Factorial Validity and Reliability of the Chemotherapy Dietary Adherence Scale: A Tool for Nutritional Assessment in Cancer Care
Download
Abstract
Background: Nutritional adherence is vital in improving clinical outcomes for chemotherapy patients. The lack of validated tools to assess dietary adherence during chemotherapy prompted the development and psychometric validation of the Chemotherapy Dietary Adherence Scale (CDAS).
Objective: To develop, refine, and validate the CDAS, assessing its reliability and factorial structure through exploratory and confirmatory factor analyses.
Methods: This two-phase, cross-sectional study was conducted in the Department of Medical Oncology at a tertiary care cancer centre. Data were collected from 245 chemotherapy patients over a period of two months. Phase 1 involved exploratory factor analysis (EFA) on an initial scale, followed by refinement to address weak inter-item correlations. Phase 2 validated the revised scale using confirmatory factor analysis (CFA). Reliability was assessed using Cronbach’s alpha, and statistical analysis was conducted using SPSS version 26 and Python’s semopy library.
Results: The study population had a mean age of 58.13 years (SD, 9.85) and consisted predominantly of skilled workers (67.1%). The 7-item CDAS demonstrated excellent internal consistency (Cronbach’s alpha = 0.83), with subscales showing strong reliability: Nutritional Adherence (α = 0.79) and Dietary Adjustment (α = 0.81). EFA revealed a two-factor structure explaining 70.5% of the total variance. CFA confirmed this structure with exceptional model fit (χ² = 12.09, df = 13, p = 0.52; CFI = 1.00; GFI = 0.98; TLI = 1.00; RMSEA = 0.00). All items showed significant factor loadings (p < 0.001), with values ranging from 1.058 to 1.262. The moderate correlation between factors (r = 0.168, p < 0.001) indicated that they represented distinct but related dimensions of dietary adherence.
Conclusions: The CDAS demonstrated robust psychometric properties, with a clear two-factor structure that captures distinct aspects of dietary adherence among chemotherapy patients. The validated scale provides a reliable and valid tool for assessing nutritional adherence and dietary adaptations during chemotherapy, potentially enhancing clinical assessment and targeted nutritional interventions.
Introduction
Proper nutrition is crucial for chemotherapy patients, as it plays a significant role in managing treatment side effects, maintaining energy levels, and supporting immune function. Chemotherapy and radiation therapy can lead to fluctuations in appetite and body weight, making dietary management essential during treatment [1].
Malnutrition is a common issue among cancer patients, with prevalence rates varying based on cancer type, stage, and treatment modalities. Studies have shown that malnutrition can affect up to 60% of patients with advanced cancer. This high prevalence underscores the need for effective nutritional interventions to improve patient outcomes [2].
Despite the recognised importance of nutrition in cancer care, there is a lack of validated tools to assess dietary adherence during chemotherapy. Existing studies on oral nutritional supplement (ONS) adherence vary in their definitions and assessment tools, creating inconsistencies [3]. This gap underscores the need for reliable instruments to assess patients’ adherence to dietary recommendations, which is essential for effectively tailoring nutritional interventions.
Developing such assessment tools is vital, as poor nutritional status can negatively impact patients’ quality of life and treatment outcomes. Malnutrition, often resulting from the disease and its treatment, can lead to decreased immunity, increased treatment toxicity, and reduced survival rates [4]. Therefore, assessing and ensuring dietary adherence is critical to comprehensive cancer care. In response to this need, the Chemotherapy Dietary Adherence Scale (CDAS) was developed to provide a standardised method for evaluating dietary adherence in chemotherapy patients. The CDAS aims to capture various aspects of dietary behaviour, including nutrient intake, meal regularity, and adjustments made in response to treatment-related side effects. By validating this scale, healthcare providers can better identify patients at risk of poor nutritional adherence and implement targeted interventions to improve clinical outcomes.
This study focuses on the psychometric validation of the CDAS, including reliability analysis and factor analysis, to ensure its effectiveness as a tool for assessing dietary adherence in patients undergoing chemotherapy. The validation process involves evaluating the scale’s internal consistency, construct validity, and factorial structure, thereby providing a robust foundation for its application in clinical practice and research settings.
By establishing a reliable and valid measure of dietary adherence, the CDAS has the potential to enhance nutritional care in oncology, ultimately contributing to improved patient outcomes and quality of life during chemotherapy.
Materials and Methods
This was a two-phase, cross-sectional study conducted in the Department of Medical Oncology over a two- month period. It aimed to develop, refine, and validate a dietary adherence scale for chemotherapy patients using confirmatory factor analysis.
The study lasted two months and was conducted in the Department of Medical Oncology at a tertiary care cancer centre. Data collection was categorised into two phases to ensure the refinement and validation of the chemotherapy dietary adherence scale.
Study Population and Sample Size
A total of 245 adult patients undergoing chemotherapy and receiving individualised dietary counselling were recruited from the oncology daycare unit. Participants were eligible if they were 18 years or older, currently undergoing chemotherapy, and capable of understanding and completing the questionnaire. Data collection was conducted during routine hospital visits, following informed consent.
Data Collection
Data were collected from eligible chemotherapy patients during their visits to the daycare oncology department. Patients were provided with the dietary adherence scale, which included questions assessing adherence to nutritional recommendations, including the intake of fruits, vegetables, whole grains, and proteins, as well as dietary adjustments made to manage treatment- related side effects. All patients received individualised diet plans developed by a qualified dietician, which formed the basis for assessing dietary adherence.
Scale Development and Validation
1. Face Validation
The Chemotherapy Dietary Adherence Scale (CDAS) underwent a rigorous face validation process involving 25 subject experts from oncology, dietetics, and community medicine. The validation criteria encompassed grammar, clarity, item construction, and alignment of items with the tool’s purpose. Experts rated each item on a 4-point scale (1 = not relevant/clear, 4 = highly relevant/clear). Based on their feedback, items were refined to ensure clarity and appropriateness. Face validation showed 85% agreement among experts regarding grammar, clarity, and content. Content validity was assessed by calculating the Content Validity Index (CVI) at the item (I-CVI = 0.81) and scale (S-CVI = 0.83) levels, indicating robust content validity. Items with I-CVI below 0.8 were revised or removed [5].
2. Statistical Validation
a. Exploratory Analysis
The EFA was performed using SPSS version 26. The Kaiser-Meyer-Olkin (KMO) value was 0.849, indicating adequate sampling for factor analysis. Bartlett’s Test of Sphericity was significant (χ² = 731.494, df = 21, p < 0.001), supporting the appropriateness of conducting EFA. Principal Component Analysis with Varimax rotation revealed two components with eigenvalues greater than 1, explaining 70.5% of the total variance. Items with low communalities or poor loadings were removed or refined, resulting in a 7-item scale.
b. Confirmatory Validation
The revised 7-item scale was validated using CFA in Python with the semopy library. Model fit indices and standardised factor loadings were evaluated. The final model demonstrated a good fit, with all loadings exceeding 0.60 and acceptable RMSEA, CFI, and TLI values, supporting the scale’s construct validity.
Psychometric Validation Framework
The psychometric validation process adhered to the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) guidelines for evaluating the quality of measurement properties of health-related patient-reported outcome measures [6]. This framework ensured the systematic evaluation of reliability, validity, and measurement error properties, which are essential for establishing the CDAS as a robust clinical assessment tool.
Statistical Analysis
The collected data were systematically entered into Microsoft Excel 2019 for initial organisation and data cleaning. The cleaned dataset was then exported and analysed using SPSS version 26, licensed to the Institution, for descriptive statistics and exploratory factor analysis (EFA) in the study’s first phase.
Confirmatory Factor Analysis (CFA) was conducted using Python Jupyter Notebook to validate the factorial structure of the Chemotherapy Dietary Adherence Scale. The CFA was performed with the semopy library to evaluate factor loadings, error variances, and fit indices. These metrics were used to assess the overall model fit and ensure the validity and reliability of the revised scale. All statistical procedures were conducted to ensure accuracy, and results were cross-checked to minimise potential errors in analysis. P-value <0.05 has been considered statistically significant.
Ethical Considerations
The Institutional Ethics Committee of [IEC: JSSMC/ PG/113/2023-24] approved the study. Before data collection, all participants provided written informed consent. Confidentiality and anonymity of patient data were strictly maintained throughout the study.
Results
According to Table 1, the study population had a mean age of 58.13 years (SD = 9.85).
| Variable | N | % |
| Age in years (Mean, SD) | 58.13 (9.85) | |
| Occupation Status | ||
| Retired | 29 | 11.80 |
| Skilled | 164 | 67.10 |
| Unemployed | 29 | 11.80 |
| Unskilled | 23 | 9.40 |
| Monthly Family Income (INR) | ||
| 1365 – 2728 | 3 | 1.20 |
| 2729 – 4550 | 20 | 8.20 |
| 4549 – 9097 | 66 | 27.10 |
| 9098 and above | 156 | 63.50 |
| Living Area | ||
| Rural | 98 | 40.00 |
| Urban | 147 | 60.00 |
| Marital Status | ||
| Divorced | 6 | 2.40 |
| Married | 216 | 88.20 |
| Unmarried | 6 | 2.40 |
| Widowed | 17 | 7.00 |
| Education Status | ||
| Diploma/Degree | 115 | 47.10 |
| Illiterate | 3 | 1.20 |
| Pre-University | 52 | 21.20 |
| Primary Education | 14 | 5.90 |
| Secondary Education | 61 | 24.70 |
| Type of Family | ||
| Joint Family | 130 | 52.90 |
| Nuclear Family | 106 | 43.50 |
| Three Generation | 9 | 3.50 |
A majority of participants were employed in skilled occupations (67.1%), while smaller proportions were retired (11.8%), unemployed (11.8%), or engaged in unskilled labour (9.4%). Most participants belonged to families with a monthly income of 9098 INR and above (63.5%), indicating a relatively higher socioeconomic status. Urban residents comprised 60%, and rural residents 40%.
Regarding marital status, 88.2% were married, with smaller proportions being divorced (2.4%), unmarried (2.4%), or widowed (7.1%). Concerning education, 47.1% held a diploma or degree, 24.7% had completed secondary education, and 1.2% were illiterate. Most participants lived in joint families (52.9%), followed by nuclear families (43.5%), with 3.5% residing in three- generation households.
The study population’s clinical profile revealed that most tumours were localised metastases (56.5%), followed by regional tumours (15.3%). 7.1% had metastases, and 21.2% had an unknown tumour status. Most participants were diagnosed with stage 2 cancer (82.4%), with fewer cases in stages 1 (9.4%) and 3 (8.2%).
In terms of diagnoses, the most common cancers were breast cancer (20.0%), ovarian cancer (12.9%), and colon cancer (9.4%), while less frequently observed were lung cancer (5.9%), kidney cancer (4.7%), and stomach cancer (4.7%). Other cancers, including oesophageal cancer (5.9%), rectal cancer (3.5%), and rare conditions like peripheral nerve sheath tumour (1.2%), were also noted. Regarding treatment modalities, most patients underwent chemotherapy (74.1%), 22.4% received surgery, and only a small proportion received radiation therapy (3.5%).
Scale Reliability and Internal Consistency
The psychometric evaluation of the Chemotherapy Dietary Adherence Scale (CDAS) demonstrated robust internal consistency reliability. The overall scale yielded a Cronbach’s alpha coefficient of 0.83, indicating strong reliability that exceeds the conventional threshold of 0.70 for established instruments. Subscale analysis revealed that the Nutritional Adherence dimension (comprising items Q1-Q3) achieved a Cronbach’s alpha of 0.79, while the Dietary Adjustment dimension (comprising items Q4-Q7) demonstrated a Cronbach’s alpha of 0.81. These findings suggest that both dimensions of the CDAS possess satisfactory internal consistency for independent assessment of distinct aspects of dietary adherence behaviour among patients undergoing chemotherapy.
Exploratory Factor Analysis
Before conducting exploratory factor analysis (EFA), we assessed sampling adequacy using the Kaiser-Meyer- Olkin (KMO) measure, which yielded a value of 0.849, substantially exceeding the recommended threshold of 0.60. Additionally, Bartlett’s Test of Sphericity was statistically significant (χ² = 731.494, df = 21, p < 0.001), confirming the appropriateness of proceeding with factor analysis.
The EFA used Principal Component Analysis with Varimax rotation to enhance interpretability. The analysis extracted two principal components with eigenvalues greater than 1.0, collectively explaining 70.5% of the total variance. The first component accounted for 53.6% of the variance, while the second component explained an additional 16.9%. A third component with an eigenvalue below the conventional cutoff accounted for 7.8% of the variance and was not retained in the final solution.
Table 1 presents the communality values and component loadings for each item. The communality values ranged from 0.691 to 0.911, indicating that the extracted components explained a substantial proportion of variance for each item. The high communality values (all > 0.60) indicate that the items were well-represented by the extracted components, supporting the structural validity of the scale (Table 2).
| Item | Communalities (Extraction) | Component Matrix | ||
| Component 1 | Component 2 | Component 3 * | ||
| I prioritised including various fruits and vegetables in my meals this week. (Q1) | 0.773 | 0.713 | 0.472 | 0.201 |
| I choose whole grains (brown rice, roti, etc.) over refined grains whenever possible. (Q2) | 0.775 | 0.743 | 0.472 | 0.016 |
| I included a good source of protein in most of my meals this week. (Q3) | 0.788 | 0.705 | 0.49 | -0.225 |
| I drank plenty of water throughout the day this week. (Q4) | 0.911 | 0.695 | -0.342 | 0.558 |
| When I experienced side effects like nausea or mouth sores, I made adjustments to my diet. (Q5) | 0.691 | 0.78 | -0.287 | -0.016 |
| I consumed adequate portions of nutrient-dense foods despite poor appetite or side effects. (Q6) | 0.775 | 0.72 | -0.384 | -0.332 |
| How closely did you follow dietary recommendations from a doctor/ dietician? (Q7) | 0.767 | 0.767 | -0.385 | -0.172 |
| Total Variance Explained | 53.60% | 16.90% | 7.80% | |
| Cumulative Variance Explained | 53.60% | 70.50% | 78.30% |
*Component 3 was not retained in the final solution as its eigenvalue was <1.0. Extraction
Examination of the component matrix revealed a clear pattern of loadings, with items Q1-Q3 demonstrating strong positive loadings on Component 2 (with secondary loadings on Component 1), while items Q4-Q7 showed strong negative loadings on Component 2 and positive loadings on Component 1. This loading pattern suggested a two-factor solution that was subsequently confirmed through confirmatory factor analysis. Based on the item content, the factors were labelled as “Nutritional Adherence” (Q1-Q3) and “Dietary Adjustment” (Q4-Q7), reflecting the conceptual dimensions of dietary behaviour during chemotherapy.
Inter-Item Correlation Analysis
Inter-item correlation analysis was performed to evaluate the relationships between individual scale items and assess the dimensionality of the CDAS. The correlation matrix, presented in Figure 1, revealed moderate to strong positive correlations among items within the same factor, providing additional evidence supporting the scale’s two-factor structure.
Figure 1. Inter-Item Correlation Matrix of the Chemotherapy Dietary Adherence. Note, Correlations within the same factor are highlighted in bold text.
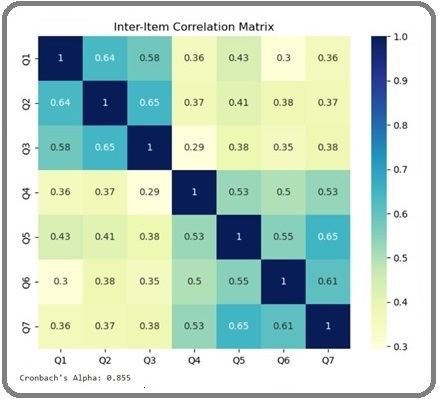
Items comprising the Nutritional Adherence factor (Q1-Q3) demonstrated stronger intercorrelations (r = 0.55 to 0.61) compared to their correlations with items from the Dietary Adjustment factor (r = 0.25 to 0.43). Similarly, items within the Dietary Adjustment factor (Q4-Q7) showed stronger associations with each other (r = 0.48 to 0.59) than with items from the Nutritional Adherence factor (r = 0.25 to 0.43). This pattern of correlations provides evidence for convergent and discriminant validity at the item level, supporting the conceptual distinction between the two dimensions of dietary adherence.
The inter-item correlation analysis also revealed the absence of multicollinearity (all r < 0.90), indicating that each item contributes unique information to the scale. Additionally, all correlations were statistically significant (p < 0.001), supporting the relevance of each item to the overall construct of dietary adherence during chemotherapy.
Confirmatory Factor Analysis
To validate the two-factor structure identified through EFA, we conducted a rigorous Confirmatory Factor Analysis (CFA) using Python with the semopy library. The CFA model specified two latent factors: Nutritional Adherence (comprising items Q1-Q3) and Dietary Adjustment (comprising items Q4-Q7), with correlated factors to account for their conceptual relationship.
The CFA results supported the hypothesised two-factor model, with excellent fit indices across multiple criteria. The chi-square test was non-significant (χ² = 12.09, df = 13, p = 0.52), indicating that the model-implied covariance matrix did not significantly differ from the observed covariance matrix. Comparative fit measures demonstrated exceptional model fit: Comparative Fit Index (CFI) = 1.00, Goodness of Fit Index (GFI) = 0.98, Adjusted Goodness of Fit Index (AGFI) = 0.97, Normed Fit Index (NFI) = 0.98, and Tucker-Lewis Index (TLI) = 1.00, all exceeding the recommended threshold of 0.95 for excellent fit. The Root Mean Square Error of Approximation (RMSEA) = 0.00, which is substantially below the conventional cutoff of 0.06, further confirms the model’s superior fit.
The substantial difference between the baseline model chi-square (744.15) and the specified model chi-square (12.09) provided additional evidence for the superiority of the two-factor structure over a null model. The parsimony-adjusted measures, Akaike Information Criterion (AIC = 29.90) and Bayesian Information Criterion (BIC = 82.42), demonstrated acceptably low values, supporting the model’s parsimony while maintaining explanatory power.
Factor Loadings and Parameter Estimates
The CFA yielded standardised parameter estimates that further validated the scale’s structure. Table 3 presents the factor loadings, standard errors, z-values, and significance levels for each item. All items demonstrated statistically significant loadings on their respective factors (p < 0.001), with unstandardised loadings ranging from 1.000 to 1.262.
| Item | Unstandardised Loading | Std. Error | z-value | p-value |
| Nutritional Adherence Factor | ||||
| Q1: I prioritised including various fruits and vegetables in my meals this week. | 1.000 * | - | - | - |
| Q2: I choose whole grains (brown rice, roti, etc.) over refined grains whenever possible. | 1.163 | 0.098 | 11.889 | <0.001 |
| Q3: I included a good source of protein in most of my meals this week. | 1.058 | 0.093 | 11.336 | <0.001 |
| Dietary Adjustment Factor | ||||
| Q4: I drank plenty of water throughout the day this week. | 1.000 * | - | - | - |
| Q5: When I experienced side effects like nausea or mouth sores, I made adjustments to my diet. | 1.147 | 0.112 | 10.262 | <0.001 |
| Q6: I consumed adequate portions of nutrient-dense foods despite poor appetite or side effects. | 1.138 | 0.118 | 9.62 | <0.001 |
| Q7: How closely did you follow dietary recommendations from a doctor/dietician? | 1.262 | 0.121 | 10.448 | <0.001 |
*Fixed parameter for model identification
The magnitude of these loadings, coupled with their high statistical significance, provides robust evidence for the construct validity of the CDAS.
Additional parameter estimates revealed significant error variances for all items (p < 0.001), ranging from 0.177 to 0.305, indicating that while the model accounts for a substantial proportion of item variance, there remains some unexplained variance for each item. The correlation between the Nutritional Adherence and Dietary Adjustment factors was 0.168 (SE = 0.028, z = 6.012, p < 0.001), indicating that while these dimensions are related, they represent distinct aspects of dietary adherence in chemotherapy patients. This moderate correlation provides evidence for discriminant validity between the two factors while acknowledging their conceptual relationship within the broader construct of dietary adherence during chemotherapy.
The variance estimates for the latent factors were also statistically significant (p < 0.001), with values of 0.301 for Nutritional Adherence and 0.249 for Dietary Adjustment. These values indicate that both factors explain meaningful variance in their respective indicators and contribute substantially to the measurement of dietary adherence. The path diagram illustrates the CDAS’s two-
factor structure, with standardised factor loadings and the correlation between factors (Figure 2).
Figure 2. Path Diagram of the Confirmatory Factor Analysis Model for the CDAS.
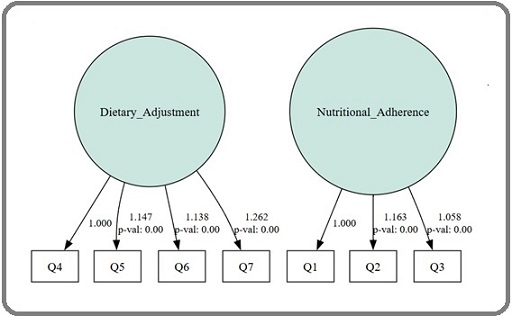
It visually represents the relationships between observed variables (Q1-Q7) and their respective latent factors (Nutritional Adherence and Dietary Adjustment), clearly depicting the scale’s structural organisation.
Discussion
The development and validation of the Chemotherapy Dietary Adherence Scale (CDAS) represents a significant advancement in assessing dietary adherence among chemotherapy patients. This study provides robust evidence for a psychometrically sound instrument that captures the multidimensional nature of dietary adherence in this vulnerable population. The psychometric validation process adhered to established guidelines for health measurement instruments, ensuring rigorous evaluation of the scale’s measurement properties.
Our study sample comprised adult chemotherapy patients with diverse cancer diagnoses, including breast (20.0%), ovarian (12.9%), and colon (9.4%) cancers, with a mean age of 58.13 years. This demographic profile aligns with Tang et al. (2024) in their validation of the Dietary Self-management Behaviour Questionnaire (DSMBQ), where participants had a mean age of 55.6 years and similar educational attainment [7].
Previous research has also emphasised the significant impact of nutritional status on quality of life among cancer patients undergoing chemotherapy, highlighting the critical need for validated assessment tools in this population [8]. The alignment of demographic characteristics between studies suggests that our findings may be generalizable to similar populations of chemotherapy patients in the region. The CDAS demonstrated excellent internal consistency reliability with an overall Cronbach’s alpha of 0.83, substantially exceeding the conventional threshold of 0.70 for established instruments. Both identified subscales, Nutritional Adherence (α = 0.79) and Dietary Adjustment (α = 0.81), showed strong reliability, supporting their use as independent measures. These findings are consistent with those of other validated instruments in cancer care, such as the EXPAD-ANEO scale for oral chemotherapy adherence, which also observed improvements in reliability metrics following expert-guided revisions [9]. The methodological approach employed in developing quality of life assessment tools for cancer patients in India provides a valuable parallel for validating patient-reported outcome measures in similar populations [8].
Our Exploratory Factor Analysis (EFA) identified a three-component structure explaining 62.71% of the variance, encompassing nutritional adherence, dietary adjustments during side effects, and adherence to professional dietary guidance. This multidimensional structure aligns with findings from other dietary adherence assessments.
Our factor analysis revealed a clear two-factor structure, explaining 70.5% of the total variance, which distinguished between Nutritional Adherence (compliance with general nutritional recommendations) and Dietary Adjustment (adaptations to treatment-related challenges). This multidimensional approach aligns with other validated dietary instruments, such as the Diabetes Diet Adherence Scale (D-DAS), which employed rigorous factor analysis to identify underlying dimensions of dietary adherence [10]. The confirmatory factor analysis demonstrated exceptional model fit across multiple indices (CFI = 1.00, GFI = 0.98, RMSEA = 0.00), providing
strong evidence for construct validity. While these indices surpass recommended thresholds for excellent fit, they should be interpreted with appropriate caution regarding potential overfitting and require replication in independent samples. The moderate correlation between factors (r = 0.168) indicates that while Nutritional Adherence and Dietary Adjustment are related, they represent distinct dimensions, warranting separate assessment. This finding has important clinical implications, as patients may adhere differently to general nutritional recommendations compared to making specific dietary adjustments during treatment-related side effects.
Furthermore, the excellent reliability and validity support the use of the CDAS as an outcome measure in intervention studies. By providing a standardised assessment of dietary adherence, the CDAS can facilitate evaluation of nutritional interventions and enable comparisons across studies and patient populations. Previous validation studies of nutritional assessment tools, such as the Visual/Verbal Analogue Scale of Food Ingesta (ingesta-VVAS), have demonstrated the importance of comprehensive validation to ensure effectiveness across different patient populations [11].
Additionally, developing and validating a Food Frequency Questionnaire (FFQ) for patients with cancer involved selecting dish items through contribution and variability analyses and validating the FFQ against average 3-day dietary records. This meticulous validation ensured the tool’s applicability in evaluating the nutritional status of cancer patients [12].
The validated CDAS addresses a critical gap in the nutritional assessment of patients undergoing chemotherapy. Unlike general dietary assessment tools such as the Automated Self-Administered 24-hour (ASA24®) Dietary Assessment Tool [13], the CDAS specifically captures behaviours relevant to the unique challenges faced by patients undergoing chemotherapy. This specificity enhances its clinical utility for identifying individuals at risk of poor dietary adherence and guiding targeted interventions.
The two-factor structure of the CDAS enables clinicians to differentiate between different aspects of dietary adherence. For instance, a patient may demonstrate good adherence to general nutritional recommendations (Factor 1) but struggle with adapting their diet during treatment-related side effects (Factor 2). This nuanced assessment can inform personalised nutritional counselling strategies that address specific challenges rather than employing a one-size-fits-all approach.
Furthermore, the excellent reliability and validity of the CDAS support its use as an outcome measure in intervention studies aimed at improving dietary adherence among patients undergoing chemotherapy. By providing a standardised assessment of dietary adherence, the CDAS can facilitate the evaluation of nutritional interventions and enable comparisons across different studies and patient populations [13].
Limitations and Future Directions
Despite these strengths, several limitations warrant consideration. This study employed a cross-sectional design, conducted at a single tertiary care centre, which limits the assessment of the scale’s temporal stability and generalizability across diverse healthcare settings. Test-retest reliability could not be assessed due to the dynamic nature of chemotherapy treatment and associated side effects, representing an important area for future investigation. While our sample size (n = 245) was adequate for psychometric analysis, validation across different cancer types and treatment protocols would strengthen the scale’s applicability.
The exceptional CFA fit indices, while supporting construct validity, warrant replication in independent samples to confirm the stability of the two-factor structure. Future research should focus on multi-centre validation studies and longitudinal assessment of the scale’s responsiveness to dietary interventions and clinical outcomes.
Additionally, research examining the relationships between CDAS scores and objective nutritional biomarkers would establish criterion validity and enhance the clinical utility of the test. Studies investigating the integration of CDAS into routine clinical practice would provide valuable insights into its real-world effectiveness in improving patient outcomes.
In conclusion, the Chemotherapy Dietary Adherence Scale (CDAS) exhibits excellent psychometric properties, with a clear two-factor structure that captures distinct aspects of dietary adherence among patients undergoing chemotherapy. The robust reliability and validity evidence from this study supports the use of the CDAS as a comprehensive assessment tool in both clinical practice and research settings. By enabling the targeted assessment of dietary adherence, the CDAS can inform personalised nutritional interventions that address the specific challenges faced by patients undergoing chemotherapy, potentially improving treatment outcomes and quality of life. Future research should validate the scale across diverse populations and investigate its predictive validity regarding clinical outcomes.
Acknowledgements
We sincerely thank all the experts who participated in the face validation process and provided valuable feedback to refine the Chemotherapy Dietary Adherence Scale (CDAS). We also thank the oncology patients who shared their insights during the cognitive interviews, thereby enhancing the relevance and clarity of the scale. Finally, we acknowledge the support of the Department of Medical Oncology and Dietetics in facilitating data collection and providing valuable resources throughout the study.
Source of Funding
Nil
Conflict of interest
None
References
- Cancer Diet: Foods to Add and Avoid During Cancer Treatment 2025. https://www.hopkinsmedicine.org/health/conditions-and-diseases/cancer/cancer-diet-foods-to-add-and-avoid-during-cancer-treatment (accessed January 24, 2025) .
- Nutrition support and clinical outcome in advanced cancer patients Laviano A, Di Lazzaro L, Koverech A. The Proceedings of the Nutrition Society.2018;77(4). CrossRef
- Definition and assessment of adherence to oral nutritional supplements in patients with neoplasms: a scoping review Liu B, Liu Z, Gui Q, Lin Y, Huang G, Lyu J, Weng N, Tang X. BMC cancer.2024;24(1). CrossRef
- 455P - Assessment of nutritional status and quality of life among cancer patients undergoing chemotherapy Prathima S., Murthy M., Maka V. V., Paibhavi P. R., Reddy H., Vungarala S.. Annals of Oncology.2019;30. CrossRef
- Enhancing Nutritional Care in Cancer: Development and Face Validation of the Chemotherapy Dietary Adherence Scale Prakash GH , D SK , Pk K, Arun V, Yadav D, M S. Asian Pacific Journal of Cancer Care.2024;9(3). CrossRef
- The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes Mokkink LB , Terwee CB , Patrick DL , Alonso J, Stratford PW , Knol DL , Bouter LM , Vet HCW . Journal of Clinical Epidemiology.2010;63(7). CrossRef
- Development and preliminary validation of the Dietary Self-management Behavior Questionnaire (DSMBQ) for breast cancer patients during chemotherapy: three rounds of survey Tang H, Zhang W, Shen H, Yan P, Li L, Liu W, Xu C, Zhao H, Shang L. BMC public health.2024;24(1). CrossRef
- Development and Validation of Quality of Life Tool among Chemotherapy Patients: A Pilot Trial Jain VK , Sharma A. JCDR.2022. CrossRef
- Validation of a scale to assess adherence to oral chemotherapy based on the experiences of patients and healthcare professionals (EXPAD-ANEO) Talens A, LÓpez-Pintor E, Guilabert M, Cantó-Sancho N, Aznar MT , Lumbreras B. Frontiers in Pharmacology.2023;14. CrossRef
- Reliability and Structure of Diabetes Diet Adherence Scale (D-DAS): A Follow-up Study among Type 2 Diabetes Patients of India Kushwaha S, Srivastava R, Bhadada SK , Sagar V, Khanna P. 2024:2024.05.25.24307586. CrossRef
- Validation Of The Visual/Verbal Analogue Scale Of Food Ingesta (Ingesta-Vvas) In Oncology Patients Undergoing Chemotherapy Wijnhoven H. A., Velden L., Broek C., Broekhuizen M., Bruynzeel P., Breen A., Oostendorp N., Heer K.. Clinical Nutrition ESPEN.2023;54. CrossRef
- Development and Validation of a Food Frequency Questionnaire for Evaluating the Nutritional Status of Patients with Cancer Lee SA, , Choi HK , Park SJ , Lee HJ . Nutrients.2023;15. CrossRef
- ASA24® Dietary Assessment Tool | EGRP/DCCPS/NCI/NIH n.d. https://epi.grants.cancer.gov/asa24/ (accessed January 24, 2025) .
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2025
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times