Real World Safety, Survival Rate, and Effectiveness of Samarium-153 (153Sm) Administration in Metastatic Castration-Resistant Prostate Cancer (mCRPC) with Bone Metastases
Download
Abstract
Background: Painful bone metastases are common in advanced prostate cancer. We report the clinical outcome after administration of Samarium-153 (153Sm), an emitter of beta-particles that concentrates in the areas of enhanced osteoblastic activity.
Methods: This analysis included patients with confirmed mCRPC with bone metastases scheduled to receive 153Sm. All patients received 1 dose of 153Sm. Primary endpoints are short- and long-term safety, including incidence of bone marrow suppression. Secondary endpoints included 5 years survival rate.
Results: Patients were enrolled from 2018 to 2019, and was followed up for 5 years. Out of 12 patients, 3 patients were lost to follow-up, and 9 patients were included. 9 patients (100%) had bone metastases only and received 153Sm injections. Most patients (88.9%) had high-volume osteoblastic lesions. The mean hot spot intensity decreased from 826,239.9 to 623,844.2 post-therapy (p = 0.036). Pain relief was significant, with the VAS score dropping from a median of 5 to 2 one day post-treatment (p = 0.003), and further improving to 1 thirty days later (p = 0.002). Drug-related treatment-emergent adverse events (TEAEs) occurred in 0% patients. At the 60-month follow-up, only 22.2% patients were found to be alive. The median time to death from samarium treatment end was recorded as 18 months.
Conclusions: In this single-center cohort, 153Sm-EDTMP provided clinically meaningful pain palliation with acceptable safety. Survival estimates are descriptive of the underlying disease trajectory and should not be interpreted as a treatment effect given the non-comparative design and small sample. Prospective, multicenter studies using standardized pain and quality-of-life endpoints are warranted.
Introduction
Prostate cancer is the most common cancer in males, and it is a significant health issue in developed countries [1]. Advanced prostate cancer has been referred to by several names over time, such as hormone-resistant prostate cancer (HRPC) and androgen-insensitive prostate cancer (AIPC) [1]. Recently, the term CRPC or castration recurrent prostate cancer were coined to acknowledge the role of intracrine/paracrine androgen synthesis in the resistance of prostate cancer cells to testosterone suppression therapy [2]. The Prostate Cancer Working Group 2 introduced the term castrate resistant prostate cancer (CRPC) in 2008 to describe the condition where prostate cancer (PCa) continues to develop clinically and/or biochemically despite having very low levels of testosterone in the bloodstream [3]. The castrate environment is typically characterized by a serum testosterone levels that is consistently below 50 ng/ dL or 1.7 nmol/dL [3].
The bone microenvironment is an important topic of research because to its association with bone metastases, which are the predominant clinical manifestation of advanced prostate cancer and a significant cause of morbidity in cancer patients [4]. The bone microenvironment facilitates the spread of metastases in castrate-resistant prostate cancer due to intricate interactions between the milieu and tumor cells. Previous research has proposed that the two-way communication between the epithelium and host stromal cells in prostate cancer might be responsible for the emergence of resistance and distinct patterns of spread. Recent experimental findings have demonstrated that a particular group of malignancies, which do not exhibit the combined loss profile linked to the p53, PTEN, or Rb gene, are connected with distinct gene expression profiles. These profiles include genes that prevent cell death (antiapoptotic genes) and genes that facilitate the spread of tumors [3, 4].
Osteoblast-rich areas of the bone are the preferred environment of prostate cancer cells [1]. The interaction between prostate cancer cells and osteoblasts in bone leads to the disruption of bone structure and the establishment of a cycle of reciprocal stimulation, promoting the growth of both prostate cancer cells and osteoblasts. Bone metastases are the main cause of illness and death in patients with prostate cancer. They contain the pool of disease that is resistant to treatment and are the most common location of disease recurrence. Treatment options for castration-resistant metastatic prostate cancer (CRMPC) are currently restricted. Docetaxel is the sole therapy that extends life after castration resistance. It provides a short-term survival benefit of 2 to 3 months and is considered the standard treatment for patients with castration-resistant metastatic prostate cancer (CRMPC) [4, 5].
The occurrence of bone pain in advanced prostate and other cancers is a common and important consequence. The successful treatment of metastatic bone disease and its accompanying symptoms remains a serious challenge for patients and their doctors [6]. The choice of treatment is partially influenced by previous treatments. Typically, a combination of systemic and local modalities is employed due to the lack of long-term effectiveness of any single treatment regimen. This review discusses the three radionuclides, namely phosphorus-32, strontium-89, and samarium-153, that are now approved for treating bone pain. It emphasizes the effectiveness of samarium Sm 153 lexidronam, which belongs to the third-generation agents in this category [4].
One approach to target the bone microenvironment and treat tumors localized in the bone is by using radiopharmaceuticals that specifically seek out and bind to bone tissue [7]. Previous research has shown that samarium-153 is efficacious in treating diffuse bony metastatic illness, specifically in cases where there is no involvement of visceral organs. This treatment targets parts of the body with high bone turnover, such as osteoblastic metastases commonly found in prostate cancer patients. The alleviation of pain often manifests within a span of 1 to 2 weeks and can persist for a duration ranging from 2 to 6 months [5, 7]. However, the use of samarium-153 for bone pain metastases in prostate cancer are limited in few countries, thus further research are needed for knowing its efficacy of real world safety. Thus, our study reports the clinical outcome after administration of Samarium-153 (153Sm), an emitter of beta-particles that concentrates in the areas of enhanced osteoblastic activity.
Materials and Methods
This was a single center study of patients given 1.0 mCi/kg intravenous (i.v.) injections of Sm-153 for palliation of bone pain secondary to metastases to bone. The study was conducted on Hasan Sadikin hospital in Indonesia to patients with painful bone metastases who also received concomitant zoledronic acid and androgen deprivation therapy (ADT).
Major eligibility criteria included: confirmed primary prostate carcinoma with painful bone metastases, positive on radionuclide bone scan, recurrence after a response to antineoplastic therapy (hormonal, chemotherapy, radiotherapy or combination), and a signed institutional review board-approved informed consent form before the initiation of any study-related procedures.
Exclusion criteria included no prior anti-neoplastic therapy, a life expectancy of < 2 months, palliative radiation therapy within 6 weeks or chemotherapy within 4 weeks of dosing; WBC < 4000/ mm3, PLT < 100.000/ mm3, serum creatinine 2.0 mg/dL; serum bilirubin >2.0 mg/ dL. Patients were eligible for retreatment with Sm-153 if pain improved by Week 4 after initial treatment but subsequently recurred by Week 8 or later (provided adequate hematologic function was present) and followed up until 60 months. Patients were not eligible for repeated Sm-153 infusion if no improvement in bone pain was noted after initial administration and if either leukopenia or thrombocytopenia were present during follow up.
Patients were enrolled from 2018 to 2019, and was followed for 5 years. The data collected were including, patient age, patient gender, prior treatment, Drug-related treatment-emergent adverse events (TEAEs), The median time to death from samarium treatment end, baseline hematology (especially PLT and WBC), and patient mortality. All patients received 1 dose of 153Sm. Primary endpoints are short- and long-term safety, including incidence of bone marrow suppression. Secondary endpoints included 5 years survival rate. Analysis performed was done descriptively. Baseline characteristics of patients enrolled in the study are summarized in Table 1.
| Variables | N(%) = 9 patients |
| Age (mean, range) | 73,16 (56-84) |
| Sex | |
| · Men | 9 (100%) |
| ·Women | - |
| Primary Tumor | |
| · Prostate Cancer | 9 (100%) |
| Prior Treatment | |
| · Surgery | 1 (11,1%) |
| · Chemotherapy | 2 (22,2%) |
| · Radiotherapy | 3 (33,3%) |
| · Chemo plus radiotherapy | 1 (11,1%) |
| · Unknown | 2 (22,2%) |
| Osteoblastic lesion | |
| · 1-3 (low volume) | 1 (11,1%) |
| · >3 (high volume) | 8 (88,9%) |
| Hot spot intensity | |
| · Intensity at ROI before therapy | 826239.9+751237 |
| ·Intensity at ROI after therapy | 623844.2+563514.9 |
| Mean change | 202396+294144.5 |
| (p = 0.036) | |
| VAS score | |
| · Prior administration | 5 (4-7) |
| · 1 day post administration | 2 (1-7) |
| Z score (prior and day 1) | -2,956 (p=0.003) |
| · 30 days post administration | 1(1-2) |
| Z score (prior and day 30) | -3.088 (p=0.002) |
| Baseline hematology (Mean, SD) | |
| · Hemoglobin | 11.6 (2.6) |
| · WBCs | 7.4 (2.7) |
| ·Platelets | 267 (115) |
| Baseline Alkaline phosphatase of Prostate tumor | 505 (570) |
| Drug-related treatment-emergent adverse events (TEAEs) | 0 (0%) |
| Median time to death from samarium treatment end (in months) | 18 months |
| Incidence of bone marrow suppression | 4 (44,4%) |
| Complaints | |
| · Resistant bone pain | 5 (55,6%) |
| · Bone marrow suppression | 4 (44,4%) |
| Mortality Rate | 78.80% |
Results
The demographics and treatment history for patients who enrolled in this study was shown in Table 1 below. Patients were enrolled from 2018 to 2019, and was followed up for 5 years. Out of 12 patients, 3 patients were lost to follow-up, and 9 patients were included. 9 patients (100%) had bone metastases only and received 153Sm injections. The patients were all male patient, with mean age 73 years old. Of the entire patients, 3 (33,3%) had received radiation both for treatment of their primary disease and for treatment of bone metastases, whereas the other case was previously received chemotherapy (2/22,2%), and surgery (1/11,1).
Most patients (88.8%) had high-volume osteoblastic lesions (>3), while a small proportion (11.2%) had low-volume lesions (1-3). The mean intensity of the hot spot at the region of interest (ROI) decreased from 826,239.9 ± 751,237 before therapy to 623,844.2 ± 563,514.9 after therapy, with a significant mean change of 202,396 ± 294,144.5 (p = 0.036) (Table 2, Figure 1).
| Intensity on ROI before therapy | Intensity on ROI at least 3 months after therapy (count) | Change | P value |
| 826239.9 ± 751237.0 | 623844.2 ± 563514.9 | 202396 ± 294144.5 | 0.036 |
| Paired T-test |
Figure 1. Changes in the Intensity of Osteoblastic Lesion before and at Least 3 Months after Administration of 153 Samarium EDTMP Therapy .
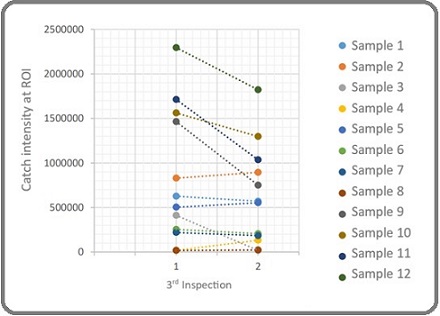
Regarding pain relief, the VAS score decreased from a median of 5 (range 4-7) prior to therapy to 2 (range 1-7) one day post-treatment, with a statistically significant Z-score of -2.956 (p = 0.003) (Table 3, Figure 2).
| VAS Before | VAS 1 day after administration | Z-score | P value |
| 5 (4-7) | 2 (1-7) | -2.956 | 0.003 |
| Wilcoxon test |
Figure 2. Changes in Respondents Pain Scale.
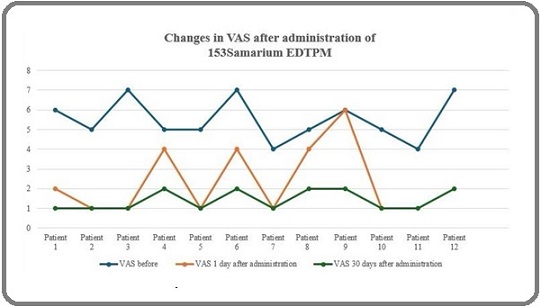
Thirty days post-treatment, the VAS score further improved to a median of 1 (range 1-2), with a significant Z-score of -3.088 (p = 0.002).
The baseline hematological values for the study participants were as follows: hemoglobin levels averaged 11.6 ± 2.6 g/dL, white blood cells (WBCs) were 7.4 ± 2.7 × 10³/µL, and platelets were 267 ± 115 × 10³/µL. The baseline alkaline phosphatase levels associated with prostate tumors were 505 ± 570 U/L.
No drug-related treatment-emergent adverse events (TEAEs) were reported. The median time to death following the end of Samarium treatment was 18 months. Bone marrow suppression was observed in 4 patients (44.4%), while complaints included resistant bone pain in 5 patients (55.6%) and bone marrow suppression in 4 patients (44.4%). The overall mortality rate in the study cohort was 77.8% with median survival 18 (95% CI 12.16-23.84) months (Figure 3).
Figure 3. Kaplan-Meier Curve for Survival.
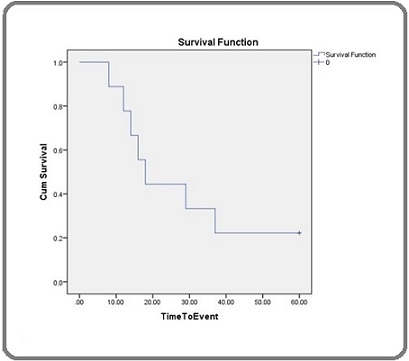
Given the absence of a comparator arm and potential selection biases, this survival estimates is presented for context only and do not imply a causal effect of ^153Sm on survival.
Discussion
Our findings reinforce the established role of ^153Sm- EDTMP as a palliative therapy for painful osseous metastases, with rapid pain relief and manageable toxicity. While we report descriptive survival outcomes to contextualize the cohort’s disease course, these data should be interpreted as reflecting disease biology and care patterns rather than a survival benefit attributable to^153Sm, given the retrospective, non-comparative design. Castrate-resistant prostate cancer (CRPC) is characterized by the advancement of the cancer despite the use of androgen deprivation therapy (ADT). This progression might manifest as a persistent increase in serum prostate-specific antigen (PSA) levels, the worsening of existing disease, and/or the emergence of new metastases. The treatment approaches vary and are illustrated in the Figure 4 below [2].
Figure 4. Proposed Approach for Patients with Castrate Resistant Prostate Cancer with Presently Available Agents. PSA, prostate-specific antigen; PSADT, PSA doubling time; CRPC, castrate-resistant prostate cancer.
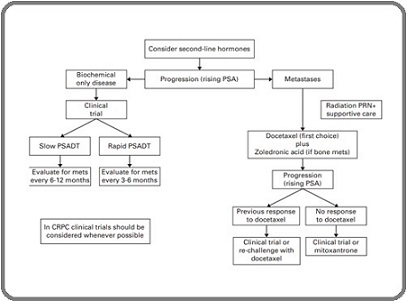
Prostate cancer-related bone metastases are frequently radiosensitive, and most men will feel some degree of pain relief when radiation is applied to a particular lesion. Research has demonstrated that administering a single dose is as effective to administering 5 separate doses in delivering relief from symptoms. Nevertheless, an increasing number of individuals necessitate additional treatment due to the reoccurrence of pain. Radio-isotopes may be investigated for certain patients with widespread bone discomfort. Due to the possibility of causing a decrease in bone marrow activity, it is necessary to have sufficient blood counts before starting treatment. The primary radioisotopes utilized are strontium and samarium. The primary benefit of samarium compared to strontium is its reduced scatter, resulting in less inhibition of the bone marrow [2].
Radionuclide therapy was developed as a substitute for external palliative radiotherapy in the management of bone discomfort caused by bone metastases of prostate cancer. The fundamental concept behind radionuclide treatment for pain is to ensure a substantial uptake of radioactive material in the tumor, resulting in a significant clinical impact, while keeping the dosage low enough to prevent significant adverse effects in other organs, typically the bone marrow. Samarium-153 ethylenediaminetetramethylenephosphonic acid (153SmEDTMP) is a radiopharmaceutical chemical that specifically binds to bone tissue and accumulates in regions where there is a higher rate of bone remodeling. It primarily localizes in the skeleton and is eliminated through the process of glomerular filtration. Sm-153 therapy can serve as a viable and secure treatment option for managing metastatic bone pain [4, 8].
Patients may receive repeated doses of samarium treatment. Repeated administration of 153Sm is both safe and efficacious. Previous research has demonstrated that patients with symptomatic bone metastases who receive repeated doses of 153Sm do not experience any notable disparities in pain relief or myelosuppression following a second or third therapy [9].
The contraindications for pain reduction using 153Sm therapy include pregnancy, lactation, acute spinal cord compression, a single metastatic lesion, renal failure, a long bone with more than 50% of affected bone metastases, a high risk of fracture, and the presence of disseminated intravascular coagulation [8, 9].
The number of osteoblastic lesions reflects the activity of cancer cells in the bone; the greater the number of bone lesions in prostate cancer patients, the more progressive the prostate cancer is. These osteoblastic lesions are linked to bone pain in patients with advanced prostate cancer. In this study, there was a significant decrease in the average intensity of osteoblastic lesions after therapy with 153 Samarium EDTMP compared to before therapy, as assessed by bone scan. This reduction in hot spot intensity suggests that Samarium-153 effectively targets areas of high osteoblastic activity, leading to a decrease in the overall burden of bone metastases.
Moreover, a reduction in pain, as measured by the Visual Analog Scale (VAS), was observed after treatment. One day post-administration, the VAS score decreased from an initial 5 (ranging from 4 to 7) to 2 (ranging from 1 to 7), with a statistically significant P-value of 0.003. This indicates that Samarium-153 provides effective short-term pain relief for patients with osteoblastic bone metastases. Further improvement was noted 30 days post-treatment, with the VAS score dropping to 1 (ranging from 1 to 2), and a P-value of 0.002, demonstrating sustained pain relief. These results suggest that Samarium-153 not only offers rapid pain relief but also maintains its efficacy over an extended period, thus significantly enhancing the quality of life for patients suffering from metastatic bone pain.
Samarium Sm-153 lexidronam has an affinity for skeletal tissue and concentrates in areas of enhanced osteoblastic activity. The radioisotope, with a half-life of 46.3 hours, emits a 103-kiloelectron volt (keV) gamma ray, suitable for external imaging, and a number of beta particles (average energy, 233 keV) appropriate for localized radiotherapy. Therefore, this drug has the potential to deliver high doses of localized radiation to regions adjacent to enhanced osteoblastic activity and supports the use of Samarium-153 as a viable treatment option for palliation in patients suffering from metastatic bone pain.
Prior study stated, the primary adverse effect linked to the use of samarium Sm-153 lexidronam is bone marrow suppression. The decline in leukocyte and platelet levels commences within 1-2 weeks following injection, reaches its lowest point by Week 3 or 4, and returns to the initial values by Week 8. Grade 3 or 4 toxic effects are observed in 10% of the patients, which is within an acceptable range in terms of safety. However, in our study bone marrow suppression was shown in 0% cases, in contrast to study by Sartor et al. [10] Sartor et al stated, Grade 2 or less WBC and PLT toxicities were observed in 92% and 97%, respectively, of patients administered 1.0 mCi/kg samarium Sm 153 lexidronam in the placebo-controlled studies [4, 5].
The results of this study indicate that the median time to death following Samarium-153 (153Sm) treatment was 18 months and the 5-year survival rate of 22.2% which provides insight into the disease trajectory in patients with bone metastasis due to castration-resistant prostate cancer (CRPC). While Samarium-153 was effective in providing pain relief for bone metastasis, this relatively short median survival and the low 5-year survival rate of 22.2% suggests that CRPC remains an aggressive and challenging cancer to control in the long term. Despite the efficacy of 153Sm in alleviating pain and improving quality of life in the short term, it is not sufficient to address the systemic progression of the disease. After treatment, while pain relief is often achieved, patients still face cancer progression, which leads to a decline in their condition relatively soon. This may also be influenced by several other factors, such as the patients’ overall health status, response to prior treatments, and the presence of other metastases [11]. In patients with CRPC, although palliative treatments like Samarium-153 can offer symptom relief, resistance to therapy and disease progression remain the primary challenges.
Pharmacological properties of SM-153 also giving several advantages. The short physical half-life of samarium Sm-153 lexidronam (1.9 days) offers the benefit of delivering a high dose rate over a brief, resulting in a greater biological effect and possibly explaining the early onset of pain relief. The shorter half-life of the radiopharmaceuticals may have several advantages. It allows for retreatment to be initiated earlier, chemotherapy to be resumed earlier, and the use of salvage medicines such colony-stimulating factors to be employed earlier [12-15].
This study is limited by its small sample size and single-center, retrospective design, which increases the risk of selection bias and residual confounding. We lacked a concurrent comparator (e.g., alternative radionuclide therapy or best supportive care), precluding causal inference for survival. Some potential confounders were incompletely captured, including detailed disease burden (e.g., quantitative skeletal tumor load), timing and lines of prior/concomitant systemic therapies (AR-axis inhibitors, taxanes), and granular performance status. Pain outcomes may be influenced by concurrent analgesic adjustments and heterogeneous documentation despite efforts to standardize abstraction. Finally, the sample size limits precision for safety estimates and subgroup analyses.
The future directions are to strengthen the evidence base, multicenter collaborations and prospective registries/ trials are needed to accrue larger cohorts, harmonize patient-reported pain and quality-of-life endpoints, and enable adjusted comparisons. Methodologic priorities include dosimetry-guided dosing, quantitative imaging of skeletal tumor burden, and incorporation of time- updated systemic therapies. Biologically rational combinations merit study including e.g., sequencing or pairing 153Sm with PSMA-targeted radioligand therapy (e.g., 177Lu-PSMA), AR-pathway inhibitors, or targeted external beam radiotherapy to explore synergy in pain control while monitoring marrow safety. Such studies should include comparator arms and pre-specified analgesic-sparing outcomes.
In conclusions, in this single-center cohort, 153Sm- EDTMP provided clinically meaningful pain palliation with acceptable safety. Survival estimates are descriptive of the underlying disease trajectory and should not be interpreted as a treatment effect given the non-comparative design and small sample. Prospective, multicenter studies using standardized pain and quality-of-life endpoints are warranted.
Acknowledgments
Statement of Transparency and Principals:
• Author declares no conflict of interest
• Study was approved by Research Ethic Committee of author affiliated Institute.
• Study’s data is available upon a reasonable request.
• All authors have contributed to implementation of this research
References
- Current perspectives on bone metastases in castrate-resistant prostate cancer Logothetis C, Morris MJ , Den R, Coleman RE . Cancer Metastasis Reviews.2018;37(1). CrossRef
- Guidelines for the management of castrate-resistant prostate cancer Saad F, Hotte SJ . Canadian Urological Association Journal = Journal De l'Association Des Urologues Du Canada.2010;4(6). CrossRef
- Definition of Castrate Resistant Prostate Cancer: New Insights Morote J, Aguilar A, Planas J, Trilla E. Biomedicines.2022;10(3). CrossRef
- Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer Sartor O, Reid RH , Hoskin PJ , Quick DP , Ell PJ , Coleman RE , Kotler JA , et al . Urology.2004;63(5). CrossRef
- Safety and efficacy of repeat administration of samarium Sm-153 lexidronam to patients with metastatic bone pain Sartor O, Reid RH , Bushnell DL , Quick DP , Ell PJ . Cancer.2007;109(3). CrossRef
- Pain in castration-resistant prostate cancer with bone metastases: a qualitative study Gater A, Abetz-Webb L, Battersby C, Parasuraman B, McIntosh S, Nathan F, Piault EC . Health and Quality of Life Outcomes.2011;9. CrossRef
- Phase I study of samarium-153 lexidronam with docetaxel in castration-resistant metastatic prostate cancer Morris MJ , Pandit-Taskar N, Carrasquillo J, Divgi CR , Slovin S, Kelly WK , Rathkopf D, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2009;27(15). CrossRef
- Samarium-153 Therapy and Radiation Dose for Prostate Cancer. Prostate Cancer - Leading-edge Diagnostic Proced Treat Parlak Y, Gumuser G, Sayit E. 2016.
- The role of bone-seeking radionuclides in the palliative treatment of patients with painful osteoblastic skeletal metastases Tomblyn M. Cancer Control: Journal of the Moffitt Cancer Center.2012;19(2). CrossRef
- Efficacy and Safety of Bone-Targeting Radioisotopes in Patients with Bone Metastases : A Meta-Analysis Ye W, Fan Y. 2021;:1-18.
- Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [177Lu]Lu-PSMA-617. A WARMTH multicenter study (the 617 trial) Ahmadzadehfar H, Rahbar K, Baum RP , Seifert R, Kessel K, Bögemann M, Kulkarni HR , Zhang J, et al . European Journal of Nuclear Medicine and Molecular Imaging.2021;48(1). CrossRef
- Samarium Sm-153 lexidronam for the palliation of bone pain associated with metastases Serafini A. N.. Cancer.2000;88(12 Suppl). CrossRef
- Erectile Dysfunction and Penile Bulb Dose Following Definitive Prostate Radiation Therapy Nouri M, , Kalantari Khandani M, Javadinia SA , Sedighi Pashaki A, Alemi M, et al . Int J Cancer Manag.2024;17(1):e147952. CrossRef
- A case report of prostate cancer with leptomeningeal metastasis Dehghani M, PeyroShabany B, Shahraini R, Fazilat-Panah D, Hashemi F, Welsh JS , Javadinia SA . Cancer Reports (Hoboken, N.J.).2022;5(8). CrossRef
- The Potential Diagnostic and Prognostic Value of Circulating MicroRNAs in the Assessment of Patients With Prostate Cancer: Rational and Progress Samami E, Pourali G, Arabpour M, Fanipakdel A, Shahidsales S, Javadinia SA , Hassanian SM , Mohammadparast S, Avan A. Frontiers in Oncology.2021;11. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2025
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times