The Correlation of Radiogenomics and Green Tea Extract Polyphenols: Redefining Head and Neck Cancer Management
Download
Abstract
Radiogenomics, an emerging paradigm that combines genomic and imaging information, is poised to advance precision oncology, particularly in head and neck cancer (HNC). This review explores the potential integration of radiogenomics with the polyphenol epigallocatechin-3-gallate (EGCG) from green tea, building on systematic and bibliometric analyses of an unprecedented amount of information (molecular and clinical milestones and advances) related to oncology and radiogenomics, spanning from 1997 to 2024. Including an antioxidant and anticancer compound, EGCG has shown promise in modifying the response to radiation and modifies certain biologic pathways. It is proposed that radiogenomic markers will be effective in modifying toxicity, potentially augmenting precision treatments in HNC, while simultaneously integrating the fields of genetics, imaging, and nutraceuticals into one new area for future research and clinical practice.
Introduction
Head and neck cancer (HNC) remains a significant public health issue across the globe, with projections of almost 900,000 new diagnoses every year. HNC arises from the pharynx, larynx, salivary glands, and oral cavity. HNC can be diagnosed at an advanced stage on account of the tumour’s aggressive biology and the limited use of diagnostics or lab tests that adequately detect early-stage disease. In the last few decades, radiation therapy, chemotherapy, and often surgery have resulted in modest advances in quality-of-life improvements in HNC patients. The prognosis for HNC is still poor. For this reason, it is important that patients are able to express their goals and needs to screen better and treat HNC at the early stage [1-4].
Radiogenomics is an emerging and rapidly expanding field that combines genetic data with imaging, allows for predictive non-invasive biomarkers discovery and tumour heterogeneity, and creates personalised precision cancer treatment [5, 6]. This radiomics approach, alongside molecular signatures, gives clinicians the ability to predict molecular and biological therapy outcomes, leading to personalised therapy interventions [7]. Given the often complex aspects of anatomical structure and molecular features of HNC, radiogenomics is likely a better option to try to predict the unpredictable nature of the treatment results and improve radiogenomics, if informed, treatment choices are more rationalised [5, 7].
Simultaneously, there is growing scientific interest in dietary agents for the prevention and treatment of cancer. Green tea, an infusion from the leaves of the Camellia sinensis plant, is one of the most widely consumed beverages, known for its taste, potential health benefits, and cultural significance. Green tea is generally recognised for its various bioactive compounds, including catechins and flavonoids, which are associated with overall health benefits that include cardiometabolic health, cancer prevention, and cognitive benefits [9], among others. Consequently, there is an interest in the chemopreventive potential of green tea extract (GTE), a source of polyphenolic compounds, particularly epigallocatechin-3-gallate (EGCG), for several types of malignancies, which could include HNC [8, 9]. EGCG is well regarded for its anti-inflammatory, anticancer, and antioxidant properties, which are explained through various cellular signalling modifications, cell proliferation inhibition, and apoptosis induction [10, 11]. Additionally, some studies have suggested that GTE may increase sensitivity to chemotherapy and radiation in a preclinical context [12].
Prior investigations into green tea catechins and radiogenomics have been conducted separately in the oncology literature, but not yet in relation to one another. In particular, limited exploration has been conducted regarding EGCG and its potential to alter radiogenomic characteristics associated with tumour growth in HNC, and there is also a lack of research on imaging biomarkers for monitoring the biological impact of EGCG as a treatment strategy. This literature gap will be addressed by systematically evaluating the current state of knowledge, including major research themes, and subsequently proposing the conceptual framework for integrating radiogenomic tools with green tea treatments of HNC.
Furthermore, there are significant differences in the contributions made by different regions to the field. There is little representation from areas with a high incidence of HNC, and research output is disproportionately concentrated in high-income nations. These results highlight the necessity of more extensive international cooperation and capacity-building to provide fair access to advancements in precision oncology [13, 14]. This analysis establishes the foundation for a new, multidisciplinary approach to HNC management that may enhance clinical results and promote individualised therapy by combining knowledge from imaging, genetics, and nutraceutical studies.
Materials and Methods
Data Collection and Screening
The data analysis was conducted in accordance with the usual PRISMA guidelines (Figure 1).
Figure 1. PRISMA Flow Diagram Illustrating the Systematic Selection of Studies on Head and Neck Cancer, Radiogenomics, Imaging Biomarkers, Green Tea Extract, and EGCG.
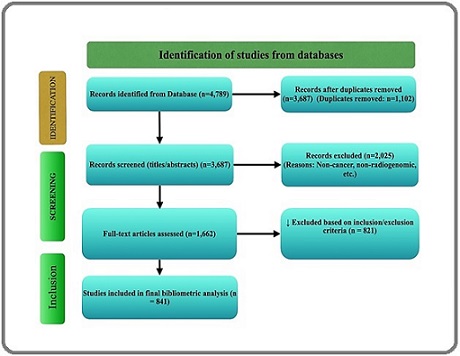
A significant amount of literature on “epigallocatechin gallate (EGCG),” “head and neck cancer,” “radiogenomics,” “imaging biomarkers,” and “green tea extract” was obtained and evaluated. 4,789 documents were found after a thorough search across databases, mostly Scopus, Web of Science. There were still 3,687 unique records for screening after 1,102 duplicates were eliminated.
Based on relevancy, 2,025 papers were excluded after screening titles and abstracts. The eligibility of the remaining 1,662 full-text publications was assessed. 821 of these were disqualified because they did not concentrate on imaging biomarkers, had unrelated molecular pathways, or had a minimal impact on citations. 841 studies were found by this technique and included in the final bibliometric analysis. Research output increased most significantly between 2013 and 2014, peaking in 2022 with 555 publications. Although the larger search scope covered works from 1997 to 2024 to record historical progression, the 2013–2023 period was chosen as the period during which a significant number of publications were recorded. Only research that satisfied specific inclusion requirements such as applicability to HNC, radiogenomics, and the anticancer properties of green tea extract was kept. After a thorough examination of the abstracts and titles, articles that did not fit these requirements were eliminated. A thorough picture of the state of HNC chemoprevention and radiogenomics research was made possible by this filtering.
Cancer stem cells and natural products; chemoprevention with green tea extract in oral malignancies; dietary practices and nutrition for cancer treatment; clinical and preclinical use of green tea compounds; molecular biology of EGCG in HNC; combination treatment; and newer phytochemicals and adjunct modalities were some of the topics covered in the literature reviewed. One major theme included the use of traditional bioactive compounds targeting cancer stem cells, particularly in pancreaticobiliary malignancies. Another major theme described the potential of green tea extract to prevent oral malignancies, including the inhibition of tumour growth and targeting receptor tyrosine kinases with EGCG.
Recommendations made by the American Cancer Society regarding lifestyle practices in cancer progression were cited by studies [8, 9]. Phytochemical and green tea extract prevention research were framed in this lifestyle context. Clinical studies showed that green tea mouthwash resulted in a significant reduction in radiation-induced mucositis in HNC patients [15]. In addition, there are multiple lines of evidence that EGCG and EGFR tyrosine kinase inhibitors demonstrated synergy [16, 17]. Molecular studies showed similarly that EGCG can target critical oncogenic pathways like DNA hypermethylation, NF-κB, and HGF/c-Met [18, 19], suggesting it can modulate gene expression and/or cellular signalling in cancer cells [20]. In addition, combination therapies such as TriCurin, which contain resveratrol, epicatechin gallate, and curcumin, have shown promising outcomes in the management of HPV-positive HNC tumours [21]. The properties of anti-inflammation and antioxidant action of other natural compounds, including curcumin, lycopene, chamomile, and aloe vera, have been recognised for their use in managing oral mucosal lesions - as such, these compounds provide additional support for cancer prevention strategies [22-25].
This bibliometric analysis highlights significant advancements in research regarding the application of radiogenomics and dietary phytochemicals for HNC therapy. It provides a comprehensive perspective on the scientific basis for natural chemopreventive medicine, specifically green tea catechins [5, 7].
Bibliometric Analysis
A density visualisation of keyword co-occurrence is shown in Figure 2, where the frequency of keywords is positively connected with font size and colour intensity.
Figure 2. Represents the Density Visualisation of Keywords in this Font Size, and the Different Colours are Positively Correlated with the Frequency of Occurrence of Keywords. 2A, Visualisation of Keyword Co-occurrence Networks in ECGC Research Using VOSviewer. 2B, Visualisation of Keyword Co-occurrence Networks in Head and Neck Neoplasm Research Using VOSviewer. 2C, Visualisation of Keyword Co-occurrence Networks in Green Tea Extract Research Using VOSviewer. 2D, Visualisation of Keyword Co-occurrence Networks in head and neck cancer and green tea extract Research Using R Studio.
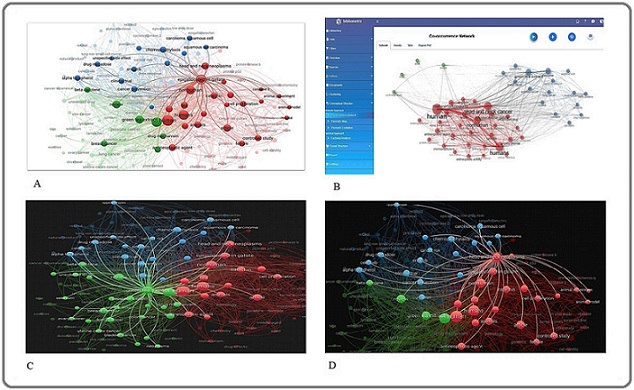
The colours of the balls are representing the different clusters that are linked together in the thematic area. The most common keywords found in the chosen literature were “green tea extract” (n = 57), “epigallocatechin gallate” (n = 43), and “head and neck neoplasm” (n = 44). The main study topics that have garnered a lot of scientific attention are shown in this figure. Important subject clusters within the field are revealed by the denser and livelier areas of the map, which represent themes with higher citation and publication densities.
Sub-figures offer more detailed information: Figures 2A–2D. VOSviewer is employed to collectively illustrate the landscape of cancer research involving natural compounds, with a central focus on green tea extract and epigallocatechin gallate (EGCG). Figure 2A presents a broad co-occurrence network identifying EGCG and green tea extract as key hubs connecting diverse domains such as mechanistic oncology, clinical research, and chemoprevention. Figure 2B refines this perspective by mapping more defined intersections among these natural agents and cancer-related keywords, emphasising interdisciplinary research convergence and collaborative focus areas. In Figure 2C, keyword associations are specifically anchored to green tea extract, revealing its wide-ranging application across various cancer types and therapeutic contexts. Meanwhile, Figure 2D narrows the analytical scope using R Studio to construct a co-occurrence map tailored to green tea extract and head and neck cancer, offering granular insight into thematic linkages in a specific oncological subdomain.
These varied visual representations show changing focus areas and new connections between radiogenomics, cancer biology, and nutraceuticals, reflecting the changing landscape of research trends.
Word Cloud Analysis
The word cloud analysis (Figure 3) identifies key theme areas of focus for cancer treatment and research.
Figure 3. Word Cloud Analysis Represents Keywords that Highlight Several Key Themes in Cancer Research and Treatment.
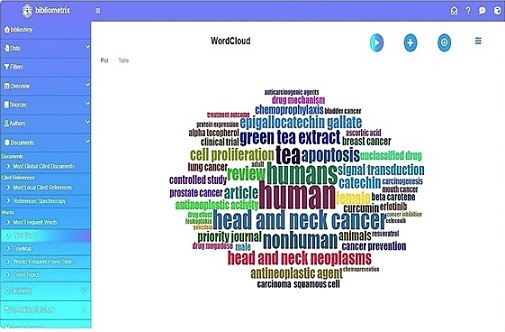
Along with mechanistic terms like “carcinogenesis” and “squamous cell carcinoma,” frequently recurring keywords include several forms of cancer, such as head and neck cancer, bladder cancer, breast cancer, lung cancer, and prostate cancer. These represent the emphasis of current studies as well as the biology of tumours.
Anticancer substances that have been thoroughly researched for their chemopreventive and antineoplastic properties, such as resveratrol, genistein, curcumin, GTE, and EGCG are at the heart of the investigation. Important molecular processes that are represented in the literature include the mechanisms of action, which include modulation of protein expression, inhibition of cell proliferation, induction of apoptosis, and regulation of signal transduction.
Terms like “treatment outcomes” and “priority drugs” highlight themes pertaining to chemoprevention, cancer prevention, and treatment optimisation. This field of study is scholarly, as evidenced by methodological terms like “controlled studies,” “journal articles,” and “review studies.” In the meantime, phrases like “humans,” “nonhuman animals,” and “gender differences” highlight the significance of responses that are distinctive to a given community.
Interestingly, substances like the targeted EGFR inhibitor erlotinib are commonly found, indicating an emphasis on precision treatments. Although less frequently used, terms like “Aloha” and “Tocoalherial” may refer to specialised study areas or local phytochemicals that are being studied.
This analysis also makes clear the importance of nutrition and food in the prevention and treatment of cancer. In order to contextualise dietary choices during and after cancer therapy, the American Cancer Society’s guidelines for nutrition and physical exercise have been regularly cited [9]. Within this framework, studies emphasised the significance of dietary phytochemicals, particularly green tea extract, for their impact on cancer progression and patient quality of life [8].
Green tea extract’s chemopreventive function in HNC was further validated by clinical and experimental studies. Green tea extract mouthwash, for example, was shown in a randomised clinical trial to be efficacious in decreasing radiation-induced mucositis in HNC patients [26]. The case for combination therapies in clinical settings is further supported by the synergistic effect of EGCG and EGFR tyrosine kinase inhibitors in inhibiting tumour growth [16].
According to molecular evidence, EGCG suppresses important oncogenic pathways that are connected to inflammation and tumour growth, such as HGF/c-Met, NF-κB, and DNA hypermethylation [18]. This lends credence to EGCG’s wider capacity to affect gene expression and control signalling cascades linked to cancer in cancerous cells [19].
Combinatorial methods utilising EGCG in conjunction with other natural substances have been the subject of additional research. Yang H. et al. verified increased anticancer activity after examining the combined effects of EGCG, curcumin, and resveratrol in the treatment of HNC [9]. Likewise, the TriCurin formulation comprising curcumin, epicatechin gallate, and resveratrol demonstrated potent tumour suppression in HPV-positive HNC patients [15].
In addition to green tea catechins, other phytochemicals like curcumin, lycopene, chamomile, and aloe vera were also evaluated, particularly for their application in managing oral mucosal lesions and reducing inflammation. These agents showed considerable antioxidant and anti-inflammatory properties, contributing further to the chemopreventive landscape [19].
When taken as a whole, word cloud analysis provides a comprehensive visual depiction of current cancer treatment research themes. The convergence of biological processes, phytochemicals, cancer kinds, and treatment approaches is skillfully shown. This bolsters the mounting data supporting the use of catechins from green tea extract in the treatment and prevention of head and neck cancer [10, 17].
Evidence of radiogenomics in Head and Neck Cancer
Radiogenomics is a novel, disruptive theory in oncology that represents the intersection of genomics and radiology, specifically with respect to HNC. The field of radiogenomics improves the capacity to personalise treatment plans across disease states by correlating imaging observations with genetic, molecular, and cellular properties of tumours [27].
Specifically, radiogenomics improves prognostication, facilitates personalised therapy selection, and reveals characteristics of tumour heterogeneity in head and neck squamous cell carcinoma (HNC) [6]. Literature has demonstrated that genetic alterations in specific oncogenes (for example, TP53, EGFR, PIK3CA), which are seen commonly in HNC, correlate reasonably well with imaging features such as tumour shape, texture, and contrast enhancement on imaging [28, 29]. p16 expression and HPV status, perhaps the two most significant prognostic characteristics in HNC, have also been correlated with radiomic features from MRI or PET/ CT [30]. Notably, HPV-positive tumours can have specific radiologic signatures that indicate more favourable biological response to radiation therapy, although that specific area of radiogenomics has not been rigorously validated [31].
In addition to predicting tumour responses to radiotherapy, radiogenomics can also be used to identify imaging biomarkers that predict responses to immunotherapy or chemotherapeutics. Multiple studies have shown that metabolic indices calculated from PET scans (most frequently standardised uptake values [SUV]) strongly correlate with responses to therapy in chemoradiotherapy settings [32]. In HPV-positive cancer environments, radiomic models that incorporate genetics (for example, GC) and imaging have shown predictive power for components of immunotherapy response.
With the use of these methods, physicians may be able to limit unnecessary exposure to unworkable therapies by identifying those patients most likely to respond to immunotherapy [33]. In addition, functional imaging techniques (e.g., dynamic contrast-enhanced MRI) provide added value in ensuring a better understanding of tumour hypoxia, known to drive radiation resistance and poor prognosis [34]. By incorporating radiomic metrics of tumour texture, volume, and shape with genomic metrics of disease-free survival, the dichotomy of overall survival, and locoregional recurrence risk of patients with HNC is augmented [35]. In addition, identifying intratumoral heterogeneity, a driver of treatment resistance, using radiogenomics can be done without performing invasive sampling. Additional imaging methodologies, including diffusion-weighted MRI and PET/CT, can be used to map aspects of the tumour microenvironment (hypoxia, angiogenesis), track the associated genetic expression profiles, or a combination of the above [36].
Radiogenomics improves clinical understanding of differences in molecular and cellular alterations that occur in distinct tumour areas by integrating genomic and other imaging features in spatial contexts. Radiogenomics also improves the development of less invasive and more precise therapeutic strategies using spatially optimised precision-guided medicines. The availability of machine learning (ML) and artificial intelligence (AI) has accelerated developments in the field of radiogenomics. Both ML and AI applications, alongside imaging and genomic features, have been utilised to establish predictive signatures to optimise tumour characterisation and predict patient-related outcomes [37]. For example, there are AI models using imaged and radiomic features of CT and MR scans to define molecular markers, such as p53 mutations and HPV status, with sufficient assurance to facilitate treatment planning and clinical decision-making [38].
To optimise therapeutic outcomes in HNC, recent clinical studies are now investigating combinations of radiogenomics techniques coupled with green tea extract (GTE). These studies suggest that GTE influences tumour- specific events, including inflammation and hypoxia, while advancing the radiogenomic profiling process with improved therapeutic efficacy and increasing the chance of a positive outcome [39].
Molecular Biomarkers Associated with Radiation Response in HNC
While radiation therapy remains an integral component of the management of HNC, biological variability assumes a multitude of forms in response to radiation comparisons among patients. There are many biomarkers in the literature that are thought to influence radiosensitivity or radioresistance that have been confirmed for the current practice of personalised therapist optimisation. For example, variable biological responses of HNC and other cancers to radiation have been shown to be associated with genes associated with DNA repair pathways, such as ERCC1, XRCC1, and NBS1 [40]. Changes in expression or mutations of these genes, which can impart increased resistance to radiation, may also lead to modifications of initial treatment proposals. Incorporating these markers into routine clinical practice promotes categorisation of patients based on expected response to radiation therapy, with the potential to elevate radiogenomic profiling to become a golden standard in precision oncology [41]. In this regard, radiogenomics standardises and reduces overtreatment and toxicity while informing the selection of appropriate drug therapies.
Radiation is one of the main treatment modalities for HNC and has the second-highest inter-individual variability of effect among the treatment modalities. Using biomarkers of radiation response may help mitigate the risk of unanticipated side effects of radiation and facilitate the development of personalised treatment regimens, as well as enhance the ability to predict treatment outcomes [42]. In HNC, radioresponse to radiation can be altered by genetic and epigenetic changes and derailed cellular signalling [43]. For example, mutations in tumour suppressor genes, such as TP53 and PTEN, that are involved in apoptosis-related and cell cycle control, have been associated with an increased resistance to radiation [44]. DNA damage response and repair genes involved in homologous recombination, such as RAD51 and BRCA1, code for proteins involved in DNA damage repair pathways, impacting radiosensitivity, and remain a challenging area for therapy and theranostic development [44, 45]. Alterations in clinically significant signalling pathways, such as the PI3K/AKT/mTOR pathway, are routinely dysregulated in HNC, which would improve cellular survival and proliferation in the context of radiation-induced stress, in conjunction with DNA repair processes, and contribute to radioresistance [46]. Immune-related factors also play a role; PD-L1 has been reported to increase the tumour immune milieu, which can influence the radiation response [47]. As per study, the understanding of molecular and immunological factors influencing therapeutic precision in HNC can be enhanced. Clinical decision-making, based on biomarkers of radiation response can maximise treatment efficacy, minimise resistance and improve overall survival and quality of life for patients [48].
Role of Genomics in Predicting Treatment Outcomes in HNC
Genomics innovations have provided an exceptional and groundbreaking paradigm shift in cancer care and treatment response prediction, including HNC. Genomic progress is crucial to developing more reliable and personalised cancer treatment protocols that rely on patients’ molecular profiles. HNC is a unique group of tumours known to be associated with changes in multiple genes or chromosomal loci that promote tumour initiation, progression, as well as treatment resistance [49]. Genetic changes will affect the patients’ tumour response to surgery, chemotherapy, radiotherapy, or immunotherapy. Due to recent innovations, clinicians will be better prepared to identify actionable genetic changes using newer technologies to acquire tumour tissue or tumour genomic profile, including blood tests with liquid biopsies and next-generation sequencing (NGS), that can incorporated into the individual decisions relying on the tumour’s molecular profile in real-time [50]. For example, one of the mutations that patients with HNC most frequently express is TP53, which has been associated with chemotherapy resistance and decreased outcomes [26, 39].
Additionally, another important chromosomal target is the epidermal growth factor receptor (EGFR) pathway that is associated with overexpressed EGFR in HNC. Therapy monoclonal antibodies to EGFR (anti-EGFR) offer some therapeutic efficacy for either primary or acquired therapy resistance [51]. Specific to the tumour profiling type, tumour response will more likely improve when the tumour molecular profile guides the tumour response.
The tumour microenvironment is also a crucial factor in determining the effectiveness of treatment. For instance, PD-L1+ immune cells have the potential to serve as predictive biomarkers for the efficacy of immunotherapy and help to identify patients who may receive the most benefit from these immune checkpoint inhibitor drugs [52]. In addition, the age of precision medicine allows genomic sequencing of functionally meaningful mutations to target even with medications. It can build more specificity for therapies to avoid ineffective treatments and unforeseen toxicities [53]. Ultimately, genetic information will be an invaluable tool for improving HNC treatment outcomes. Oncologists would be able to treat patients much more precisely for the specific tumour, can predict response to therapy, lessen side effects, and enhance quality of life and overall survival of patients [54].
Clinical Trials in Radiogenomics for HNC
Clinical investigations into radiogenomics are gaining traction as variables to potentially aid in the detection of cancer and personalise therapeutic strategies for enhanced patient outcomes. These studies are exploring the potential relationships between molecular markers such as HPV and p16, genetic variants such as TP53, EGFR, and PIK3CA, and imaging biomarkers from MRI, PET, or CT [55].
The primary objective of these clinical investigations is to exploit imaging information to predict treatment response, particularly amongst patients undergoing immunotherapy or radiation therapy [56]. Currently, studies are ongoing that focus on whether tumour shape, metabolic activity, or certain radiomic signals could be used as non-invasive surrogate markers to predict treatment response in the context of chemoradiation [57].
Beyond predictive studies, prognostic studies are evaluating the relationship between radiogenomic markers and overall survival, recurrence, or treatment resistance [6]. For example, the use of dynamic contrast-enhanced MRI to assess tumour hypoxia, shown to correlate with poor prognosis or treatment resistance, is also being connected to gene expression patterns to develop more precise risk stratification models [58].
Furthermore, adaptive treatment strategies, which involve changing the specific treatment strategies (e.g., radiation dose and timing) over time based on the tumour’s genetic profile and imaging response in real-time, are also a focus of active clinical trials [59]. When using these adaptive strategies, individualised alterations may implement enhanced outcomes or require less unnecessary toxicity.
In addition, some clinical trials are also combining artificial intelligence (AI) and machine learning (ML) strategies into radiogenomics. These techniques can process multi-gene imaging data points simultaneously into a more responsive, patient-specific treatment plan, aimed at individual patient needs [60]. In this manner, radiogenomics can significantly achieve the goals of precision oncology, or the practice of involving treatment decisions between biologies or an individual tumour type and the patient’s needs. In efforts to bridge the widening knowledge gap between radiology and genomics, radiogenomics should ideally assist with optimising treatment selection, improving outcomes as a result in a more desirable treatment plan, and most importantly, strengthening individual management practices of cancer [61].
Green Tea Extract as a Chemopreventive Agent
It is well recognised that green tea extract (GTE) has a high concentration of polyphenols, a group of compounds that have received considerable attention because of their chemopreventive potential [especially epigallocatechin gallate (EGCG)]. The health benefits of GTE are primarily attributed to its polyphenols’ potent antioxidant properties, which allow polyphenols to effectively scavenge reactive oxygen species (ROS), nitrogen radicals, superoxide anions, and metal ions (Table 1) [62].
| Mechanism/Effect | Molecular Target / Outcome | Relevant Studies |
| Antioxidant Activity | Scavenges ROS, protects DNA from oxidative stress | [79] |
| Anti-inflammatory and Anti-mutagenic | Reduces inflammatory signaling and mutagenic events linked to carcinogenesis | [59] |
| Radiosensitization | Enhances radiation-induced DNA damage, inhibits repair pathways, promotes apoptosis | [88] |
| Apoptosis Induction | Modulates Bax/Bcl-2 expression; activates caspase-3 and cytochrome c pathways | [82] |
| Cell Cycle Arrest | Inhibits proliferation through modulation of MAPK/ERK and PI3K/AKT/mTOR pathways | [80], [81] |
| Anti-angiogenesis | Inhibits VEGF signaling and MMP activity, particularly MMP-2 and MMP-9 | [55], [66] |
| EGFR Pathway Inhibition | Suppresses EGFR phosphorylation, disrupting downstream Akt/NF-κB signaling | [64], [65] |
| Suppression of Cancer Stem Cells (CSCs) | Targets CSCs involved in recurrence and metastasis | [90] |
| Epigenetic Modulation | Influences DNA methylation and histone acetylation | [79], [83] |
| Synergy with Other Therapies | Enhances effects of chemotherapeutics (e.g., 5-FU, cisplatin), EGFR inhibitors (e.g., erlotinib), and other polyphenols (e.g., curcumin, resveratrol) | [70], [74]–[77], [91] |
| Immunomodulation | Boosts T-cell and dendritic cell activity; reduces Tregs and MDSCs | [23], [87] |
EGCG is potentially the most well-characterised (and studied) polyphenolic compound due to its reported chemopreventive effects against head and neck squamous cell carcinoma (HNSCC) [63]. Overexpression of the epidermal growth factor receptor (EGFR) has been identified in up to 90% of HNC cases, and it is thought that EGCG impacts EGFR activity primarily by blocking EGFR phosphorylation, an important step in EGFR signalling [64]. The result is downstream inhibition of key signal transduction pathways that regulate cell survival, proliferation, and metastasis, such as Akt phosphorylation and NF-κB activation/translocation into the nucleus [65]. In addition, EGCG disrupts angiogenesis and inhibits migration into the tumour microenvironment by inhibiting production of vascular endothelial growth factor (VEGF) and subsequently blocking VEGF receptor (VEGFR) activation [66]. Moreover, EGCG has been shown to downregulate matrix metalloproteinases (MMPs), most notably MMP-2, and MMP-9, that have been positively correlated with aggressive and invasive oral malignancies ia inhibition of the MAPK pathway [56].
Preclinical studies, both in vitro and in vivo, consistently show that GTE has strong chemopreventive efficacy, particularly in EGCG [67]. An early study published in 1987 demonstrated that topical EGCG dramatically reduced tumour growth in animal models [68]. Oral administration of EGCG-containing GTE has also inhibited tumour initiation and metastasis [69]. In addition, EGCG has been shown to increase the cytotoxic effect of standard chemotherapeutic agents like 5-fluorouracil (5-FU) while preferentially inhibiting growth in HNC cell lines [70]. Early clinical trials demonstrated patients tolerated up to 1.0 g of GTE (approx. 7–8 cups of green tea) per day [71]. Additionally, high-risk oral cancer patients receiving high-dose GTE had better clinical response rates. However, there was no significant increase in cancer-free survival, indicating further optimisation of dose and treatment regimens is warranted [72].
The restricted bioavailability of EGCG is a major barrier when it comes to reaping benefits for translation into the clinic. The therapeutic effectiveness of EGCG is limited by the fact that only 12-28% of orally administered EGCG remains in plasma in unbound form [73]. Nevertheless, GTE has shown synergistic effects when used in combination with varied chemotherapeutic agents [74]. These combinations largely influence the therapeutic outcomes in preclinical animals using GTE with luteolin, genistein, curcumin, and cisplatin and EGFR inhibitors [75]. Furthermore, EGCG has been found to enhance the efficacy of chemotherapeutic agents such as vincristine sulfate by resensitising multidrug-resistant HNC cells to chemotherapeutics [76]. Combinations of EGCG and erlotinib (which is an EGFR inhibitor) have shown promising results as synergistic tumour-suppressive effects have been found in preclinical animal models of premalignant HNC, and are currently being evaluated in a modern phase I chemoprevention clinical trial [77, 78].
Mechanistic Insights of Green Tea Extract Against HNC
The anticancer ability of green tea extract (GTE) - particularly its dominant catechin epigallocatechin gallate (EGCG) has been extensively explored for head and neck cancers (HNC). Some of the suspected mechanisms of EGCG activity are: growth inhibition, angiogenesis inhibition, epigenetic modification, apoptosis induction, reduction in oxidative stress, and inhibition of metastasis; these are summarised in Figure 4 [76, 78].
Figure 4. Action Mechanism of Green Tea (EGCG) in Head and Neck Cancer Prevention and Therapy.
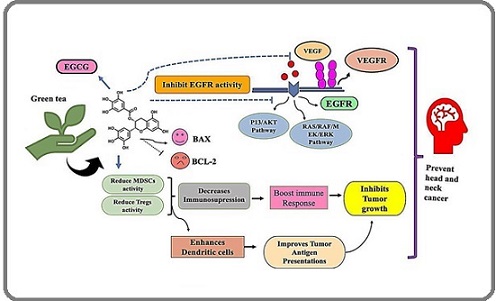
In preclinical studies, there is substantial evidence demonstrating that GTE catechins can decrease oxidative DNA damage and free radicals, which are two significant players in the initiation of carcinogenesis, especially in tissues that have been exposed to environmental carcinogens, as in head and neck tissues [79]. In vitro studies demonstrate that EGCG inhibits upstream receptors (blockade) in important signalling pathways, such as MAPK/ERK and PI3K/AKT/mTOR pathways, both of which are frequently dysregulated in HNC and cancers [80, 81]. EGCG may also induce apoptosis by regulating apoptotic proteins; EGCG has been shown to activate mediators of programmed cell death, cytochrome c and caspase-3, downregulate its anti-apoptotic counterpart Bcl-2, and upregulate anti-apoptotic Bax [82]. Furthermore, EGCG has significant anti-angiogenic activity by inhibiting vascular endothelial growth factor (VEGF) and matrix metallopeptidases (MMPs), thereby inhibiting the tumour supply [55].
Interestingly, EGCG inhibits the forced overexpression of progesterone receptors in tumour cells, thereby decreasing cancer cell invasion and migration [55, 72]. Moreover, data suggest that EGCG has epigenetic effects by altering DNA methylation and histone acetylation, modifying expression of genes involved in cancer development [79, 83].
EGCG has shown the ability to significantly reduce EGFR signalling, which is frequently over-expressed in HNSCC, in preclinical studies. Within tumour cells, EGCG signalling inhibition induces lower viability, increased apoptosis, and decreased cell proliferation [77, 84]. The effect of GTE regimen on prevention of metastases to lymph nodes is further verified by animal studies, demonstrating continuously decreased tumour volume, angiogenesis, and lymphatic spread [85]. Notably, EGCG has two roles: it increases oxidative stress in malignant tissues, making tumour cells more susceptible to standard therapies, while also promoting healthy cells with its antioxidant effects [86]. In HNC models, the chemosensitising and radiosensitising effect has been shown, indicating it is a promising adjunct to radiation and chemotherapy [18].
Beyond direct anticancer properties, EGCG has demonstrated immunomodulatory features, decreasing the immune-suppressive activity of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), while increasing activation of T and dendritic cells [23]. Due to its broad application as an anti-immunosenescence and anticancer agent, EGCG is recognised as a candidate agent in cancer immunotherapy [87, 91].
In conclusion, GTE catechins, with special note to EGCG, display diverse anticancer effects and roles as potential adjunct treatments for HNC . Additional clinical studies and optimisation of pharmacology will be needed to demonstrate their therapeutic potential and develop standard protocols for their inclusion in cancer treatment [92].
Chemoprevention Using Radiogenomics
Camellia sinensis (green tea), especially its extract (GTE), has particularly compelling potential in the chemoprevention of HNC. In particular, the potential of epigallocatechin gallate (EGCG) as a multi-targeted anticancer agent should be highlighted. The principal mechanism of GTE in head and neck cancer chemoprevention is its antioxidant capacity to reduce reactive oxygen species (ROS) and subsequent DNA damage; these two factors are paramount in preventing the initiation and progression of cancer [79]. EGCG also demonstrates anti-inflammatory, anti-mutagenic, and anti-angiogenic activities aimed at inhibiting tumour development and metastasis [59]. Notably, EGCG has demonstrated increased radiosensitivity in cancer cells by managing radiation-induced apoptosis, inhibiting DNA repair pathways, and enhancing DNA damage (Table 2) [88].
| Active Compound | Source | Type of Study | Anticancer Target | Molecular Mechanism | Reference |
| Green Tea Catechins (GTCs) | Camellia sinensis | In vitro, In vivo, Clinical | HNC Stem Cells, Tumour Invasion, Angiogenesis | Inhibition of VEGF, suppression of EMT, induction of apoptosis via XIAP degradation, modulation of Wnt/β-catenin signaling, sensitization to radiation | [82, 83] |
| Epigallocatechin Gallate (EGCG) | Camellia sinensis | In vitro, In vivo, Clinical | HNC Stem Cells, Tumour Invasion, Radiation Sensitization | Inhibition of VEGF, suppression of EMT, modulation of Wnt/β-catenin and Notch signaling pathways, induces apoptosis, enhances radiation-induced DNA damage | [84, 85] |
| Epicatechin (EC) | Camellia sinensis | In vitro | HNC Stem Cells, Tumour Growth | Antioxidant activity, inhibition of cancer cell proliferation, suppression of VEGF expression, enhances chemotherapy and radiotherapy effectiveness | [86, 87] |
| Epicatechin Gallate (ECG) | Camellia sinensis | In vitro, In vivo | HNC Stem Cells, Tumour Invasion | Modulates oxidative stress, suppresses inflammatory pathways (NF-κB), enhances radiation-induced cell death, inhibits tumour growth | [88, 89] |
| Catechin (C) | Camellia sinensis | In vitro, In vivo | HNC Stem Cells, Tumour Invasion, Radiation Sensitization | Suppresses stemness markers, inhibits NF-κB signaling, induces apoptosis, enhances radiosensitivity | [90, 92] |
| Green Tea Extract (GTE) | Camellia sinensis | In vitro, In vivo | HNC Stem Cells, Tumour Progression, Radiation Sensitization | Inhibits metastasis and invasion, suppresses CSC-like properties, potentiates radiation therapy by enhancing DNA damage response and apoptosis | [94] |
This could be a critical benefit when treating HNCs where resistance to traditional therapy is an issue. GTE catechins have been shown to manage cell survival signalling and target the cancer stem cell subpopulations responsible for tumour recurrence and treatment-related resistance, and regulate biological pathways managing both cell cycle and apoptosis pathways [89, 90].
An exciting and novel area of research is the convergence of radiogenomics and chemoprevention. Radiogenomics is the assessment of genetic variability relating to a patient’s response to radiation therapy, and a similar approach can be taken to identify genes that would alter treatment response to EGCG and other polyphenol chemicals. This could provide a personalised chemoprevention strategy.
Precision medicine aims to provide individualised healthcare to patients. Combination therapies of precision medicine can be developed through the integration of pharmacogenomics with GTE-based therapeutics. The integration of pharmacogenomics with GTE-based therapeutics would improve clinical outcomes, allow clinicians more refinements in dosing methodologies, and allow scientists to characterise the nature of EGCG interactions with DNA damage and/or repair pathways induced by radiation [92-94]. In summary, GTE-based chemoprevention and a radiogenomic-based approach is a novel method to optimise response to therapy, particularly refractory/head and neck cancer (HNC). The integration of genomic characterisation with the biological activity of natural products has great promise in the future of precision medicine and personalised oncology.
In discussing the potential of green tea extract polyphenols in head and neck cancer management, insights from studies on other natural compounds highlight their supportive roles in cancer care. For instance, Salek et al. (2021) demonstrated that crocin, a carotenoid compound, significantly reduced anxiety, depression, and chemotherapy-related toxicity in breast cancer patients, suggesting that plant-derived compounds can enhance patient quality of life during treatment. Similarly, Ebrahimi et al. (2024) found that crocin improved outcomes in esophageal squamous cell carcinoma, indicating its potential to mitigate treatment-related side effects, which may parallel the mechanisms of green tea polyphenols in alleviating toxicity and improving therapeutic efficacy in head and neck cancer [95-98].
In conclusion, this bibliometric analysis underscores the increasing significance of radiogenomics in the landscape of HNC research, while also amplifying the previously untapped therapeutic potential of bioactives from green tea extract (GTE), primarily EGCG. By integrating genomic profiling with modern imaging modalities, radiogenomics offers a strong tool for improving diagnosis, therapy planning, and personalised patient care in HNC. Likewise, EGCG has revealed a myriad of anticancer effects such as enhancing the efficacy of chemotherapy and radiation therapy, altering signalling pathways, and inhibiting tumour growth. Collectively, these findings suggest the opportunity for synergy between naturopathic options and radiogenomics.
Future projects should examine the molecular and radiomic interactions of EGCG through the lens of AI- based imaging technologies to strengthen predictive modelling and investigate precision patient stratification. Together, refinement of methodologies, larger-scale clinical trials, and collaboration across disciplines between nutrition scientists, geneticists, radiologists, and oncologists will be a principal factor to yield benefit in this field.
Significantly, the aim of the Sustainable Development Goal (SDG) is complemented by the integrative approach, particularly SDGs 3 (Good Health and Well-Being), 9 (Industry, Innovation and Infrastructure), 12 (Responsible Consumption and Production), and 17 (Partnerships for the Goals). This study supports the development of precision forms of holistic approaches to cancer treatment with the potential to increase rates of survival and enhance quality of life for individuals with head and neck cancer, aligning with the SDGs, which support healthy, good societal outcomes.
Acknowledgments
The authors thank Graphic Era (Deemed to be University), Dehradun, Uttarakhand, for all their support during this study.
Funding information
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of Interest
Author declares no conflict of interest
References
- World Health Organization (WHO). Global Cancer Statistics, 2023.https://www.who.int/news-room/fact-sheets/detail/cancer (accessed 27 January 2025) .
- Head and neck squamous cell carcinoma Johnson DE , Burtness B, Leemans CR , Lui VWY , Bauman JE , Grandis JR . Nature Reviews. Disease Primers.2020;6(1). CrossRef
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray F, Ferlay J, Soerjomataram I, Siegel RL , Torre LA , Jemal A. CA: a cancer journal for clinicians.2018;68(6). CrossRef
- Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer Vermorken JB , Remenar E, Herpen C, Gorlia T, Mesia R, Degardin M, Stewart JS , et al . The New England Journal of Medicine.2007;357(17). CrossRef
- Radiomics: Images Are More than Pictures, They Are Data Gillies RJ , Kinahan PE , Hricak H. Radiology.2016;278(2). CrossRef
- Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach Aerts HJWL , Velazquez ER , Leijenaar RTH , Parmar C, Grossmann P, Carvalho S, Bussink J, et al . Nature Communications.2014;5. CrossRef
- Radiomics: the bridge between medical imaging and personalized medicine Lambin P, Leijenaar RTH , Deist TM , Peerlings J, Jong EEC , Timmeren J, Sanduleanu S, et al . Nature Reviews. Clinical Oncology.2017;14(12). CrossRef
- Nutrition and cancer: a review of the evidence for an anti-cancer diet Donaldson MS . Nutrition Journal.2004;3. CrossRef
- Cancer prevention by tea: animal studies, molecular mechanisms and human relevance Yang CS , Wang X, Lu G, Picinich SC . Nature Reviews. Cancer.2009;9(6). CrossRef
- Tea polyphenols for health promotion Khan N, Mukhtar H. Life Sciences.2007;81(7). CrossRef
- Direct inhibition of insulin-like growth factor-I receptor kinase activity by (-)-epigallocatechin-3-gallate regulates cell transformation M. Li , Z. He , S. Ermakova , et al . Cancer Epidemiol. Biomarkers Prev.2007;16:598-605.
- Chemoprevention of mouse urinary bladder carcinogenesis by fermented brown rice and rice bran Kuno T, Hirose Y, Yamada Y, Hata K, Qiang SH , Asano N, Oyama T, et al . Oncology Reports.2006;15(3). CrossRef
- Green Tea and Its Extracts in Cancer Prevention and Treatment Schulze J, Melzer L, Smith L, Teschke R. Beverages.2017;3(1). CrossRef
- Long noncoding RNA repressor of adipogenesis negatively regulates the adipogenic differentiation of mesenchymal stem cells through the hnRNP A1-PTX3-ERK axis Pan Y, Xie Z, Cen S, Li M, Liu W, Tang S, Ye G, et al . Clinical and Translational Medicine.2020;10(7). CrossRef
- Green tea: an effective synergist with anticancer drugs for tertiary cancer prevention Fujiki H, Suganuma M. Cancer Letters.2012;324(2). CrossRef
- Phase I trial of GREEN TEA EXTRACT polyphenol (EGCG) and erlotinib in patients with high-risk oral premalignant lesions. Cancer Prev Res (Phila.) 2009;2:931–41 Tsao AS , Liu D, Martin J, et al . .
- Mechanisms of cancer prevention by tea constituents Lambert JD , Yang CS . The Journal of Nutrition.2003;133(10). CrossRef
- EGCG suppresses cellular proliferation by targeting molecular signaling pathways, Nutr Cancer. 2011;63:414–24 Chen D, Wan SB , Yang H, et al. . .
- Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications Singh BN , Shankar S, Srivastava RK . Biochemical Pharmacology.2011;82(12). CrossRef
- Effect of black and green tea polyphenols on c-jun phosphorylation and H(2)O(2) production in transformed and non-transformed human bronchial cell lines: possible mechanisms of cell growth inhibition and apoptosis induction Yang G. Y., Liao J., Li C., Chung J., Yurkow E. J., Ho C. T., Yang C. S.. Carcinogenesis.2000;21(11). CrossRef
- PI3K/Akt signalling pathway and cancer Fresno Vara JA , Casado E, Castro J, Cejas P, Belda-Iniesta C, González-Barón M. Cancer Treatment Reviews.2004;30(2). CrossRef
- GREEN TEA EXTRACT consumption and cancer prevention Schwartz GG , Kushi LH . Annu. Rev. Public Health.2002;23:245-267.
- New cancer treatment strategy using combination of green tea catechins and anticancer drugs Suganuma M, Saha A, Fujiki H. Cancer Science.2011;102(2). CrossRef
- Tea antioxidants in cancer chemoprevention Katiyar S. K., Mukhtar H.. Journal of Cellular Biochemistry. Supplement.1997;27.
- Angiogenesis inhibited by drinking tea Cao Y., Cao R.. Nature.1999;398(6726). CrossRef
- Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals Chow HS , Hakim IA , Vining DR , Crowell JA , Ranger-Moore J, Chew WM , Celaya CA , et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2005;11(12). CrossRef
- Radiomics: extracting more information from medical images using advanced feature analysis Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, Stiphout RGPM , Granton P, Zegers CML , et al . European Journal of Cancer (Oxford, England: 1990).2012;48(4). CrossRef
- Behind the numbers: Decoding molecular phenotypes with radiogenomics--guiding principles and technical considerations Kuo MD , Jamshidi N. Radiology.2014;270(2). CrossRef
- Quantitative imaging biomarkers: a review of statistical methods for technical performance assessment Raunig DL , McShane KM , Pennello G, Gatsonis C, Carson PL , Voyvodic JT , Wahl RL , et al . Statistical Methods in Medical Research.2015;24(1). CrossRef
- Radiomics in medical imaging-"how-to" guide and critical reflection Timmeren JE , Cester D, Tanadini-Lang S, Alkadhi H, Baessler B. Insights into Imaging.2020;11(1). CrossRef
- SUV as a biomarker for prognosis in head and neck cancer patients treated with chemoradiation, Head Neck. 2014;36:291–98. Baschnagel AM , Williams L, Hanna A. .
- Using Machine Learning Algorithms to Predict Immunotherapy Response in Patients with Advanced Melanoma Johannet P, Coudray N, Donnelly DM , Jour G, Illa-Bochaca I, Xia Y, Johnson DB , et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2021;27(1). CrossRef
- Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome O'Connor JPB , Rose CJ , Waterton JC , Carano RAD , Parker GJM , Jackson A. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2015;21(2). CrossRef
- Radiomics in head and neck cancer: from exploration to application Wong AJ , Kanwar A, Mohamed AS ., Fuller CD . Translational Cancer Research.2016;5(4). CrossRef
- Integration of imaging and genomic data to characterize tumor heterogeneity, Curr Opin Biotechnol. 2018;49:146–53. Parikh RR , Yip SS , Hu JC , et al . .
- Artificial intelligence in cancer imaging: Clinical challenges and applications Bi WL , Hosny A, Schabath MB , Giger ML , Birkbak NJ , Mehrtash A, Allison T, et al . CA: a cancer journal for clinicians.2019;69(2). CrossRef
- Radiomics in lung cancer for oncologists Pinta C, Barrios-Campo N, Sevillano D. Journal of Clinical and Translational Research.2020;6(4).
- Epigallocatechin Gallate (EGCG), an Active Phenolic Compound of Green Tea, Inhibits Tumor Growth of Head and Neck Cancer Cells by Targeting DNA Hypermethylation Agarwal A, Kansal V, Farooqi H, Prasad R, Singh VK . Biomedicines.2023;11(3). CrossRef
- Role of ERCC1, XRCC1, and NBS1 in predicting resistance to radiation therapy in head and neck cancer Liu Y, Song J, Chang X, et al . Int J Radiat. Oncol Biol Phys.2014;89:690-8.
- Evolving Role and Translation of Radiomics and Radiogenomics in Adult and Pediatric Neuro-Oncology Ak M., Toll S. A., Hein K. Z., Colen R. R., Khatua S.. AJNR. American journal of neuroradiology.2022;43(6). CrossRef
- Strategies to improve radiotherapy with targeted drugs Begg AC , Stewart FA , Vens C. Nature Reviews. Cancer.2011;11(4). CrossRef
- Genomics and the continuum of cancer care McDermott U, Downing JR , Stratton MR . The New England Journal of Medicine.2011;364(4). CrossRef
- p53 in health and disease Vousden KH , Lane DP . Nature Reviews. Molecular Cell Biology.2007;8(4). CrossRef
- DNA repair pathways as targets for cancer therapy Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA . Nature Reviews. Cancer.2008;8(3). CrossRef
- Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy Sharabi AB , Lim M, DeWeese TL , Drake CG . The Lancet. Oncology.2015;16(13). CrossRef
- Human papillomavirus and survival of patients with oropharyngeal cancer Ang KK , Harris J, Wheeler R, Weber R, Rosenthal DI , Nguyen-Tân PF , Westra WH , et al . The New England Journal of Medicine.2010;363(1). CrossRef
- Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1 Agrawal N, Frederick MJ , Pickering CR , Bettegowda C, Chang K, Li RJ , Fakhry C, et al . Science (New York, N.Y.).2011;333(6046). CrossRef
- The molecular landscape of head and neck cancer Leemans CR , Snijders PJF , Brakenhoff RH . Nature Reviews. Cancer.2018;18(5). CrossRef
- TP53 mutations and survival in squamous-cell carcinoma of the head and neck Poeta ML , Manola J, Goldwasser MA , Forastiere A, Benoit N, Califano JA , Ridge JA , et al . The New England Journal of Medicine.2007;357(25). CrossRef
- Platinum-based chemotherapy plus cetuximab in head and neck cancer Vermorken JB , Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, et al . The New England Journal of Medicine.2008;359(11). CrossRef
- Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck Ferris RL , Blumenschein G, Fayette J, Guigay J, Colevas AD , Licitra L, Harrington K, et al . The New England Journal of Medicine.2016;375(19). CrossRef
- A randomized phase III trial of oral S-1 plus cisplatin versus docetaxel plus cisplatin in Japanese patients with advanced non-small-cell lung cancer: TCOG0701 CATS trial Kubota K., Sakai H., Katakami N., Nishio M., Inoue A., Okamoto H., Isobe H., et al . Annals of Oncology: Official Journal of the European Society for Medical Oncology.2015;26(7). CrossRef
- Clinical analysis and interpretation of cancer genome data Van Allen EM , Wagle N, Levy MA . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2013;31(15). CrossRef
- Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology Limkin E. J., Sun R., Dercle L., Zacharaki E. I., Robert C., Reuzé S., Schernberg A., et al . Annals of Oncology: Official Journal of the European Society for Medical Oncology.2017;28(6). CrossRef
- Radiomic Machine-Learning Classifiers for Prognostic Biomarkers of Head and Neck Cancer Parmar C, Grossmann P, Rietveld D, Rietbergen MM , Lambin P, Aerts HJWL . Frontiers in Oncology.2015;5. CrossRef
- Current Issues in the Utility of Blood Oxygen Level Dependent MRI for the Assessment of Modulations in Tumor Oxygenation Baudelet C, Gallez B. Current Medical Imaging.2005;1(3). CrossRef
- Adaptive radiotherapy: State-of-the-art imaging and treatment planning Paganelli C, Whelan B, Peroni M, Paganelli C, Whelan B, Peroni M, et al . Clin Oncol.2018;30:662-73.
- Scinderin promotes the invasion and metastasis of gastric cancer cells and predicts the outcome of patients Liu J, Liu J, Chen J, Wu Y, Yan P, Ji C, Wang Y, et al . Cancer Letters.2016;376(1). CrossRef
- Novel clinical trial designs emerging from the molecular reclassification of cancer Nikanjam M, Kato S, Allen T, Sicklick JK , Kurzrock R. CA: A Cancer Journal for Clinicians.2025;75(3):243-67.
- The chemistry of tea flavonoids Balentine D. A., Wiseman S. A., Bouwens L. C.. Critical Reviews in Food Science and Nutrition.1997;37(8). CrossRef
- Studies on prevention of obesity, metabolic syndrome, diabetes, cardiovascular diseases and cancer by tea Yang CS , Wang H, Sheridan ZP . Journal of Food and Drug Analysis.2018;26(1). CrossRef
- Molecular Targets of Epigallocatechin-Gallate (EGCG): A Special Focus on Signal Transduction and Cancer Negri A, Naponelli V, Rizzi F, Bettuzzi S. Nutrients.2018;10(12). CrossRef
- Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo Thangapazham RL , Singh AK , Sharma A, Warren J, Gaddipati JP , Maheshwari RK . Cancer Letters.2007;245(1-2). CrossRef
- Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Cancer Research.2006;66(2). CrossRef
- A phase II trial of GREEN TEA EXTRACT extract in patients with asymptomatic early stage chronic lymphocytic leukemia Shanafelt TD , Call TG , Zent CS , et al . J Clin Oncol.2009;27:3808-3814.
- EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NFκB, and VEGF expression Gu JW , Makey KL , Tucker KB , Chinchar E, Mao X, Pei I, Thomas EY , Miele L. Vascular cell.2013;5(1):9.
- Piceatannol induces heme oxygenase-1 expression in human mammary epithelial cells through activation of ARE-driven Nrf2 signaling Lee H, Park S, Almazari I, Kim E, Na H, Surh Y. Archives of Biochemistry and Biophysics.2010;501(1). CrossRef
- An Elucidation of the Anti-Photoaging Efficacy and Molecular Mechanisms of Epigallocatechin Gallate Nanoparticles in a Balb/c Murine Model Xia F, Wang F, Kuo L, Huang P, Liu A, Wang G, Tang X, Guan K, Xie Y, Wang J. Foods.2025;14(13):2150.
- Green tea polyphenol EGCG causes anti-cancerous epigenetic modulations in acute promyelocytic leukemia cells Borutinskaitė V, Virkšaitė A, Gudelytė G, Navakauskienė R. Leukemia & Lymphoma.2018;59(2). CrossRef
- Dietary polyphenols may affect DNA methylation Fang M, Chen D, Yang CS . The Journal of Nutrition.2007;137(1 Suppl). CrossRef
- Natural Compounds in Prostate Cancer Prevention and Treatment: Mechanisms of Action and Molecular Targets Fontana F, Raimondi M, Marzagalli M, Di Domizio A, Limonta P. Cells.2020;9(2). CrossRef
- Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Cancer Research.2006;66(2). CrossRef
- Phase I trial of daily oral Polyphenon E in patients with asymptomatic Rai stage 0 to II chronic lymphocytic leukemia Shanafelt TD , Call TG , Zent CS , LaPlant B, Bowen DA , Roos M, Secreto CR , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2009;27(23). CrossRef
- Mechanisms of growth inhibition of human lung cancer cell line, PC-9, by tea polyphenols Okabe S., Suganuma M., Hayashi M., Sueoka E., Komori A., Fujiki H.. Japanese Journal of Cancer Research: Gann.1997;88(7). CrossRef
- Evolution of phenolic compounds from color and flavor problems to health benefits Soto-Vaca A, Gutierrez A, Losso JN , Xu Z, Finley JW . Journal of Agricultural and Food Chemistry.2012;60(27). CrossRef
- EGCG inhibits CSC-like properties through targeting miR-485/CD44 axis in A549-cisplatin resistant cells Jiang P, Xu C, Chen L, Chen A, Wu X, Zhou M, Haq IU , Mariyam Z, Feng Q. Molecular Carcinogenesis.2018;57(12). CrossRef
- Genetics and genomics of radiotherapy toxicity: towards prediction West CM , Barnett GC . Genome Medicine.2011;3(8). CrossRef
- EGCG enhances cancer cells sensitivity under 60Coγ radiation based on miR-34a/Sirt1/p53 Kang Q, Zhang X, Cao N, Chen C, Yi J, Hao L, Ji Y, Liu X, Lu J. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association.2019;133. CrossRef
- A Biological Perspective of TLR8 Signaling in Host Defense and Inflammation Bian F, Yan D, Wu X, Yang C. Infectious Microbes & Diseases.2023;5(2). CrossRef
- Epigallocatechin-3-gallate attenuates head and neck cancer stem cell traits through suppression of Notch pathway Lee SH , Nam HJ , Kang HJ , Kwon HW , Lim YC . European Journal of Cancer (Oxford, England: 1990).2013;49(15). CrossRef
- Synergistic inhibition of head and neck tumor growth by GREEN TEA EXTRACT extract and erlotinib Shimizu M, Adachi S, Masuda M, et al . Int J Cancer.2008;123(6):1480-1489.
- A comprehensive insight into apoptosis: molecular mechanisms, signaling pathways, and modulating therapeutics Mosadegh M, Noori Goodarzi N, Erfani Y. Cancer Investigation.2025;43(1):33-58.
- Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases Shimizu M, Adachi S, Masuda M, Kozawa O, Moriwaki H. Molecular Nutrition & Food Research.2011;55(6). CrossRef
- Anticarcinogenic potentials of tea catechins Li X, Liu C, Dong S, Ou C, Lu J, Ye J, Liang Y, Zheng X. Frontiers in Nutrition.2022;9. CrossRef
- Antioxidant effects of epicatechin in cancer treatment Ryu JH , Lee HJ , Seo JH , et al . Molecules.2011;25(14):3146.
- Epicatechin gallate modulates oxidative stress in cancer cells Huang Y, Zheng J, Mo J J, et al . Molecule.2011;25(14):3146.
- (‑)‑Epigallocatechin‑3‑gallate inhibits bladder cancer stem cells via suppression of sonic hedgehog pathway Sun X, Song J, Li E, Geng H, Li Y, Yu D, Zhong C. Oncology Reports.2019;42(1). CrossRef
- Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer Almatroodi SA , Almatroudi A, Khan AA , Alhumaydhi FA , Alsahli MA , Rahmani AH . Molecules (Basel, Switzerland).2020;25(14). CrossRef
- Effects of Green Tea Extract Epigallocatechin-3-Gallate on Oral Diseases: A Narrative Review Li Y, Cheng L, Li M. Pathogens (Basel, Switzerland).2024;13(8). CrossRef
- Cancer Prevention with Green Tea and Its Principal Constituent, EGCG: from Early Investigations to Current Focus on Human Cancer Stem Cells Fujiki H, Watanabe T, Sueoka E, Rawangkan A, Suganuma M. Molecules and Cells.2018;41(2). CrossRef
- Informing the new developments and future of cancer immunotherapy : Future of cancer immunotherapy Kumar A, Swain CA , Shevde LA . Cancer Metastasis Reviews.2021;40(2). CrossRef
- The Effect of Intragastric Gavage of High Dose Green Tea Extract on Serum Status of Magnesium, Calcium, and Zinc Khaleel AK , Shaari RB , Nawi MAA , Al-Yassiri AMH . Asian Pacific journal of cancer prevention: APJCP.2022;23(9). CrossRef
- Expression Level of Caspase Genes in Colorectal Cancer Asadi M, Shanehbandi D, Asvadi Kermani T, Sanaat Z, Zafari V, Hashemzadeh S. Asian Pacific journal of cancer prevention: APJCP.2018;19(5). CrossRef
- Anti-Cancer Effects of Green Tea by Either Anti- or Pro- Oxidative Mechanisms Hayakawa S, Saito K, Miyoshi N, Ohishi T, Oishi Y, Miyoshi M, Nakamura Y. Asian Pacific journal of cancer prevention: APJCP.2016;17(4). CrossRef
- A Randomized, Controlled, Parallel-Group, Trial on the Long-term Effects of Melatonin on Fatigue Associated With Breast Cancer and Its Adjuvant Treatments Sedighi Pashaki A, Sheida F, Moaddab Shoar L, Hashem T, Fazilat-Panah D, Nemati Motehaver A, Ghanbari Motlagh A, et al . Integrative Cancer Therapies.2023;22. CrossRef
- A Randomized, Controlled, Parallel-Group, Trial on the Effects of Melatonin on Fatigue Associated with Breast Cancer and Its Adjuvant Treatments Sedighi Pashaki A, Mohammadian K, Afshar S, Gholami MH , Moradi A, Javadinia SA , Keshtpour Amlashi Z. Integrative Cancer Therapies.2021;20. CrossRef
- Amelioration of anxiety, depression, and chemotherapy related toxicity after crocin administration during chemotherapy of breast cancer: A double blind, randomized clinical trial Salek R, Dehghani M, Mohajeri SA , Talaei A, Fanipakdel A, Javadinia SA . Phytotherapy research: PTR.2021;35(9). CrossRef
- Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Concurrent Use of Crocin During Chemoradiation for Esophageal Squamous Cell Carcinoma Ebrahimi N, Javadinia SA , Salek R, Fanipakdel A, Sepahi S, Dehghani M, Valizadeh N, Mohajeri SA . Cancer Investigation.2024;42(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2025
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times