Serum IDO1 and Biochemical Markers Correlation in Iraqi Women with Breast Cancer
Download
Abstract
Background: Breast cancer (BC) is the leading cause of cancer-related mortality among women worldwide. The type of BC depends on the specific breast cells undergoing malignant transformation and can originate in different regions of the breast. Indoleamine 2,3-dioxygenase 1 (IDO1) is an enzyme that catabolizes tryptophan and is overexpressed in several metastatic tumors, suggesting its potential role in cancer progression. This study aimed to assess serum IDO1 levels across different stages and ages in Iraqi women with BC, evaluating its diagnostic and prognostic value. Additionally, the correlation between IDO1 and clinicopathological features, as well as lipid and liver enzyme profiles, was examined.
Materials and Methods: A case-control study was conducted on 90 newly diagnosed Iraqi women with BC and 90 age-matched healthy controls. Serum IDO1 concentrations were measured using enzyme-linked immunosorbent assay (ELISA). Lipid profiles and liver enzyme activities (ALT, AST, ALP) were also evaluated. Statistical analyses were performed using SPSS software.
Results: Serum IDO1 levels were significantly elevated in BC patients compared to healthy controls (19.25 ± 7.36 vs. 2.16 ± 0.34 pg/mL; p < 0.001), with levels increasing progressively across advanced disease stages. Patients also exhibited elevated ALT, AST, ALP, triglycerides, total cholesterol, LDL-C, and VLDL-C, along with reduced HDL-C compared to controls. Serum IDO1 showed significant positive correlations with age, ALT, AST, ALP, TG, TC, and HDL-C, but not with BMI, LDL-C, or VLDL-C. Diagnostic performance analysis revealed a cut-off value of 12.65 ng/mL for IDO1, with 80.0% sensitivity, 81.1% specificity, and an AUC of 0.867 (95% CI: 0.814–0.919; p < 0.001).
Conclusion: Elevated serum IDO1 is a significant predictor of BC outcomes and may reflect its role in disease pathogenesis. IDO1 demonstrates strong potential as a diagnostic and prognostic biomarker, as well as a promising target for immunotherapeutic strategies in breast cancer management.
Introduction
According to the World Health Organization, Breast cancer (BC) was the most complex cancer disease globally in and the most common cause of mortality among women. According to unequivocal scientific evidence, the implementation of an effective preventive operative program could save lives of millions women afflicted breast cancer [1]. BC stands is the most prevalent and diverse malignancy women worldwide, representing a significant health issue. Improved understanding of tumor pathophysiology and the development of innovative medicines are essential for progressing breast cancer treatment [2]. Despite the rise of breast cancer incidence with advancing age, around 7 to 10% of women diagnosed with breast cancer are under the age of 40. This subgroup of patients exhibit distinct risk factors, tumor biology, clinical prognosis, and specific psychosocial issues, including fertility preservation, family planning, and employment reintegration [3]. Nonetheless, age should not be the primary criterion determining treatment aggressive- ness other considerations, including as the biologic aggressiveness of the tumor, potential long-term toxicities, and the preferences of the patient, must also be taken into account [4]. Fertility preservation techniques must be addressed with the patient prior to initiating any cancer therapy. Despite the significant percentage of breast cancer patients under 40 years of age, limited clinical trials have specifically examined the disease characteristics and outcomes of this demographic, and the majority of medicines routinely administered to these younger women were evaluated in older patients. Furthermore, young women diagnosed with breast cancer face heightened risks of sexual and psychological distress, and necessary for the clinicians to address these issues concerns to effectively support patients during the long diagnostic and therapeutic process [5, 6]. Consequently, it is essential to adhere to diagnostic and therapeutic protocol specifically tailored young women. Specific procedures should be adhered to while treating pregnant patients diagnosed with breast cancer [7]. Indoleamine 2,3-dioxygenase (IDO) is an immunosuppressive enzyme that catabolizes L-tryptophan into kynurenines (Kyn) [8, 9]. A deficiency of tryptophan impairs the cytotoxicity of T lymphocytes (T cells) [10]. Tryptophan metabolism produces compound that induces T cell apoptosis in vitro [11]. IDO participate in suppression of T-cell immunological responses by promoting the development of regulatory T cells (T-reg) [12]. IDO is involved in pathway of tumor immune evasion mechanisms [13, 14]. IDO overexpression lead to immunosuppression and immunotolerance [14]. IDO-expressing dendritic cells are present in the tissues and draining lymph nodes of patients with breast cancer [15]. IDO originating from tumor, possess the ability to obstruct the antitumor immune responses and facilitate metastasis [16, 17]. Numerous researchers have detected IDO expression in breast, colorectal, ovarian, and endometrial cancers [18-21]. IDO inhibition represent a developing antitumor approach within cancer immunotherapy. Currently, multiple IDO inhibitors are undergoing clinical trials [22]. Numerous studies indicate that high IDO1 expression in tumor tissues typically correlates with a significantly worse prognosis in patients [2]. Consequently, obstructing or ablating the IDO1 immune-inhibitory pathway may represent a significant method for anticancer immunotherapy, with multiple IDO1 inhibitors currently undergoing clinical trials [22]. A multitude of research has demonstrated that indoleamine 2,3-dioxygenase 1 (IDO1) catalyzes the metabolism of tryptophan (Trp) within the tumor microenvironment, hence interfering the function of cytotoxic sputum cells and resulting. immunosuppression [23].
Materials and Methods
The study employed was a case–control design with 180 participants aged 35 to 65 years conducted between December 2023 and April 2024. The total participants were categorized into two groups: Ninety individuals newly diagnosed with breast cancer encompassing [all stages: stage I (n=23), stage II (n=36), stage III (n=24), and stage IV (n=7)]. Diagnosed were made by mammography and histopathological examination at the Hospital of Oncology and Hematology in Najaf Al-Ashraf, Iraq. Additionally, ninety healthy participants, matched by age served as controls. This study excluded individuals who were pregnant, afflicted chronic diseases, autoimmune disorders, microbial infections, smokers, using oral contraceptives or any hormonal medications, and undergoing surgery.
Prior to sample collection for the study, ethical principles in accordance with Declaration of Helsinki were adhered. Patients granted both verbal and written consent before the collection of sample. The study methodology, as along with the subject information and consent form, received approval from a local Ethics Committee following clearance from the ethics commission (IRB) of the Faculty of Science, University of Kufa, Iraq. Additionally, approved by the hospital’s administration the medical records encompass, demographic data, medical history, history of exposure, signs and symptoms, and laboratory findings.
Subsequently, Five milliliters of blood from each individual’s veins were obtained. Gel tubes were utilized to transport the blood subsequent to a breast cancer diagnosis and prior to initiation of any treatment. The blood is centrifuged at 3000 rpm for ten minutes to separate the sera into four tubes (Eppendorf tubes). It is subsequently stored at -8°C until the examination. The activities of AST, ALT, and ALP was measured spectrophotometrically utilizing Roche/Hitachi cobas c system in vitro diagnostic. The serum concentrations of total cholesterol (TC), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) were quantified using an enzymatic colorimetric approach with a commercial kit from Emclab (Germany). Level of low-density lipoprotein cholesterol (LDL-C) and very low-density lipoprotein cholesterol (VLDL-C) were calculated using Friedewald’s formula. Serum IDO1 was measured using enzyme-linked immunosorbent assay (ELISA) kits (Melsin, China. Assay range 1.25-40 ng/mL). Anthropometric measurements including age, weight (kg), height (cm), waist circumference (cm), and hip (cm) were assessed. Furthermore, the body mass index was calculated by dividing weight in kilograms by the square of individual’s in meters: BMI = (weight in kg)/ (height in meters²) [24].
The Kolmogorov-Smirnov test was employed to analyze the distribution types of the results group. The student t-test was utilized to evaluated differences in scale variables across diagnostic categories. The analysis of variance (ANOVA) test was employed to assess the differences in scale variables among diagnostic groups. The results were presented as (mean ± standard deviation) for normally distributed values. The Pearson’s correlation coefficients (r) assess the relationship between the scale variable and the parametric parameter to determine their correlation another variable. Statistical significance for all hypothesis tests was determined with p-values below 0.05 (two-tailed). Receiver operating characteristic (ROC) curves were utilized to assess the diagnostic efficacy of the evaluated biomarkers in diagnosing the disease. The cut-off values of the concentrations yield optimal sensitivity and specificity based The data was compiled and analyzed by using on the area under the curve (AUC). IBM’s Statistical Package for Social Sciences, version 27 (SPSS, Chicago, Illinois, USA).
Results
The baseline characteristics of 90 breast cancer patients diagnosed for the first time, compared with 90 healthy women in the study group, are detailed in Table 1, which summarizes various clinicopathological parameters of the patients presented as (mean ± SD).
| Parameters | Patients group | Controls group | P- value |
| ( mean ± SD ) | ( mean ± SD ) | ||
| Number | 90 | 90 | ------- |
| Age (years) | 48.344 ± 6.657 | 49.988 ± 8.074 | p˃ 0.05 (NS) |
| BMI (kg/m 2 ) | 28.758 ± 4.209 | 28.956 ± 6.559 | p˃ 0.05 (NS) |
| ALT (IU/L) | 22.185 ± 7.882 | 7.566 ± 3.187 | P˂ 0.0001 |
| AST (IU/L) | 20.557 ± 4.773 | 7.683 ± 3.078 | P˂ 0.0001 |
| ALP (IU/L) | 241.233 ± 62.521 | 86.733 ± 31.102 | P˂ 0.0001 |
| TG (mg/dL) | 209.387 ± 25.511 | 196.721 ± 33.714 | P˂ 0.005 |
| TC (mg/dL) | 235.642 ± 28.936 | 214.047 ± 34.246 | P˂ 0.0001 |
| HDL-C (mg/dL) | 34.559 ± 5.067 | 48.661 ± 5.537 | P˂ 0.0001 |
| LDL-C (mg/dL) | 159.205 ± 26.159 | 126.041 ± 30.651 | P˂ 0.0001 |
| VLDL-C (mg/dL) | 41.877 ± 5.102 | 39.344 ± 6.742 | P˂ 0.0001 |
| IDO1 (ng/mL) | 19.197 ± 7.212 | 11.703 ± 3.866 | P˂ 0.0001 |
Data displayed as mean ± SD, were SD denotes: Standard Deviation, BMI: Body Mass Index, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, ALP: Alkaline phosphatase, TG: Triglyceride, TC: Total Cholesterol, HDL-C: High Density Lipoprotein-Cholesterol, LDL-C: Low density Lipoprotein-Cholesterol, VLDL-C: Very Low Density Lipoprotein-Cholesterol, IDO1: Indoleamine 2,3-dioxygenase.
The mean age at breast cancer diagnosis was years (range, 35-65 years). No significant difference was found between the study groups regarding mean age and BMI (p < 0.1378 and p< 0.8096, respectively). The IDO1 level was significantly higher in the BC group compared to the control group (19.197 ± 7.212 vs. 11.703 ± 3.866 ng/mL, P < 0.0001;Table 1), respectively. Clinical data including liver enzymes and lipid profiles, were significantly elevated in the BC women compared to healthy women groups. In Table 2 and Figure 1, illustrates that serum IOD1 levels significantly escalated with the progression of BC stages in women.
| Variables | Patients Stages (No.= 90) | ||||
| Stage I | Stage II | Stage III | Stage IV | p-value | |
| (No.= 23) | (No.= 36) | (No.= 24) | (No.= 7) | ||
| Age (years) | 43.739 ± 5.941 | 49.388 ± 6.442 | 50.041 ± 5.834 | 52.285 ± 6.156 | a) 0.001 |
| b) 0.001 | |||||
| c) 0.002 | |||||
| d) 0.001 | |||||
| e) 0.687 | |||||
| h) 0.397 | |||||
| BMI (kg/m 2 ) | 27.815 ± 3.574 | 29.122 ±4.561 | 29.292 ± 4.232 | 28.154 ±4.489 | a) 0.251 |
| b) 0.235 | |||||
| c) 0.853 | |||||
| d) 0.879 | |||||
| e) 0.582 | |||||
| h) 0.533 | |||||
| ALT (IU/L) | 14.095 ± 4.031 | 20.586 ± 4.558 | 28.379 ± 5.175 | 35.757 ± 1.912 | a) 0.0001 |
| b) 0.0001 | |||||
| c) 0.0001 | |||||
| d) 0.0001 | |||||
| e) 0.0001 | |||||
| h) 0.0001 | |||||
| AST (IU/L) | 17.982 ± 4.478 | 20.077± 4.923 | 22.937 ± 3.682 | 23.328 ± 3.552 | a) 0.079 |
| b) 0.0001 | |||||
| c) 0.006 | |||||
| d) 0.016 | |||||
| e) 0.078 | |||||
| h) 0.837 | |||||
| ALP (IU/L) | 185.913 ± 46.193 | 235.778 ± 54.168 | 296.458 ± 42.601 | 261.714±38.573 | a) 0.0001 |
| b) 0.0001 | |||||
| c) 0.0001 | |||||
| d) 0.0001 | |||||
| e) 0.197 | |||||
| h) 0.097 | |||||
| TG (mg/dL) | 211.196 ±29.001 | 201.235 ±22.372 | 217.036 ± 26.149 | 219.140±16.535 | a) 0.138 |
| b) 0.424 | |||||
| c) 0.462 | |||||
| d) 0.01 | |||||
| e) 0.085 | |||||
| h) 0.845 | |||||
| TC (mg/dL) | 231.408 ± 32.218 | 230.633 ± 28.745 | 243.430 ± 27.695 | 248.615±13.983 | a) 0.92 |
| b) 0.154 | |||||
| c) 0.168 | |||||
| d) 0.094 | |||||
| e) 0.132 | |||||
| h) 0.675 | |||||
| HDL-C (mg/dL) | 34.224 ± 4.874 | 32.335 ± 5.151 | 37.261 ± 4.011 | 37.832 ± 2.338 | a) 0.131 |
| b) 0.028 | |||||
| c) 0.075 | |||||
| d) 0.0001 | |||||
| e) 0.005 | |||||
| h) 0.775 | |||||
| LDL-C (mg/dL) | 154.944 ± 28.872 | 158.051 ± 26.590 | 162.761 ± 25.969 | 166.954±13.637 | a) 0.66 |
| b) 0.312 | |||||
| c) 0.294 | |||||
| d) 0.499 | |||||
| e) 0.416 | |||||
| h) 0.712 | |||||
| VLDL-C (mg/dL) | 42.239 ± 5.800 | 40.247 ± 4.474 | 43.407 ± 5.229 | 43.828 ± 3.307 | a) 0.138 |
| b) 0.424 | |||||
| c) 0.462 | |||||
| d) 0.01 | |||||
| e) 0.085 | |||||
| h) 0.845 | |||||
| IDO1 (ng/mL) | 11.872±0.804 | 16.052±1.048 | 26.608±2.265 | 34.014±1.629 | a) 0.001 |
| b) 0.0001 | |||||
| c) 0.0001 | |||||
| d) 0.0001 | |||||
| e) 0.0001 | |||||
| h) 0.0001 |
Data displayed as mean ± SD, where SD denotes as: Standard Deviation, BMI: Body Mass Index, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, ALP: Alkaline phosphatase, TG: Triglyceride, TC: Total Cholesterol, HDL-C: High Density Lipoprotein-Cholesterol, LDL-C: Low density Lipoprotein-Cholesterol, VLDL-C: Very Low Density Lipoprotein-Cholesterol, IDO1: Indoleamine 2,3-dioxygenase. Significant differences among stages; a) Stage I compared to Stage II, b) Stage I compared to. Stage III, c) Stage I compared to Stage IV, d) Stage II compared to Stage III, e) Stage II compared to Stage IV, h) Stage III compared to Stage IV.
Figure 1. Comparison of Serum IDO1 Levels in Patients' stages.
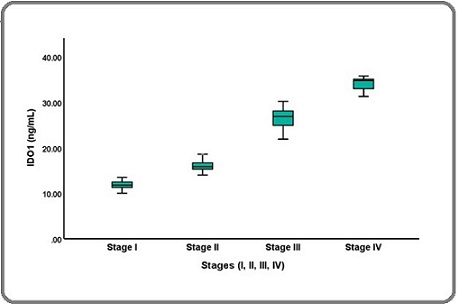
The activities of ALT, AST, and ALP were significantly elevated in all stages of the patients. However, there were no significant differences in mean values of BMI, TG, TC, LDL-C, and VLDL-C across all stages, a significant difference was observed in HDL-C between Stage II and Stage III as well as between Stage II and Stage IV. Table 3 shows the correlation among the measured parameters and variables.
| Parameters | r | p-value | Parameters | r | p-value |
| Age (years) | 0.362 ** | 0.001 | TG (mg/dL) | 0.192 | 0.069 |
| BMI (kg/m 2 ) | 0.068 | 0.522 | TC (mg/dL) | 0.217 * | 0.04 |
| ALT (IU/L) | 0.798 ** | 0.0001 | HDL-C (mg/dL) | 0.373 ** | 0.001 |
| AST (IU/L) | 0.363 ** | 0.0001 | LDL-C (mg/dL) | 0.13 | 0.223 |
| ALP (IU/L) | 0.577 ** | 0.0001 | VLDL-C (mg/dL) | 0.192 | 0.069 |
**. Correlation is significant at the 0.01 level (2-tailed). *. Correlation is significant at the 0.05 level (2-tailed). r: Pearson correlation coefficient.
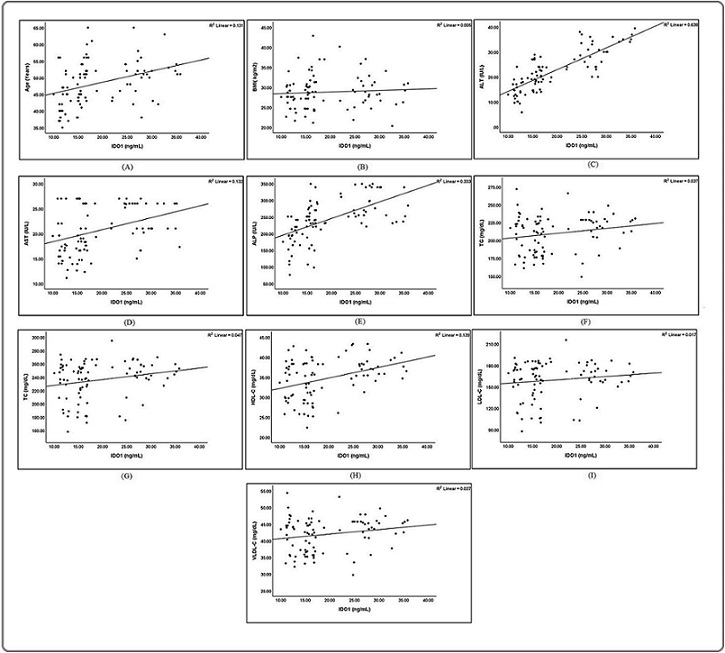
The findings of this study indicated toward positive correlation between IDO1and all examined parameters with exception of BMI, LDL-C and VLDL-C Figure 2.
Figure 2. Correlation between IDO1 levels and A: Age, B: BMI, C: ALT, D: AST, E: ALP, F: TG, G: TC, H: HDL-C, I: LDL-C, J: VLDL-C.
Receiver operating characteristics (ROC) analysis was conducted to evaluate the efficacy of the measured IDO1 in identifying BC patients by assessing its diagnostic sensitivity and specificity. Figure 3 illustrates the plotted ROC curves.
Figure 3. Receiver Operating Characteristic Curve for Breast Cancer Patients vs. healthy control.
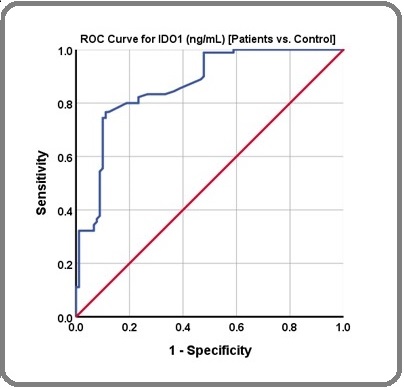
Table 4 presents the coordinates of the ROC results along with the concentration cutoff that yields optimal sensitivities and specificities.
| Variables | IDO1(ng/mL) |
| Area Under Curve (AUC) | 0.867 |
| Cut-off value | 12.65 |
| P-value | p ˂ 0.0001 |
| Specificity | 80.00% |
| Sensitivity | 81.10% |
| Confidence interval 95% | 0.814-0.919 |
The results indicates that IDO1 levels exceeding the projected cut-off values suggest that subjects may have BC, with sensitivities of 80.0 % and specificities of 81.1 %, p < 0.0001 at a cut-off value of 12.65 ng/mL.
Discussion
Several prognostic and predictive factors have been reported in breast cancer patients, including those associated with survival outcomes and the risk of treatment-related adverse effects [25-29]. Indoleamine 2,3-dioxygenase 1 is often activated in various types of human cancers, including BC, and influences the innate immune system, tumor cells, and stromal cells [30]. The presence of IDO1 is frequently linked to a negative prognosis. The influence on immunosuppression is intricate as it involves the suppression of natural killer and CD8+ effector T cells, while enhancing the function of myeloid- derived suppressor and CD4+ T-reg cells [31]. It has been documented that tumors-derived IDO possesses the ability to inhibit the immunological responses against tumor [32]. IDO proposed to play a crucial role in the pathogenesis of breast cancer. Inflammatory cytokines indicates the ability to create an inflammatory environment that promotes tumor proliferation. Interferon‐gamma (IFN-γ) recognized for its significant role in antitumor immune responses, while Interleukin 6 (IL-6) is considered a pro-tumorigenic cytokine. Recent studies indicate that IDO1 is linked to the induction of IL-6, while IFN-γ serves as a primary catalyst for IDO1 induction. Despite extensive research on the effects of Trp deprivation, the functions of Kyn and other Trp catabolizes remain ambiguous [20]. Godin-Ethier et al. reported that Kyn serves as a natural ligand for the aryl hydrocarbon receptor, a transcription factor integral to proinflammatory processes and finding particularly relevant to inflammatory carcinogenesis [30]. IDO overexpression correlate with reduced overall survival in patients with solid tumors [19]. Huang et al [33] confirmed that elevated IDO expression correlates with improved survival rates in breast cancer patients, aligning with results reported by Jaquemiere et al [34].
Yu et al. observed that the influx of T-regs into the tumor microenvironment by IDO1 activation in primary BC may suppress the local immune response and facilitate metastasis [35]. Song et al. demonstrated that L-Kyn, a catabolite of IDO1, can trigger apoptosis in NK cells by stimulating the generation of ROS, thus facilitating immune evasion [36]. Recent years demonstrated that the upregulation of IDO1 in many malignancies leads to Trp deprivation in the local environment, thereby inhibiting the T cell-mediated immune response. IDO1 expression and clinical outcomes exhibit significant correlations across several cancers, including BC. The involvement of IDO1 in various carcinomas is controversial due to inconsistent results. In colorectal cancer, endometrial, ovarian, and esophageal carcinomas, the expression of IDO1 is indicative of a poor clinical prognosis [37]. In contrast, patients with hepatocellular carcinoma and elevated IDO1 levels demonstrates a favorable prognosis. While most experimental studies indicate a positive correlation between IDO1 activity and breast tumor progression, one study reported contrary findings [38] and another observed no significant differences in IDO1 expression between tumorigenic and non-tumorigenic tissues [39]. The same research group contradictory findings IDO1 activation in patients with invasive BC, indicating that IDO1 activity was significantly reduced in patients exhibiting higher number of metastatic abrasions compared to those with no or fewer metastatic abrasions [40]. Previous research demonstrated a favorable correlation between IDO1 expression and the number of metastatic abrasions [41]. The concurrent expression of cytokeratin 18, IL-6 and IDO1 in BC patients prior to neoadjuvant chemotherapy; identifying a correlation between IL-6 and IDO1 expression in advanced BC and an reduced response to neoadjuvant treatment [42]. Wei et al. demonstrated a positive correlation between the expression of IDO1 and CD105 in Michigan cancer foundation-7 malignant BC cell lines. This was associated with the initial metastasis of tumor draining tumor-draining lymph nodes, lymph node metastasis stage, and histological grade. Beside inhibiting immunological responses, IDO1 may facilitate tumor progression by enhancing angiogenesis through the proliferation of CD105-expressing human umbilical vein endothelial cells [43]. Dill et al. demonstrated that IDO1 expression was highest in triple-negative breast cancer (TNBC), with roughly 70% of Programmed death-ligand 1 (PD-L1) breast neoplasms exhibiting IDO1expression. The majority of breast neoplasms with low PD-L1 expression were negative for IDO1 [44]. In 2020, Wei et al. proposed that IDO1 and tumor-infiltrating immune cells serve as a significant prognostic markers in BC [45]. Costa et al. indicated that 40% of human epidermal growth factor receptor-2-positive breast tumors overexpress IDO1, suggesting the potential of a synergistic treatment approach similar to that shown in TNBC models [46]. Research conducted by Sakurai et al. demonstrated that high IDO1 expression in breast cancer is associated with clinical stage and may significantly contribute to the immunosuppression in affected patients [47]. The mechanisms involving this pathogenic process remain unidentified. It is proposed that local T-cell-mediated immunotolerance induced by elevated IDO1 level in the tumor microenvironment may serve as a primary immunoregulatory mechanism facilitating tumor metastasis [19]. IDO1 facilitates the degradation of the essential amino acid tryptophan resulting in the synthesis of immunosuppressive Kyn and the depletion of Trp. IDO1 expression and activity facilitate aggressive tumor growth, inferior therapeutic efficacy and poor prognosis [48]. The microenvironment of breast tumor comprises several cell types, including lymphocytes. IDO1 has been linked to the tumor evasion from the immune system [49]. Another immunosuppressive enzyme investigated in BC is IDO1. It is a Trp catabolic enzyme expressed during fetal development to protect against maternal T lymphocyte destruction. IDO1 expression is suppressed under normal physiological conditions in adults, however it is observed during inflammatory settings and in different tumors. IDO1 expression is linked with tumor evasion by suppressing the antigen specific immune response [50]. A limitation of this study is the small sample size in the advanced-stage groups of BC patients. Consequently, future research involving larger patient populations is recommended to validate the observed associations between IDO1 and BC.
In conclusion, the elevated level of IDO1 in BC women was identified as the most significant predictor of prognosis and a valuable instrument for prognostic evaluation and potentially indicating its involvement in disease pathogenesis. It can produce an inflammatory microenvironment by serving as a central interface for inflammatory cytokines. Furthermore, elevated IDO levels are significantly linked with reduced overall patient survival. IDO may serve as a potential biomarker and immunotherapeutic target in patients with breast cancer.
Acknowledgements
The authors express their gratitude to the participating patients and all the medical staff at the National Hospital for Oncology and Hematology in Najaf Al-Ashraf, Iraq for their cooperation in sample collecting and execution of the required laboratory tests.
Declaration of interest
The authors declare no conflict of interest.
Funding
This research was self-financed
References
- Breast cancer Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano Gi. Lancet (London, England).2021;397(10286). CrossRef
- Breast cancer organoids derived from patients: A platform for tailored drug screening Tzeng YT , Hsiao J, Tseng L, Hou M, Li C. Biochemical Pharmacology.2023;217. CrossRef
- AI-based selection of individuals for supplemental MRI in population-based breast cancer screening: the randomized ScreenTrustMRI trial Salim M, Liu Y, Sorkhei M, Ntoula D, Foukakis T, Fredriksson I, et al . Nature Medicine.2024;30(9). CrossRef
- Comprehensive Inherited Risk Estimation for Risk-Based Breast Cancer Screening in Women Mars N, Kerminen S, Tamlander M, Pirinen M, Jakkula E, Aaltonen K, Meretoja T, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2024;42(13). CrossRef
- Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021 Burstein H. J., Curigliano G., Thürlimann B., Weber W. P., Poortmans P., Regan M. M., Senn H. J., et al . Annals of Oncology: Official Journal of the European Society for Medical Oncology.2021;32(10). CrossRef
- Breast Cancer Prediction Based on Multiple Machine Learning Algorithms Zhou S, Hu C, Wei S, Yan X. Technology in Cancer Research & Treatment.2024;23. CrossRef
- Diagnosis and Treatment of Breast Cancer in Young Women Rossi L, Mazzara C, Pagani O. Current Treatment Options in Oncology.2019;20(12). CrossRef
- Simultaneous Foxp3 and IDO expression is associated with sentinel lymph node metastases in breast cancer Mansfield AS , Heikkila PS , Vaara AT , von Smitten KA , Vakkila JM , Leidenius MH . BMC cancer.2009;9. CrossRef
- Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy Muller AJ , DuHadaway JB , Donover PS , Sutanto-Ward E, Prendergast GC . Nature Medicine.2005;11(3). CrossRef
- Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division Lee GK , Park HJ , Macleod M, Chandler P, Munn DH , Mellor AL . Immunology.2002;107(4). CrossRef
- Inhibition of T cell proliferation by macrophage tryptophan catabolism Munn D. H., Shafizadeh E., Attwood J. T., Bondarev I., Pashine A., Mellor A. L.. The Journal of Experimental Medicine.1999;189(9). CrossRef
- Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Mellor A. L., Munn D. H.. Immunology Today.1999;20(10). CrossRef
- IDO expression by dendritic cells: tolerance and tryptophan catabolism Mellor Al , Munn DH . Nature reviews. Immunology.2004;4(10). CrossRef
- Tolerance, DCs and tryptophan: much ado about IDO Grohmann U, Fallarino F, Puccetti P. Trends in Immunology.2003;24(5). CrossRef
- Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape Katz JB , Muller AJ , Prendergast GC . Immunological Reviews.2008;222. CrossRef
- Monitoring tryptophan metabolism in chronic immune activation Schröcksnadel K, Wirleitner B, Winkler C, Fuchs D. Clinica Chimica Acta; International Journal of Clinical Chemistry.2006;364(1-2). CrossRef
- Expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer Nakamura T, Shima T, Saeki A, Hidaka T, Nakashima A, Takikawa O, Saito S. Cancer Science.2007;98(6). CrossRef
- Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, et al . Journal of Immunology (Baltimore, Md.: 1950).2013;190(7). CrossRef
- Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER , et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2006;12(4). CrossRef
- Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, Ishii N, et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2005;11(16). CrossRef
- Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer Ino K., Yoshida N., Kajiyama H., Shibata K., Yamamoto E., Kidokoro K., Takahashi N., et al . British Journal of Cancer.2006;95(11). CrossRef
- Targeting the indoleamine 2,3-dioxygenase pathway in cancer Moon YW , Hajjar J, Hwu P, Naing A. Journal for Immunotherapy of Cancer.2015;3. CrossRef
- IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance Munn DH , Mellor AL . Trends in Immunology.2016;37(3). CrossRef
- Body Mass Index: Obesity, BMI, and Health: A Critical Review Nuttall FQ . Nutrition Today.2015;50(3). CrossRef
- Amelioration of anxiety, depression, and chemotherapy related toxicity after crocin administration during chemotherapy of breast cancer: A double blind, randomized clinical trial Salek R, Dehghani M, Mohajeri SA , Talaei A, Fanipakdel A, Javadinia SA . Phytotherapy research: PTR.2021;35(9). CrossRef
- A Randomized, Controlled, Parallel-Group, Trial on the Long-term Effects of Melatonin on Fatigue Associated With Breast Cancer and Its Adjuvant Treatments Sedighi Pashaki A, Sheida F, Moaddab Shoar L, Hashem T, Fazilat-Panah D, Nemati Motehaver A, Ghanbari Motlagh A, et al . Integrative Cancer Therapies.2023;22. CrossRef
- A Randomized, Controlled, Parallel-Group, Trial on the Effects of Melatonin on Fatigue Associated with Breast Cancer and Its Adjuvant Treatments Sedighi Pashaki A, Mohammadian K, Afshar S, Gholami MH , Moradi A, Javadinia SA , Keshtpour Amlashi Z. Integrative Cancer Therapies.2021;20. CrossRef
- A Review of Intraoperative Radiotherapy After Neoadjuvant Chemotherapy in Patients with Locally Advanced Breast Cancer: From Bench to Bedside Keramati A, Javadinia SA , Gholamhosseinian H, Fanipakdel A, Homaei Shandiz F, Taghizadeh-Hesary F. Indian Journal of Gynecologic Oncology.2020;18(4). CrossRef
- Outcome of hypofractionated breast irradiation and intraoperative electron boost in early breast cancer: A randomized non-inferiority clinical trial Fadavi P, Nafissi N, Mahdavi SR , Jafarnejadi B, Javadinia SA . Cancer Reports (Hoboken, N.J.).2021;4(5). CrossRef
- Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives Godin-Ethier J, Hanafi L, Piccirillo CA , Lapointe R. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2011;17(22). CrossRef
- Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer Prendergast GC , Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, Muller AJ . Cancer immunology, immunotherapy: CII.2014;63(7). CrossRef
- Strong Correlation of Indoleamine 2,3-Dioxygenase 1 Expression with Basal-Like Phenotype and Increased Lymphocytic Infiltration in Triple-Negative Breast Cancer Kim S, Park S, Cho MS , Lim W, Moon B, Sung SH . Journal of Cancer.2017;8(1). CrossRef
- Serum tryptophan decrease correlates with immune activation and impaired quality of life in colorectal cancer Huang A., Fuchs D., Widner B., Glover C., Henderson D. C., Allen-Mersh T. G.. British Journal of Cancer.2002;86(11). CrossRef
- High expression of indoleamine 2,3-dioxygenase in the tumour is associated with medullary features and favourable outcome in basal-like breast carcinoma Jacquemier J, Bertucci F, Finetti P, Esterni B, Charafe-Jauffret E, Thibult M, Houvenaeghel G, et al . International Journal of Cancer.2012;130(1). CrossRef
- Upregulated expression of indoleamine 2, 3-dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T cells in situ and lymph node metastasis Yu J, Sun J, Wang SE , Li H, Cao S, Cong Yi, Liu J, Ren X. Clinical & Developmental Immunology.2011;2011. CrossRef
- L-kynurenine-induced apoptosis in human NK cells is mediated by reactive oxygen species Song H, Park H, Kim Y, Kim KD , Lee H, Cho D, Yang J, Hur DY . International Immunopharmacology.2011;11(8). CrossRef
- The Clinicopathological and Prognostic Significance of IDO1 Expression in Human Solid Tumors: Evidence from a Systematic Review and Meta-Analysis Yu C, Fu S, Chen X, Ye J, Ye Y, Kong L, Zhu Z. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology.2018;49(1). CrossRef
- Tryptophan catabolism increases in breast cancer patients compared to healthy controls without affecting the cancer outcome or response to chemotherapy Onesti CE , Boemer F, Josse C, Leduc S, Bours V, Jerusalem G. Journal of Translational Medicine.2019;17(1). CrossRef
- Gene expression of indoleamine and tryptophan dioxygenases and three long non-coding RNAs in breast cancer Ghafouri-Fard S, Taherian-Esfahani Z, Dashti S, Kholghi Oskooei V, Taheri M, Samsami M. Experimental and Molecular Pathology.2020;114. CrossRef
- [Indoleamine 2,3-Dioxygenase Activity during Fulvestrant Therapy for Multiple Metastatic Breast Cancer Patients] Sakurai K, Fujisaki S, Adachi K, Suzuki S, Masuo Y, Nagashima S, Hara Y, et al . Gan to Kagaku Ryoho. Cancer & Chemotherapy.2016;43(10).
- [Analysis of indoleamine 2, 3-dioxygenase expression in breast cancer patients with bone metastasis] Sakurai K, Fujisaki S, Nagashima S, Maeda T, Shibata M, Gonda K, Tomita R, et al . Gan to Kagaku Ryoho. Cancer & Chemotherapy.2012;39(12). CrossRef
- Indoleamine-2,3-dioxygenase and Interleukin-6 associated with tumor response to neoadjuvant chemotherapy in breast cancer Li F, Wei L, Li S, Liu J. Oncotarget.2017;8(64). CrossRef
- High Indoleamine 2,3-Dioxygenase Is Correlated With Microvessel Density and Worse Prognosis in Breast Cancer Wei L, Zhu S, Li M, Li F, Wei F, Liu J, Ren X. Frontiers in Immunology.2018;9. CrossRef
- IDO expression in breast cancer: an assessment of 281 primary and metastatic cases with comparison to PD-L1 Dill EA , Dillon PM , Bullock TN , Mills AM . Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2018;31(10). CrossRef
- Prognosis significance of indoleamine 2, 3-dioxygenase, programmed death ligand-1 and tumor-infiltrating immune cells in microenvironment of breast cancer Wei L, Wu N, Wei F, Li F, Zhang Y, Liu J, Ren X. International Immunopharmacology.2020;84. CrossRef
- Clinical development of immunotherapies for HER2+ breast cancer: a review of HER2-directed monoclonal antibodies and beyond Costa RLB , Czerniecki BJ . NPJ breast cancer.2020;6. CrossRef
- [Study of indoleamine 2,3-dioxygenase expression in patients with breast cancer] Sakurai K, Amano S, Enomoto K, Kashio M, Saito Y, Sakamoto A, Matsuo S, et al . Gan to Kagaku Ryoho. Cancer & Chemotherapy.2005;32(11).
- Chemoresistance was correlated with elevated expression and activity of indoleamine 2,3-dioxygenase in breast cancer Zhao Y, Wei L, Liu J, Li F. Cancer Chemotherapy and Pharmacology.2020;85(1). CrossRef
- Expression of immune checkpoints (PD-L1 and IDO) and tumour-infiltrating lymphocytes in breast cancer Alkhayyal N, Elemam NM , Hussein A, Magdub S, Jundi M , Maghazachi AA , Talaat IM , Bendardaf R. Heliyon.2022;8(9). CrossRef
- Directing Traffic: How to Effectively Drive T Cells into Tumors Anandappa AJ , Wu CJ , Ott PA . Cancer Discovery.2020;10(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2025
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times