Thyroglobulin and Antithyroglobulin Antibody Dynamics Following Radioactive Iodine Therapy in Post-Thyroidectomy Papillary Thyroid Carcinoma Patients
Download
Abstract
Background: In some cases of papillary thyroid carcinoma (PTC), residual tissues are observed to persistently after total thyroidectomy has been performed. In an attempt to provide further treatment, radioactive iodine (RAI) therapy is administered to obtain remission. Serum thyroglobulin (Tg) and antithyroglobulin antibodies (TgAb) are widely used biomarkers to evaluate treatment response. This study aimed to evaluate the dynamic changes in serum Tg and TgAb levels following RAI therapy in post-thyroidectomy PTC patients, and to determine the predictive value of preablation biomarkers on postablation outcomes.
Methods: This observational analytic study employed a prospective cohort design. It was conducted with 51 patients with PTC who have undergone total thyroidectomy. Tg and TgAb levels were measured both pre- and one-month post-ablation. Associations were analyzed using univariate and multivariate methods.
Result: The mean preablation Tg level was 82.2 ± 196.1 ng/mL, decreasing slightly to 73.7 ± 198.9 ng/mL post-ablation. However, Tg levels increased in 29.5% of patients following RAI therapy. A significant correlation was observed between preablation and postablation Tg levels (P < 0.001). Importantly, 56.9% of patients had persistently positive TgAb before and after ablation, with no observed change in status. Moreover, TgAb positivity showed no significant association with postablation Tg levels (P = 0.55), suggesting its limited utility as a post-therapy prognostic indicator.
Conclusion: Preablation Tg level shows a significant correlation with postablation Tg outcomes, supporting their predictive value. However, RAI therapy showed limited effectiveness in reducing TgAb levels in certain patients, with preablation Tg being a predictor of postablation levels.
Introduction
Thyroid carcinoma is the most common malignancy of the endocrine system [1-3], with papillary thyroid carcinoma (PTC) being the most prevalent subtype of differentiated thyroid carcinoma (DTC) [1, 2], It generally carries a favorable prognosis, particularly when managed with total thyroidectomy followed by adjuvant radioactive iodine (RAI) therapy [4]. Despite definitive surgery, residual thyroid tissue may remain, necessitating ablation for optimal disease control [5, 6]. In this context, serum thyroglobulin (Tg) is widely recognized as a reliable tumor marker to evaluate the presence of remnant tissue or recurrent disease [7-9]. Similarly, antithyroglobulin antibodies (TgAb) are increasingly studied for their potential prognostic implications, particularly in patients whose Tg measurements are unreliable due to antibody interference [10].
Although Tg dynamics post-RAI have been extensively studied, the behavior of TgAb remains less understood. Prior studies have suggested that a declining TgAb trend may indicate favorable prognosis, yet the clinical value of persistently positive TgAb remains unclear [11, 12]. Moreover, variations in assay methods, patient populations, and timing of post-therapy evaluation have contributed to inconsistencies in existing literature.
This study addresses a regional knowledge gap by examining Tg and TgAb dynamics in Indonesian PTC patients, a population that is underrepresented in global datasets. We also emphasize early Tg and TgAb responses within one-month post-RAI, a time frame less frequently analyzed in prior studies. Importantly, we explore whether preablation Tg levels can serve as a short-term predictor of ablation success, and whether TgAb status offers meaningful insight in this early period.
The objective is to evaluate the effectiveness of RAI therapy based on early biomarker changes and to determine the prognostic value of Tg and TgAb levels in a cohort from Indonesia.
Materials and Methods
Study Design
This study was a prospective observational cohort conducted at Abdul Wahab Sjahranie Hospital, Samarinda, Indonesia, from June 2018 to February 2021. The study aimed to analyze the dynamics of Tg and TgAb in post- thyroidectomy PTC patients receiving standardized RAI therapy.
Participants
Eligible participants were adults (≥18 years) with histopathologically confirmed PTC who had undergone total thyroidectomy and received at least one cycle of RAI therapy. Exclusion criteria included the presence of distant metastases or any diagnosed autoimmune disease prior to surgery, to reduce confounding effects on TgAb levels.
Sample Size Justification
A total of 76 patients were initially enrolled based on a sample size calculation using the Slovin formula [13-15]. Following the exclusion of cases with incomplete data, 51 subjects were included in the final analysis.
Intervention Protocol
Following total thyroidectomy, patients received suppressive levothyroxine therapy, which was withdrawn four weeks prior to RAI to allow for TSH elevation. Thyroid scintigraphy with I-131 was performed to confirm adequate uptake. A standardized dose of 85 mCi sodium iodide (I-131) was then administered. Serum Tg and TgAb levels were assessed twice: at baseline (preablation) and at one month post-RAI.
Time Interval Consideration
Tg and TgAb levels were reassessed one month post- ablation to capture early biochemical changes. While longer follow-up may better reflect long-term remission, early post-RAI trends can still offer valuable insight into short-term therapeutic response, as supported by prior literature.
Biomarker Measurement
Tg and TgAb were measured using an enzyme-linked immunosorbent assay (ELISA) method. The assay sensitivity was 0.04 ng/mL for Tg and detectable up to 5000 ng/mL; for TgAb, the assay range was 0–4000 IU/mL, with values >115 IU/mL considered positive. Although thyroid-stimulating hormone (TSH) levels were not consistently available across all patients, levothyroxine withdrawal and clinical monitoring ensured an appropriate hypothyroid state prior to RAI administration.
Data Handling and Validation
Data were collected and initially entered using Microsoft Excel 2010. To ensure data integrity and minimize transcription errors, double data entry was performed independently by two trained data clerks. Discrepancies were resolved through cross-checking against the original clinical records. Statistical analyses were subsequently performed using SPSS version 25.0 (IBM Corp., Chicago, IL, USA).
Statistical Analysis
Continuous variables were evaluated for normality and are presented as means ± standard deviation (SD) or medians where appropriate. Tg levels were categorized into three groups: <1 ng/mL, 1–10 ng/mL, and >10 ng/ mL. TgAb status was categorized as positive or negative. Categorical data were analyzed using chi-square or Fisher’s exact test. Correlations between pre- and postablation Tg levels were assessed using univariate and multivariate analyses. A P value < 0.05 was considered statistically significant.
Results
A total of 76 patients were initially identified, of whom 51 met inclusion criteria and had complete data for analysis. The cohort was predominantly female (82.4%), with a mean age of 55.7 ± 8.6 years. Tumor characteristics and other clinicopathological features are summarized in Table 1.
| Variables | n (%) |
| Age (year) | |
| mean ± SD | 55.7 ± 8.6 |
| min – max | 41 – 57 |
| Sex n (%) | |
| Male | 9 (17.6) |
| Female | 42 (82.4) |
| Primary Tumor n (%) | |
| T1 | 36 (70.6) |
| T2 | 4 (7.8) |
| T3 | 9 (17.6) |
| T4 | 2 (3.9) |
| Regional Lymph Nodes n (%) | |
| NX atau N0 | 40 (78.4) |
| N1 | 11 (21.6) |
| Antithyroglobulin | |
| Positive | 29 (56.9) |
Changes in Tg Levels Post-RAI
The mean preablation Tg level was 82.2 ± 196.1 ng/ mL, which decreased slightly to 73.7 ± 198.9 ng/mL following RAI therapy. This average decline is illustrated in Figure 1, which presents a bar graph of Tg values before and after ablation, with error bars representing standard deviation.
Figure 1. Bar graph and box plot showing the characteristics of pre- and postablation Tg serum levels (mean ± SD preablation Tg = 82.2 ± 196.1 ng/mL and mean ± SD postablation Tg = 73.7 ± 198.9 ng/mL).
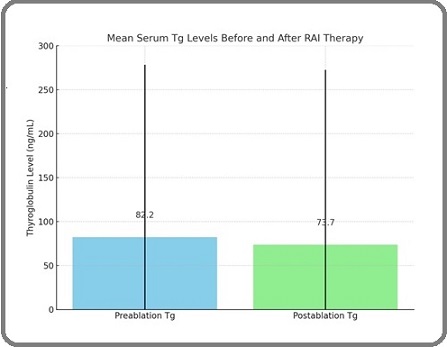
Although the group-level average showed a decrease, individual responses varied substantially. A total of 25 patients (49%) maintained Tg levels <1 ng/ mL both before and after RAI, while 15 patients (29.5%) experienced an increase in Tg following therapy, and only 1 patient (1.9%) demonstrated a decrease in Tg to a lower category post-RAI. These findings suggest that absolute average reductions obscure relative variations across individuals. The relationship between pre- and postablation Tg levels is detailed in Table 2, which shows a statistically significant correlation (P < 0.001, chi-square test). Notably, all patients with preablation Tg>10 ng/mL remained above that threshold post-therapy, indicating that pre-treatment Tg may serve as a predictor of persistence.
| Postablation Tg (ng/mL) | p-value * | ||||
| < 1 | 1 – 10 | > 10 | |||
| Preablation Tg (ng/mL) | < 1 | 19 (76.0) | 0 (0.0) | 6 (24.0) | 0.55 |
| 1 – 10 | 1 (9.1) | 1 (9.1) | 9 (81.9) | ||
| > 10 | 0 (0.0) | 0 (0.0) | 15 (100) |
Note: *Chi-square test shows p < 0.001; Tg: thyroglobulin
TgAb Dynamics
TgAb was positive in 29 of 51 patients (56.9%) prior to ablation and remained persistently positive at one month post-RAI in all cases. Among those with postablation Tg <1 ng/mL, 64.0% were TgAb positive, while 46.7% of patients with Tg ≥10 ng/mL were also TgAb positive. However, the association between TgAb status and postablation Tg was not statistically significant (P = 0.55), as shown in Table 3.
| Variable | TgAb | p-value * | ||
| Negative | Positive | |||
| Postablation Tg | < 1 | 9 (36.0) | 16 (64.0) | 0.55 |
| (ng/mL) | 1-10 | 5 (45.4) | 6 (54.6) | |
| ≥ 10 | 8 (53.3) | 7 (46.7) |
Note: *Chi-square test
These findings suggest that TgAb status did not predict short-term therapeutic response in this cohort.
Exploratory Multivariate Analysis
To assess potential confounders, a logistic regression model was constructed to identify predictors of high postablation Tg levels (>10 ng/mL). Variables included age, tumor stage, lymph node status, and TgAb positivity. Only preablation Tg emerged as a significant independent predictor (OR 3.21; 95% CI: 1.58–6.91; P = 0.004).
Other variables did not reach statistical significance. TSH levels were excluded due to incomplete data in the majority of subjects.
Discussion
The findings of this study reinforce the role of Tg as a reliable biomarker in evaluating the early biochemical response following RAI therapy in patients with PTC. As observed in this cohort, preablation Tg levels were significantly correlated with postablation Tg outcomes, consistent with the findings of Kim et al. [16], who reported that elevated pre-Tg was predictive of persistent disease. The utility of Tg as an early indicator of ablation efficacy is further supported by studies that suggest threshold values such as 6 ng/mL or 10 ng/mL may inform post-therapy risk stratification [17, 18].
The present study observed no statistically significant association between TgAb status and postablation Tg levels, echoing the findings of Dewi et al. and Kim et al. [19, 20], who noted that TgAb may persist for months even years after successful ablation. These persistent antibodies are not necessarily indicative of treatment failure but may reflect immunologic memory or slow clearance. Notably, all TgAb-positive patients in our cohort remained positive at one month post-RAI, underscoring the limited value of TgAb as a short-term response marker.
One limitation of our study lies in the timing of postablation biomarker measurement, which was conducted at one month. While this early window was chosen to evaluate short-term dynamics, some studies suggest that Tg and TgAb levels stabilize more reliably at 3–6 months post-RAI [20-23]. Thus, interpretation of short-term values must be made cautiously, and longitudinal monitoring remains essential in confirming disease status or remission.
In terms of clinical application, our findings support the use of preablation Tg as a prognostic marker, which may help clinicians identify patients at higher risk of incomplete ablation. Conversely, the lack of early TgAb decline should not be interpreted as treatment failure, particularly within the first few months post-therapy. Follow-up protocols may benefit from delaying reliance on TgAb kinetics until a later stage in the surveillance timeline. For TgAb-positive patients, clinicians should consider adjunct imaging or alternate surveillance markers, especially given the possibility of assay interference. Immunometric assays, such as ELISA and IRMA, are known to underestimate Tg in the presence of TgAb, potentially resulting in falsely low Tg values and underestimation of disease burden [19, 20].
Although formal ATA risk stratification was not part of the original study design, we performed a post hoc assessment using available tumor size, lymph node status, and histopathological features. The majority of patients in our cohort would likely fall under the low-risk ATA category, as most had T1 tumors with no nodal involvement. Our findings, particularly the strong correlation between preablation and postablation Tg levels in these patients are consistent with previous studies reporting favorable outcomes in low-risk PTC patients. However, due to the small number of patients with higher-stage disease, the prognostic implications of Tg and TgAb dynamics across ATA risk levels could not be robustly compared. Future studies with stratified cohorts are warranted to validate risk-based differences in early biomarker response.
This study has several important limitations. First, the relatively small sample size (n = 51) may reduce the statistical power to detect meaningful differences, especially in subgroup analyses. Second, while an exploratory multivariate analysis was attempted, a comprehensive risk-adjusted model could not be fully implemented due to missing data on key variables such as TSH levels and RAI dose standardization. Third, limited detail was provided on the analytical performance of the Tg assay, such as its sensitivity, functional detection limit, and inter-assay variability, which may affect the interpretability and reproducibility of Tg measurements. Additionally, potential confounding factors, including variations in TSH stimulation and adequacy of RAI dosing, were not fully accounted for and could have influenced the biomarker responses observed.
Despite these constraints, this study adds valuable insight by presenting early Tg and TgAb dynamics in Indonesia PTC cohort, a population that remains underrepresented in existing literature. It highlights the importance of individualized interpretation of biomarker trends and the limitations of relying solely on antibody kinetics in early surveillance. Future studies should aim for larger, multicenter cohorts with serial follow-up to validate these early findings and better define optimal timelines for clinical decision-making.
In conclusion, preablation Tg levels were significantly associated with postablation Tg outcomes, indicating their value as early predictors of RAI therapy response in PTC. In contrast, TgAb remained persistently positive and showed no correlation with postablation Tg, limiting their short-term prognostic utility. These findings are limited by the small sample size, short follow-up interval, and potential assay interference in TgAb-positive patients. Further studies with larger cohorts and longer follow-up are needed to confirm these results and guide clinical decision-making.
Competing interests
No competing interests were reported.
Funding
Self-funding
Ethics approval
The study was approved by the Research Ethics Committee of the Abdoel Wahab Sjahranie, Samarinda, Indonesia, number: No: 270/KEPK-AWS/I/2021. We promised that the participants’ data were anonymized or maintained with confidentiality, the rights or interests of participants were not invaded, and informed consent was taken from all individual participants.
References
- Long-Term Effect of TIMP3 Gene Expression on Thyroid Cancer: A Cure Model Analysis Dindarloo MM , Fendereski A, Kashi Z, Yazdani-Charati J. Asian Pacific journal of cancer prevention: APJCP.2024;25(10). CrossRef
- Serum Biomarkers in Thyroid Malignancies: Evaluating Thyroid-Stimulating Hormone Receptor (TSHR) and Vascular Adhesion Protein-1 (VAP-1) as Potential Diagnostic Biomarker Shihab RN , Zain Elabdeen AAE , Jabbar SA . Asian Pacific journal of cancer prevention: APJCP.2025;26(3). CrossRef
- Epidemiology of Thyroid Cancer in Kazakhstan and in Areas Adjacent to the Former Semipalatinsk Nuclear Test Site in 2013-2023 Espenbetova MZ , Bidakhmetova AM , Krykpayeva AS , Yespenbetova BA , Toleutayeva DM , Serikbayev AS , Tukinova AR , Uasheva LB . Asian Pacific journal of cancer prevention: APJCP.2025;26(2). CrossRef
- Long-Term Survival Rate for Moroccan Patients with Differentiated Thyroid Cancer Tabiti H, Guensi A, Bendahhou K. Asian Pacific journal of cancer prevention: APJCP.2025;26(3). CrossRef
- Radioactive Iodine Therapy for Thyroid Malignancies. In: StatPearls. Treasure Island (FL): StatPearls Publishing Palot Manzil FF , Kaur H. 2025.
- Residual Thyroid Tissue on Postoperative Diagnostic 131 I Radioactive Whole-Body Scan After Surgery in Differentiated Thyroid Cancer: A Tertiary Referral Centre Experience Prasad R, Rao V, Subash A, Majumdar KS , Sinha P, Kallur K, Nayar RC . Indian Journal of Surgical Oncology.2022;13(1). CrossRef
- Use of thyroglobulin as a tumour marker Indrasena BSH . World Journal of Biological Chemistry.2017;8(1). CrossRef
- Low correlation between serum thyroglobulin and 131I radioiodine whole body scintigraphy: implication for postoperative disease surveillance in differentiated thyroid cancer Thai JN , De Marchena IR , Nehru VM , Landau E, Demissie S, Josemon R, Peti S, Brenner AI . Clinical Imaging.2022;87. CrossRef
- THYROGLOBULIN AS A TUMOR MARKER IN DIFFERENTIATED THYROID CANCER - CLINICAL CONSIDERATIONS Prpić M, Franceschi M, Romić M, Jukić T, Kusić Z. Acta Clinica Croatica.2018;57(3). CrossRef
- Thyroglobulin levels before radioactive iodine therapy and dynamic risk stratification after 1 year in patients with differentiated thyroid cancer Bandeira L, Padovani RDP , Ticly AL , Cury AN , Scalissi NM , Marone MMS , Ferraz C. Archives of Endocrinology and Metabolism.2017;61(6). CrossRef
- Prognostic Significance of Thyroglobulin Antibodies in Differentiated Thyroid Cancer Reverter JL , Rosas-Allende I, Puig-Jove C, Zafon C, Megia A, Castells I, Pizarro E, Puig-Domingo M, Granada ML . Journal of Thyroid Research.2020;2020. CrossRef
- Analysis of the prognostic value of thyroglobulin antibody change trends during follow-up after 131I treatment in patients with differentiated thyroid carcinoma Ge H, Chen W, Lin Z, Li Y, Chen S. Frontiers in Oncology.2025;15. CrossRef
- Factors associated with Leptospira serodiagnosis in febrile patients at public health centers in Makassar, Indonesia: a cross-sectional study Cahyaningtyas C, Muslich LT , Madjid B, Sultan AR , Hamid F, Hatta M. The Pan African Medical Journal.2024;49. CrossRef
- Comparing the adaptive capacity of traditional irrigated rice fields farmers in urban and rural areas to climate change in Bali, Indonesia Sriartha IP , Giyarsih SR , Purnamawati IGA . Cogent Soc Sci.2023;9:2275936. CrossRef
- Calculating the Sample Size in Quantitative Studies Adhikari GP . Sch J.2021;:14-29. CrossRef
- Undetectable pre-ablation thyroglobulin levels in patients with differentiated thyroid cancer: it is not always what it seems Pitoia F, Bueno MF , Abelleira E, Salvai ME , Bergoglio L, Luster M, Niepomniszcze H. Arquivos Brasileiros De Endocrinologia E Metabologia.2013;57(4). CrossRef
- Antithyroglobulin Antibody as a Marker of Successful Ablation Therapy in Differentiated Thyroid Cancer Dewi AR , Darmawan B, Kartamihadja AHS , Hidayat B, Masjhur JS . World Journal of Nuclear Medicine.2017;16(1). CrossRef
- Age and prognosis of papillary thyroid carcinoma: retrospective stratification into three groups Cho JS , Yoon JH , Park MH , Shin SH , Jegal YJ , Lee JS , Kim HK . Journal of the Korean Surgical Society.2012;83(5). CrossRef
- Clinical implications of anti-thyroglobulin antibody measurement before surgery in thyroid cancer Jo K, Lim D. The Korean Journal of Internal Medicine.2018;33(6). CrossRef
- Thyroid carcinoma Tuttle RM , Ball DW , Byrd D, Dilawari RA , Doherty GM , Duh Q, Ehya H, et al . Journal of the National Comprehensive Cancer Network: JNCCN.2010;8(11). CrossRef
- Differences in the Impact of Age on Mortality in Well-Differentiated Thyroid Cancer Yan H, Winchester DJ , Prinz RA , Wang C, Nakazato Y, Moo-Young TA . Annals of Surgical Oncology.2018;25(11). CrossRef
- Utility of Stimulated Thyroglobulin in Reclassifying Low Risk Thyroid Cancer Patients' Following Thyroidectomy and Radioactive Iodine Ablation: A 7-Year Prospective Trial Jammah AA , Masood A, Akkielah LA , Alhaddad S, Alhaddad MA , Alharbi M, Alguwaihes A, Alzahrani S. Frontiers in Endocrinology.2020;11. CrossRef
- The Relationship Between Serum Levels of Thyroglobulin Antibody and the Risk of Recurrence in Patients With Differentiated Thyroid Cancer Fatemeh A, Mohammad Ali Y, Hassan M, Masoud M, Parvin L, Leila V, Maliheh DM , Zahra A, Ghazaleh T. Cancer Reports (Hoboken, N.J.).2025;8(5). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2025
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times