens of colorectal carcinoma were included; the availability of adequate tissue material and clinical data was required for the selection of these specimens. Specimens with insufficient clinical data, inadequate or extensively necrotic tissue material, or patients who received pre-operative anti-cancer therapy were excluded.
Ethical considerations
The study was started after approval by the Committee of Medical Ethics at Faculty of Medicine, Sohag University. The study’s registration number is (Soh- Med-22-9-16). The study was listed in Clinical Trials. Gov PRS (NCT05601388).
Immunohistochemical staining
For the immunostaining procedure, 4μm tissue sections were prepared from each paraffin block and placed on coated slides. Tissue sections were deparaffinized and rehydrated using hot xylene and descending grades of alcohol, respectively. By incubating in 3% hydrogen peroxide for 10 minutes, endogenous peroxidase was inhibited. The slides were subsequently treated with the antigen retrieval solution (citrate buffer, pH 6.0); the buffer was heated in the microwave for 15 minutes. After washing in phosphate buffer solution (PBS), the sections were incubated with rabbit anti-Human IMP3 polyclonal antibody (0.1 mg/ml concentration, Chongqing Biospes, Cat No. #YPA1463, China, dilution of 1:100) at 4ºC overnight. A standard labeled streptavidin-biotin system was used for immunodetection. Diaminobenzidine (DAB) was used as a chromagen for visualization of the stained sections, and then the sections were counterstained with Mayer’s Hematoxylin. Finally, sections were dehydrated, cleared, and mounted. A positive control was fetal liver taken from an aborted 16-week-old fetus. A negative control was employed by skipping the primary antibody step.
Immunohistochemical interpretation
IMP3 was expressed as a brownish cytoplasmic stain within the tumor cells. IMP3 expression was evaluated semi quantitatively based on both the staining intensity and the percentage of positive cells. The staining intensity was scaled as follows (0, 1+, 2+, and 3+), and the percentage of positive tumor cells was estimated for each tissue spot. The final score was based on the sum of the scores obtained by evaluating the intensity of staining and the percentage of stained cells. The results were interpreted in the following way: negative expression referred to a staining intensity of 0 and 1+ in ≤10% of tumor cells; weak positive expression had a staining intensity of 1+ in >10% and ≤70% of tumor cells or staining intensity of 2+ in ≤30% of tumor cells; moderate positive scores had a staining intensity of 1+ in >70% of tumor cells, a staining intensity of 2+ in >30%, and ≤70% of tumor cells, or a staining intensity of 3+ in ≤30% of tumor cells; and strong expression was defined as a staining intensity of 2+ in >70% of tumor cells or a staining intensity of 3+ in >30% of tumor cells. All tumors exhibiting at least weak expression were considered IMP3-positive [17].
Statistical analysis
Version 26 of the Statistical Package for Social Science (SPSS) program was used for data analysis. Mean, standard deviation (SD), median, and range were used to express quantitative data. Numbers and percentages were used to express qualitative data. The clinicopathological features were compared to IMP3 expression using the Chi-square test (χ2) or Fisher’s exact test when indicated. P value of 0.05 or less was regarded as statistically significant.
Results
Patients’ characteristics
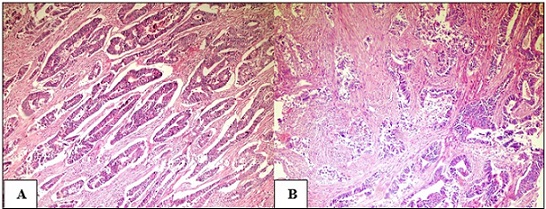
The patients’ ages ranged from 32 to 75 years; the mean age ± SD and median were 53.9 ± 9 and 54 years, respectively. The male-to-female ratio in the studied cases was 1.5:1, with 42 (60%) males and 28 (40%) women. Colorectal adenocarcinoma was the histological subtype detected in all cases. The following histological grades were assigned based on the representative H&E-stained sections: 35 (50%) cases were well-differentiated, 27 (38.6%) cases were moderately-differentiated, and 8 (11.4%) were poorly-differentiated (Figure 1).
Figure 1. (A) Well-differentiated colorectal adenocarcinoma.(B) moderately-differentiated colorectal adenocarcinoma invading the muscle fibers (H and E X100)..
On applying the TNM staging system of the AJCC (8th edition), 26 (37.1%) cases were T2, 36 (51.4%) cases were T3, and 8 (11.4%) cases were T4a. The presence of LNM was detected in 42 (60%) of the cases. Vascular and perineural invasions were found in 43 (61.4%) and 12 (17.1%) cases, respectively. Only six (8.6%) specimens showed mucoid differentiation. Table 1 describes the clinicopathological characteristics of the studied cases.
| Clinicopathological parameters | N | % |
| Age (years) | ||
| ≤50 | 27 | 38.60 |
| >50 | 43 | 61.40 |
| Sex | ||
| Male | 42 | 60 |
| Female | 28 | 40 |
| Mucoid differentiation | ||
| Present | 6 | 8.60 |
| Absent | 64 | 91.40 |
| Degree of differentiation | ||
| Well-differentiated | 35 | 50 |
| Moderately-differentiated | 27 | 38.60 |
| Poorly-differentiated | 8 | 11.40 |
| T stage | ||
| T2 | 26 | 37.10 |
| T3 | 36 | 51.40 |
| T4a | 8 | 11.40 |
| N stage | ||
| N0 | 28 | 40 |
| N1 | 28 | 40 |
| N2 | 14 | 20 |
| LVI | ||
| Positive | 43 | 61.40 |
| Negative | 27 | 38.60 |
| Perineural invasion | ||
| Positive | 12 | 17.10 |
| Negative | 58 | 82.90 |
LVI, lymphovascular invasion
Immunohistochemical results
Cytoplasmic IMP3 expression was detected within the tumor cells. In contrast, adjacent histologically normal colonic tissue lacked IMP3 expression (Figure 2A).
Figure 2. (A) Strong cytoplasmic immunostaining of IMP3 in neoplastic cells and its absence in apparently normal colonic mucosa adjacent to tumor tissue, X100.(B) Negative IMP3 expression in well-differentiated colorectal adenocarcinoma, X400. (C) Positive moderate cytoplasmic IMP3 expression in moderately- differentiated colorectal adenocarcinoma, X200.(D) Positive strong cytoplasmic IMP3 expression in poorly-differentiated colorectal adenocarcinoma, X200 .
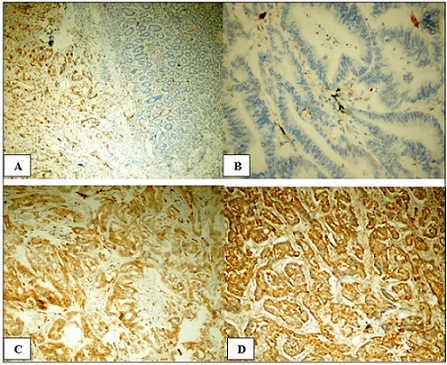
Positive IMP3 expression was found in 48 (68.6%) of the neoplastic cells; 23 (32.9%) cases revealed strong expression. While moderate and weak scores were detected in 14 (20%) and 11 (15.7%) of the cases, respectively. Negative expression was detected in 22 (31.4%) of the cases.
The analyzed clinicopathological characteristics of the studied cases were compared with the statistical data of IMP3 immunostaining. IMP3 expression was significantly correlated with the degree of tumor differentiation (p= 0.003). IMP3 expression was more frequent in high-grade tumors (Figure 2C & 2D); about 82% of cases that revealed negative IMP3 expression were well-differentiated tumors (Figure 2B). Also, we found a highly statistically significant correlation between IMP3 expression and the T category of the applied TNM staging system (p<0.001). IMP3 reactivity was detected more frequently in advanced tumor stages (T3 & T4a) compared to low tumor stages (T2). Additionally, a statistically significant correlation was observed between IMP3 expression and both lymph node status (p=0.001) and LVI (p=0.006). On the other hand, statistical analysis of IMP3 expression in correlation to patient age, sex, mucoid differentiation, and perineural invasion did not reveal any statistically significant differences. The statistical relationship between IMP3 expression and clinicopathological data is summarized in Table 2.
| Clinicopathological parameters | IMP3 expression | P value | |||
| Negative (N=22) | Positive (N=48) | ||||
| Weak | Moderate | Strong | |||
| (N=11) | (N=14) | (N=23) | |||
| Age (years) | |||||
| ≤50 | 10 (45.5%) | 5 (45.5%) | 4 (28.6%) | 8 (34.8%) | 0.708 |
| >50 | 12 (54.5%) | 6 (54.5%) | 10 (71.4%) | 15 (65.2%) | |
| Sex | |||||
| Male | 13 (59.1%) | 8 (72.7%) | 8 (57.1%) | 13 (56.5%) | 0.822 |
| Female | 9 (40.9%) | 3 (27.3%) | 6 (42.9%) | 10 (43.5%) | |
| Mucoid differentiation | |||||
| Present | 1 (4.5%) | 1 (9%) | 2 (14.3%) | 2 (8.7%) | 0.791 |
| Absent | 21 (95.5%) | 10 (91%) | 12 (85.7%) | 21 (91.3%) | |
| Degree of differentiation | |||||
| Well-differentiated | 18 (81.8%) | 5 (45.5%) | 7 (50%) | 5 (21.7%) | 0.003* |
| Moderately-differentiate | 2 (9.1%) | 5 (45.5%) | 7 (50%) | 13 (56.5%) | |
| Poorly-differentiated | 2 (9.1%) | 1 (9%) | 0 | 5 (21.7%) | |
| T stage | |||||
| T2 | 17 (77.3%) | 2 (18.2%) | 1 (7.1%) | 6 (26.1%) | <0.001** |
| T3 | 5 (22.7%) | 7 (63.6%) | 8 (57.1%) | 16 (69.6%) | |
| T4a | 0 | 2 (18.2%) | 5 (35.7%) | 1 (4.3%) | |
| N stage | |||||
| N0 | 15 (68.2%) | 0 | 2 (14.3%) | 11 (47.8%) | 0.001* |
| N1 | 4 (18.2%) | 9 (81.8%) | 6 (42.9%) | 9 (39.1%) | |
| N2 | 3 (13.6%) | 2(18.2%) | 6 (42.9%) | 3 (13.1%) | |
| LVI | |||||
| Positive | 7 (31.8%) | 9 (81.8%) | 11 (78.6%) | 16 (69.6%) | 0.006* |
| Negative | 15 (68.2%) | 2 (18.2%) | 3 (21.4%) | 7 (30.4%) | |
| Perineural invasion | |||||
| Positive | 2 (9.1%) | 2 (18.2%) | 4 (28.6%) | 4 (17.4%) | 0.512 |
| Negative | 20 (90.9%) | 9 (81.8%) | 10 (71.4%) | 19(82.6) |
LVI, lymphovascular invasion; p value was calculated by Pearson chi-square test or Fisher's exact when indicated, p<0.05 is considered significant.
Discussion
The worldwide prevalence of CRC is rising, making it a serious public health issue. According to statistics, it is the third most common cancer worldwide. CRC is associated with high morbidity and mortality rates due to the high incidence of metastatic spread. Recent research has been actively focused on developing therapeutic drugs that particularly limit CRC progression and dissemination [18, 19].
IMP3 expression was investigated in various tumors. It was reported that IMP3 enhances the proliferation, migration, and invasiveness of cancer cells. Previous studies reported a link between IMP3 expression in tumor cells and the aggressive behavior of various neoplasms, so it may serve as a novel prognostic biomarker and therapeutic target for these tumors [20, 21]. Few previous studies highlighted the prognostic value of IMP3 expression in CRC, reporting that IMP3 plays a key role in the development and progression of CRC and can serve as a valuable prognostic biomarker to identify patients with a risk of developing metastasis or recurrence [22].
In our study, we investigated the expression of IMP3 in seventy specimens of colorectal adenocarcinoma using IHC. The relationship between its expression and various clinicopathological data from the studied cases was evaluated, and the results were analyzed statistically. According to our findings, 48 (68.6%) of the studied cases revealed positive IMP3 expression, while 22 (31.4%) cases showed negative expression. Similarly, studies by Huang et al., Burdelski et al., and Mohamed et al. found positive IMP3 expression in 72.3%, 63.4%, and 68.8% of cases, respectively [15, 17, 23]. However, Lochhead et al. reported a lower percentage of positivity, where only 35% of CRC cases exhibited IMP3 positive expression [24]. The use of different primary antibodies may be the cause of this variation in the percentage of expression.
IMP3 immunoreactivity was detected mainly in the cytoplasm of tumor cells. In contrast, adjacent histologically normal colonic tissue lacked IMP3 expression. This is in concern with Nechifor et al. and Wei et al., who found negative IMP3 expression in normal colonic mucosa and diffuse positive IMP3 expression in adenocarcinoma [25, 26].
We correlated our findings with the clinicopathological characteristics of the studied cases. There was a statistically significant correlation between IMP3 expression and the degree of differentiation. This finding is consistent with the findings of Mohamed et al. [23], Lochhead et al. [24], and Wei et al. [27], who reported increased IMP3 expression in poorly differentiated tumors compared to well-differentiated tumors. Meanwhile, Huang et al., Zhang et al., and Lin et al. reported an insignificant correlation between IMP3 expression and histological grade [15, 28, 29]. Also, we found that IMP3 expression was significantly correlated with advanced T stage and N stage. These findings are in agreement with studies by Wei et al. [27], Zhang et al. [28], Lin et al. [29], and Yuan et al. [30]. However, Akyol et al. concluded that there was no statistically significant correlation between IMP3 expression and tumor stage or LNM and attributed their results to the small number of included cases [31]. The association between IMP3 expression and poorly differentiated and advanced stages of CRC may have a promising role in developing novel anti-cancer therapeutic agents that target IMP3.
Furthermore, a significant correlation was detected between IMP3 expression and LVI. This indicates that IMP3-positive cells may play an important role in developing metastatic emboli. This finding is in agreement with previous studies by Huang et al. and Bevanda Glibo et al. that reported the involvement of IMP3 in tumor progression and metastatic dissemination [15, 22].
No statistically significant correlation was observed between IMP3 positivity or staining intensity and other clinicopathological parameters such as patient age, gender, mucoid differentiation, and neural invasion. These recorded findings were similar to those of both Huang et al. and Wei et al. [15, 27].
In conclusion, Up-regulated IMP3 expression is strongly linked to unfavorable prognosis in colorectal adenocarcinoma, suggesting that the progression and invasion of CRC may be influenced by this protein. It may be helpful to target this molecule in future therapeutic approaches to reduce CRC invasion and metastasis.
List of abbreviations
CRC: Colorectal carcinoma, IHC: Immunohistochemistry/ immunohistochemical, IMP3:Insulin-like growth factor II m-RNA-binding protein 3, LNM: Lymph node metastasis, LVI: Lymphovascular invasion, PBS: Phosphate-buffered saline, DAB:Diaminobenzidine.
Limitations of the study
The sample size was not large enough, which in turn affected the statistical power of the results.
Recommendations
Further large-scale prospective studies on IMP3 expression in colorectal adenoma and carcinoma to clarify its diagnostic role in distinguishing carcinoma from adenoma with high-grade dysplasia in challenging cases, especially in endoscopic biopsies, are recommended. In addition, follow-up of patients is important to emphasize the correlation between IMP3 expression and patient survival and disease outcome.
Acknowledgments
Not applicable.
Statements and Declarations Ethical approval
The study was performed according to the guidelines approved by the Ethical Committee of Sohag Faculty of Medicine.
Consent to participate
Written informed consent was obtained from all participants included in the study.
Consent for publication
Not applicable.
Availability of data and materials
The required data and materials were all available.
Competing interests
The authors declare that they have no competing interests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors.
Authors Contribution
Noha ED Hassab El-Naby and Nagwa Abd EL- Sadek Ahmed designed the study, analyzed the data, and drafted the manuscript. Maisa Hashem Mohammed and Nagwa Abd EL-Sadek Ahmed were responsible for histopathological assessment and figure analysis. Amira Maher and Abdelrahman Mohammad Galal assisted with the design of the study and analysis of the data, collected the clinical and demographic data, and edited the manuscript.
References
- Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL , Soerjomataram I , Jemal A. CA Cancer J Clin.2024 May-Jun;74(3):229-263. CrossRef
- Cancer statistics, 2022 Siegel RL , Miller KD , Fuchs HE , Jemal A. CA: a cancer journal for clinicians.2022;72(1). CrossRef
- Mapping Cancer in Africa: A Comprehensive and Comparable Characterization of 34 Cancer Types Using Estimates From GLOBOCAN 2020 Sharma R, Aashima N, Nanda M, Fronterre C, Sewagudde P, Ssentongo AE , Yenney K, et al . Frontiers in Public Health.2022;10. CrossRef
- General insight into cancer: An overview of colorectal cancer (Review) Alzahrani SM , Al Doghaither HA , Al-Ghafari AB . Molecular and Clinical Oncology.2021;15(6). CrossRef
- The 2019 WHO classification of tumours of the digestive system Nagtegaal ID , Odze RD , Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM , et al . Histopathology.2020;76(2). CrossRef
- Hepatic metastasis from colorectal cancer Kow AWC . Journal of Gastrointestinal Oncology.2019;10(6). CrossRef
- Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements Malki A, ElRuz RA , Gupta I, Allouch A, Vranic S, Al Moustafa A. International Journal of Molecular Sciences.2020;22(1). CrossRef
- IMPs: an RNA-binding protein family that provides a link between stem cell maintenance in normal development and cancer Degrauwe N, Suvà M, Janiszewska M, Riggi N, Stamenkovic I. Genes & Development.2016;30(22). CrossRef
- Oncofetal protein IMP3, a new cancer biomarker Gong Y, Woda BA , Jiang Z. Advances in Anatomic Pathology.2014;21(3). CrossRef
- Expression of IMP3 and IGF2 in giant cell tumor of spine is associated with tumor recurrence and angiogenesis Zhang K., Zhou M., Chen H., Wu G., Chen K., Yang H.. Clinical & Translational Oncology: Official Publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico.2015;17(7). CrossRef
- IMP3 Predicts Invasion and Prognosis in Human Lung Adenocarcinoma Yan J, Wei Q, Jian W, Qiu B, Wen J, Liu J, Fu B, Zhou X, Zhao T. Lung.2016;194(1). CrossRef
- IMP3 Protein Overexpression Is Linked to Unfavorable Outcome in Laryngeal Squamous Cell Carcinoma Maržić D, Marijić B, Braut T, Janik S, Avirović M, Hadžisejdić I, Tudor F, Radobuljac K, Čoklo M, Erovic BM . Cancers.2021;13(17). CrossRef
- Prognostic value of IMP3 immunohistochemical expression in triple negative breast cancer Sjekloča N, Tomić S, Mrklić I, Vukmirović F, Vučković L, Lovasić IB , Maras-Šimunić M. Medicine.2020;99(7). CrossRef
- Immunohistochemical analysis of IMP3 and p53 expression in endoscopic ultrasound-guided fine needle aspiration and resected specimens of pancreatic diseases Senoo J, Mikata R, Kishimoto T, Hayashi M, Kusakabe Y, Yasui S, Yamato M, et al . Pancreatology: official journal of the International Association of Pancreatology (IAP) ... [et al.].2018;18(2). CrossRef
- Analysis of IMP3 expression in primary tumor and stromal cells in patients with colorectal cancer Huang X, Wei Q, Liu J, Niu H, Xiao G, Liu L. Oncology Letters.2017;14(6). CrossRef
- AJCC 8th Edition: Colorectal Cancer Weiser MR . Annals of Surgical Oncology.2018;25(6). CrossRef
- IMP3 overexpression occurs in various important cancer types and is linked to aggressive tumor features: A tissue microarray study on 8,877 human cancers and normal tissues Burdelski C, Jakani-Karimi N, Jacobsen F, Möller-Koop C, Minner S, Simon R, Sauter G, et al . Oncology Reports.2018;39(1). CrossRef
- Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors Rawla P, Sunkara T, Barsouk A. Przeglad Gastroenterologiczny.2019;14(2). CrossRef
- Global colorectal cancer burden in 2020 and projections to 2040 Xi Y, Xu P. Translational Oncology.2021;14(10). CrossRef
- Prognostic value of high IMP3 expression in solid tumors: a meta-analysis Chen L, Xie Y, Li X, Gu L, Gao Y, Tang L, Chen J, Zhang X. OncoTargets and Therapy.2017;10. CrossRef
- The Oncogenic Functions of Insulin-like Growth Factor 2 mRNA-Binding Protein 3 in Human Carcinomas Wang P, Wang X, Liu M, Zeng Z, Lin C, Xu W, Ma W, et al . Current Pharmaceutical Design.2020;26(32). CrossRef
- IMP3 protein is an independent prognostic factor of clinical stage II rectal cancer Bevanda Glibo D, Bevanda D, Vukojević K, Tomić S. Scientific Reports.2021;11(1). CrossRef
- Prognostic value of IMP3 and cyclin D1 expression in patients with colorectal cancer Mohamed SY , Hafez A, Elwan A, Abdelhamid MI , Ahmed NH , Abdelmaksoud BA , et al . Middle East J Cancer.2022;13(1):57-66. CrossRef
- Insulin-like growth factor 2 messenger RNA binding protein 3 (IGF2BP3) is a marker of unfavourable prognosis in colorectal cancer Lochhead P, Imamura Y, Morikawa T, Kuchiba A, Yamauchi M, Liao X, Qian ZR , et al . European Journal of Cancer (Oxford, England: 1990).2012;48(18). CrossRef
- Expression of insulin-like growth factor-II mRNA-binding protein 3 (IMP3) in various normal and neoplastic human tissues Nechifor-Boila A, Nechifor-Boila A, Loghin A, Catana R, Borda A. Revista Romana de Medicina de Laborator.2012;20:163-172.
- IMP3 expression in biopsy specimens as a diagnostic biomarker for colorectal cancer Wei Q, Zhou H, Zhong L, Shi L, Liu J, Yang Q, Zhao T. Human Pathology.2017;64. CrossRef
- IMP3 expression in biopsy specimens of colorectal cancer predicts lymph node metastasis and TNM stage Wei Q, Huang X, Fu B, Liu J, Zhong L, Yang Q, Zhao T. Int J Clin Exp Pathol.2015;8(9):11024-11032. CrossRef
- RNA-binding protein IMP3 is a novel regulator of MEK1/ERK signaling pathway in the progression of colorectal Cancer through the stabilization of MEKK1 mRNA Zhang M, Zhao S, Tan C, Gu Y, He X, Du X, Li D, Wei P. Journal of experimental & clinical cancer research: CR.2021;40(1). CrossRef
- Insulin-like growth factor-II mRNA-binding protein 3 predicts a poor prognosis for colorectal adenocarcinoma Lin L, Zhang J, Wang Y, Ju W, Ma Y, Li L, Chen L. Oncology Letters.2013;6(3). CrossRef
- Diffuse expression of RNA-binding protein IMP3 predicts high-stage lymph node metastasis and poor prognosis in colorectal adenocarcinoma Yuan R, Wang C, Chou C, Chang K, Lee P, Jeng Y. Annals of Surgical Oncology.2009;16(6). CrossRef
- Diagnostic value of IMP3 expression in colorectal carcinoma and adenoma Akyol H, Özercan IH . Eastern Journal Of Medicine.2022;27(3). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2025
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times