Supplementary Antioxidants for Preventing Cancer: Beneficial or Detrimental?
Download
Abstract
The global market for supplementary antioxidants, currently valued at approximately USD 4.88 billion, is projected to double by 2030. Despite this growing investment, the clinical efficacy and safety of these supplements in cancer prevention remain contentious. This review evaluates the evidence for key antioxidant supplements, including beta-carotene, coenzyme Q10, folic acid, glutathione, selenium, vitamin C, vitamin E, and zinc, examining their roles in cancer prevention based on randomized controlled trials, systematic reviews, and experimental studies. While dietary antioxidants consistently show protective effects, supplemental forms often fail to replicate these benefits and, in some cases, increase cancer risk. For instance, beta-carotene and vitamin E are associated with increased cancer incidence in certain populations. Conversely, limited evidence suggests potential benefits of zinc, glutathione, and coenzyme Q10, though further high-quality research is needed. This review underscores the complexity of antioxidant supplementation and emphasizes prioritizing dietary sources for cancer prevention. It calls for rigorous studies to clarify the clinical and molecular impacts of these widely used supplements.
Introduction
The role of antioxidants in cancer prevention has garnered significant attention, particularly given the well-documented benefits of fresh fruits and vegetables in reducing different cancers risk [1-5]. These foods are rich in naturally occurring antioxidants, which help mitigate oxidative damage, a key factor in carcinogenesis [6]. Motivated by these findings, researchers and the supplement industry have sought to replicate these benefits through antioxidant supplements.
Despite their promise, concerns persist about the clinical efficacy and potential detrimental effects of these supplements. While some studies suggest that specific supplements may confer protective effects [7], more others highlight risks, such as increased cancer incidence in certain populations [8-10]. This controversy is further compounded by the vast and heterogeneous literature on the topic, encompassing varying study designs, populations, and outcomes.
In addition to clinical uncertainties, the economic burden of antioxidant supplements is non-trivial, with billions spent annually worldwide despite inconsistent evidence supporting their benefits [11]. This review aims to provide a comprehensive analysis of the clinical and mechanistic evidence surrounding antioxidant supplements in cancer prevention, evaluating their potential benefits and risks while emphasizing the need for evidence-based recommendations. By examining the literature critically, we hope to clarify the complexities of this topic and inform better health decisions.
Materials and Methods
To provide a comprehensive overview of the clinical impacts of antioxidant supplements in cancer prevention, a systematic literature search was conducted across PubMed, MEDLINE, Embase, and Web of Science databases. The search included studies published up to January 2025, with results limited to articles written in English. The keywords used in the search encompassed “antioxidant supplements,” “cancer prevention,” “randomized controlled trials,” “systematic review”, and their equivalent terms.
The retrieved studies were evaluated and categorized based on the Oxford Centre for Evidence-Based Medicine (OCEBM) 2011 Levels of Evidence [12]. Each selected study was critically assessed for quality, relevance, and findings to synthesize evidence on the benefits or risks of supplementary antioxidants.
This mini-review primarily focuses on key antioxidant supplements, including beta-carotene, coenzyme Q10, folic acid, glutathione, selenium, vitamin C, vitamin E, and zinc. Priority was given to randomized controlled trials (RCTs) and systematic reviews of RCTs as the highest levels of evidence. In cases where such studies were unavailable, lower levels of evidence, including observational studies and case-control studies, were considered to ensure comprehensive data collection and conclusions.
Clinical Evidence
Supplementary ß- Carotene: Detrimental (level of evidence: 1)
Evidence does not support the preventive role of supplementary ß-carotene in preventing cancer. Nevertheless, clinical evidence has demonstrated its carcinogenic effect in specific contexts. The CARET study was conducted on a hypothesis that ß-carotene- rich fruits and vegetables can reduce cancer incidence. This study examined the preventive role of daily 30 mg ß-carotene and 25,000 IU vitamin A in preventing lung cancer. Surprisingly, the study was terminated due to 28% more cancer incidence in the experimental arm (relative risk [RR] 1.36; 95%CI: 1.07–1.73) [9]. A similar study, the ATBC trial, investigated the preventive role of lower dose of ß-carotene in lung cancer. This phase III clinical trial demonstrated that ß-carotene supplementation (20 mg per day) significantly increased the risk of lung cancer development (RR 1.16, 95%CI 1.02–1.33), particularly among heavy smokers (RR 1.25, 95%CI 1.07–1.46) and individuals with high alcohol consumption (RR 1.35, 95%CI 1.01–1.81) [10]. A recent met-analysis on eight RCTs confirmed that supplementary ß-carotene increases the risk of lung cancer (RR 1.19, 95%CI 1.08–1.32). This negative effect was not found for other cancer types [13].
Supplementary Coenzyme Q10: Beneficial (level of evidence: 2)
Clinical evidence evaluating the role of coenzyme Q10 (CoQ10) supplementation in cancer prevention is limited. In a randomized controlled trial involving 120 participants with precancerous lesions identified through Pap smears, Modarres Gilani et al. reported that a three-month supplementation regimen with multivitamins, minerals, and CoQ10 significantly reduced the risk of cervical cancer. The Pap smear normalization rate was 83% in the experimental group compared to 53% in the placebo group [14]. However, the exact composition of the multivitamin and mineral supplements used in the study was not disclosed, complicating the interpretation of these results and making it difficult to isolate the role of CoQ10. Conversely, Chai et al. reported an association between higher pre-diagnostic serum CoQ10 levels and an increased incidence of breast cancer in postmenopausal women [15]. However, these observations must be interpreted cautiously because of potential confounding by higher rate of smoking and alcohol consumption in the case group. Smoking- and ethanol-induced oxidative stress could lead to increased consumption of CoQ10, potentially distorting the results by reducing serum CoQ10 levels in the case group [16-18].
These conflicting findings highlight the need for more robust clinical trials to clarify the role of CoQ10 supplementation in cancer prevention and to determine whether its effects are beneficial, detrimental, or potentially context-dependent.
Supplementary Folic Acid: No Effect (level of evidence: 1)
Clinical evidence indicates no preventive effect of folic acid supplements on cancer development. A comprehensive meta-analysis of 49,621 participants across 13 clinical trials demonstrated that folic acid supplementation has no significant impact on the incidence of site-specific cancers within the first 5 years of treatment (RR 1.06, 95% CI 0.99–1.13) [19]. Similarly, another meta-analysis of 11 clinical trials found that folic acid supplements did not reduce colorectal cancer (CRC) incidence (RR 1.07, 95% CI 0.86–1.43) [20]. In contrast, dietary folate appears to play a protective role in CRC prevention. A recent meta-analysis of 931,469 participants revealed that for every 100 μg increase in dietary folate intake, the risk of CRC significantly decreases (RR 0.97, 95% CI 0.95–0.99). This protective effect was sustained with a daily dietary folate intake of up to 500 μg [21]. Therefore, while folic acid supplementation offers no significant cancer prevention benefits, dietary folate intake may help reduce CRC.
Supplementary Glutathione: Beneficial (level of evidence: 3) The clinical benefits of supplemental glutathione in cancer prevention remain largely unexplored, with, to our knowledge, the study by Fan et al. being the only investigation on this topic. In this study, a six-month course of supplemental glutathione prevented the progression of lung nodules to lung tumors in all 30 participants and reduced the size of lung nodules in 13 participants (43.3%) [7].
Supplementary Selenium: No Effect (level of evidence: 1)
According to the most recent Cochrane review, selenium supplementation neither decreases nor increases the overall risk of cancer. This meta-analysis, which included 27,232 participants across 83 studies, reported an overall RR for selenium intake and all cancer types of 1.01 (95% CI 0.93–1.10). Similarly, no significant benefit was observed for specific cancer types, including colorectal cancer (RR 0.99, 95% CI 0.69–1.43), non-melanoma skin cancer (RR 1.16, 95% CI 0.30–4.42), lung cancer (RR 1.16, 95% CI 0.89–1.50), breast cancer (RR 2.04, 95% CI 0.44–9.55), bladder cancer (RR 1.07, 95% CI 0.76–1.52), and prostate cancer (RR 1.01, 95% CI 0.90–1.14) [22].
Supplementary Vitamin C: No Effect (level of evidence: 1)
The role of vitamin C in cancer prevention has been extensively studied, with a clear distinction between its effects when consumed through dietary sources, mainly fresh fruits and vegetables, versus supplementation. Dietary sources of vitamin C consistently demonstrate strong associations with reduced cancer risk. A recent meta-analysis reported significant reductions in the risk of breast cancer (RR 0.72, 95% CI 0.60–0.85), colorectal cancer (RR 0.55, 95% CI 0.42–0.73), and prostate cancer (RR 0.88, 95% CI 0.77–1.00) with higher intake of vitamin C-rich foods. However, no clinical benefit was found for supplemental vitamin C [23]. Another meta-analysis on seven RCTs including 62,619 participants did not found clinical benefit for vitamin C supplements in cancer prevention (RR 1.00, 95% CI 0.95–1.05) [24]. A recent umbrella review concluded that higher dietary vitamin C intake is associated with a reduced risk of several cancer types, including breast, endometrial, esophageal, gastric, prostate, and total cancer risk. This benefit was not demonstrated in case of supplemental vitamin C [25]. In summary, while vitamin C from dietary sources provides a protective effect against various cancers, supplementation has not demonstrated similar efficacy.
Supplementary Vitamin E: Detrimental (level of evidence: 2)
In contrast to the protective effects of dietary vitamin E in cancer prevention [26], supplemental vitamin E has been associated with an increased risk of cancer development. In August 2001, the SELECT trial was initiated to evaluate the preventive efficacy of supplemental vitamin E and selenium in reducing the risk of prostate cancer. Surprisingly, after a 10-year follow-up of 35,533 participants, the study revealed a significant increase in prostate cancer incidence among those in the experimental group receiving only vitamin E supplements (hazard ratio [HR] 1.17, 99% CI 1.004–1.36) [8]. Another RCT found no clinical benefit in reducing the risk of total cancer, as well as breast, colon, and lung cancers, with the administration of 600 IU of vitamin E supplements [27].
Supplementary Zinc: Beneficial (level of evidence 4)
Emerging evidence suggests that zinc supplementation may have a protective role against certain cancers. In two retrospective cohort studies, Hosui et al. demonstrated that zinc supplementation significantly reduced the incidence of hepatocellular carcinoma (HCC) in patients with chronic liver disease (3-year HCC incidence: 7.6% in the zinc group vs. 19.2% in the non-zinc group) and in patients with treated HCV-positive infections (3-year HCC incidence: 0% in the zinc group vs. 5.6% in the non-zinc group) [28, 29]. Additionally, two case-control studies reported an association between high serum zinc levels and a reduced risk of lung [30] and breast cancers, particularly in BRCA1 mutation carriers [31].
Experimental Evidence
This section discusses on how different supplementary antioxidants prevent or even increase cancer formation (Figure 1).
Figure 1. Overview of the Beneficial and Detrimental Effects of Supplementary Antioxidants on Cellular PathwaysandCancerProgression.Beneficialantioxidants, such as CoQ10, glutathione, and zinc, enhance immune responses, inhibit epithelial-mesenchymal transition (EMT), activate apoptosis, and support mitochondrial metabolism and DNA repair. Conversely, detrimental antioxidants, including β-carotene and vitamin E, can disrupt cellular homeostasis by depleting mild-to-mod- erate reactive oxygen species (ROS) levels, generating pro-oxidant tocopheroxyl radicals, and suppressing immune functions, potentially leading to DNA mutations, mitochondrial dysfunction, and cancer development. Neutral antioxidants, such as vitamin C, folic acid, and selenium, exhibit neither significant beneficial nor harmful effects on cancer-related pathways.
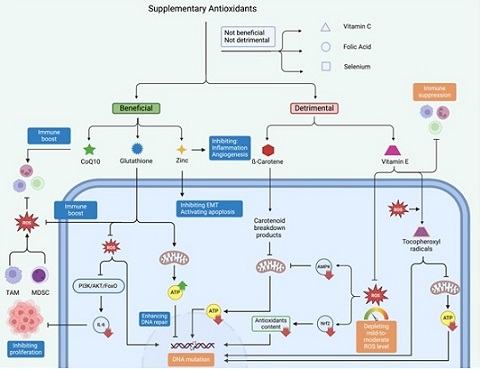
ß-Carotene: Mitochondria are the primary source of ATP in eukaryotic cells. Emerging evidence has linked mitochondrial dysfunction in human cells to cancer formation [32]. Routine daily activities can expose normal cells to various carcinogens, including radiation, chemicals, and biological agents, which can lead to genetic mutations. Under physiologic conditions, human cells maintain DNA integrity through the activity of several ATP-dependent enzymes such as XRCC1, PARP-1, ATM, and DNA ligases [33]. As such, factors impairing mitochondrial function can potentially enhance the risk of carcinogenesis.
Studies have shown that carotenoid breakdown products (CBPs) can disrupt mitochondrial function. Siems et al. demonstrated that CBPs impair mitochondrial membrane potential, leading to dysfunction of the adenine nucleotide translocator (ANT). This dysfunction hinders ATP export to the cytosol, reducing nuclear ATP levels and thereby impairing the activity of DNA integrity enzymes [34]. ß-Carotene can be degraded into CBPs either enzymatically or non-enzymatically through interactions with reactive oxygen species (ROS) [35]. This non-enzymatic degradation may explain why ß-carotene has more detrimental effects in smokers and individuals with high alcohol consumption [10], as both smoking and alcohol induce oxidative stress [36, 37].
Coenzyme Q10: Modarres Gilani et al. demonstrated that oral supplements containing CoQ10 effectively contributed to the regression of precancerous cervical lesions [14]. This beneficial outcome may be attributed to the immune-enhancing properties of CoQ10, which potentially support cancer regression by protecting tumor-associated lymphocytes from oxidative DNA damage [38, 39].
Glutathione: Fan et al. demonstrated that glutathione supplementation effectively prevents the progression of lung nodules to lung tumors. The study showed that glutathione restores mitochondrial metabolism and lowers serum IL-6 levels by reducing cellular ROS and inhibiting the PI3K/AKT/FoxO signaling pathway [7].
Additionally, glutathione may aid in nodule regression by activating anti-tumor immune cells. Within the tumor microenvironment (TME), myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs) can suppress the anti-tumor immune response by releasing ROS [40]. This suppression occurs through the nitration of T-cell receptors by MDSCs and the downregulation of the CD3ζ chain on T cells by TAMs [41, 42]. High levels of glutathione can counteract these effects, creating an environment conducive to a robust anti-tumor immune response. Therefore, similar to CoQ10, supplementary glutathione can prevent the progression of preliminary cancer by boosting the immune system. Therefore, similar to CoQ10, supplemental glutathione may help prevent the progression of early-stage cancer by enhancing the immune system.
Vitamin E: While vitamin E is widely recognized for its antioxidant properties, excessive intake may paradoxically promote cancer formation through several mechanisms. Low-to-moderate levels of ROS are essential for normal cellular physiology, contributing to key processes such as signaling and metabolism. These include the Nrf2/Notch pathway, which regulates the physiological antioxidant response [43], the AMPK/PGC- 1α signaling pathway, which helps restore ATP levels to preserve DNA integrity [44, 45], and pathways involved in immune regulation [46]. As such, excessive vitamin E intake can disrupt these regulatory pathways, leading to DNA mutations and mitochondrial dysfunction, thereby increasing the risk of cancer formation (see above).
Furthermore, excess vitamin E can act as a pro-oxidant by generating tocopheroxyl radicals during interactions with ROS [47]. These radicals can damage critical cellular components, such as DNA and mitochondria. This disruption undermines the cell’s ability to maintain metabolic homeostasis and DNA stability, fostering conditions that promote cancerous transformations.
Zinc: Valenzano et al. showed that oral zinc gluconate influenced gene expression, favoring reduced inflammation, suppression of epithelial-mesenchymal transition (EMT), activation of apoptosis, and inhibition of angiogenesis in the context of Barrett’s esophagus in humans [48]. In another study, Bai et al. demonstrated that zinc can prevent lung cancer formation through slowing telomere attrition and interaction with 8 cancer-related genes [30].
In conclusions, this review highlights the varying effects of supplementary antioxidants on cancer prevention, ranging from no overall benefits to potential detrimental effects or context-dependent protective roles. Supplements like beta-carotene and vitamin E have shown increased risks of cancer in specific contexts, supported by high levels of evidence. Others, such as folic acid and selenium, demonstrate no significant impact. In contrast, supplements like CoQ10, glutathione, and zinc suggest beneficial effects, albeit with limited or lower-quality evidence.
Notably, the protective effects of dietary antioxidants are consistently supported, emphasizing the importance of obtaining these nutrients through natural sources. The findings underscore the complexity of antioxidant interactions in cancer prevention and the critical need for high-quality research to guide evidence-based recommendations. Prioritizing whole-food dietary sources over supplementation remains the most reliable approach to optimize health benefits.
Declarations
CRediT authorship contribution statement: F.TH: Conceptualization, Supervision, Investigation, Writing- Original draft preparation, Writing- Reviewing and Editing; P.F: Visualization and Writing- Original draft preparation
Ethics approval and consent to participate: Not applicable. This article contains no studies performed by authors with human participants or animals. It is a narrative review, synthesizing insights from previously published articles.
Clinical trial number
Not applicable.
Data Availability Statement
No data was collected or analyzed for this review article. All information provided is based on a comprehensive review of relevant published literature.
Conflicts of Interest
All authors declare no competing interests.
Funding
This study was not supported by any external funding
References
- Fruit and vegetable intake in relation to gastric cancer risk: A comprehensive and updated systematic review and dose-response meta-analysis of cohort studies Naemi Kermanshahi M, Safaei E, Tutunchi H, Naghshi S, Mobarak S, Asadi M, Sadeghi O. Frontiers in Nutrition.2023;10. CrossRef
- Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies Liu J, Wang J, Leng Y, Lv C. International Journal of Cancer.2013;133(2). CrossRef
- Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies Aune D, Lau R, Chan DSM , Vieira R, Greenwood DC , Kampman E, Norat T. Gastroenterology.2011;141(1). CrossRef
- Fruits, vegetables and breast cancer risk: a systematic review and meta-analysis of prospective studies Aune D., Chan D. S. M., Vieira A. R., Rosenblatt DAN , Vieira R., Greenwood D. C., Norat T.. Breast Cancer Research and Treatment.2012;134(2). CrossRef
- Dietary Factors and Risk of Glioma in Adults: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies Zhang W, Jiang J, Li X, He Y, Chen F, Li W. Frontiers in Nutrition.2022;9. CrossRef
- “Natural Antioxidants for the Prevention and Treatment of Cancer,” in Handbook of Oxidative Stress in Cancer: Therapeutic Aspects, S. Chakraborti, Ed., Singapore: Springer Nature Singapore O. Cioanca , I.-D. Morariu , L. Hritcu . 2022;:277–289. CrossRef
- Glutathione inhibits lung cancer development by reducing interleukin-6 expression and reversing the Warburg effect Fan C, Chen G, Reiter RJ , Bai Y, Zheng T, Fan L. Mitochondrion.2024;79. CrossRef
- Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Klein EA , Thompson IM , Tangen CM , Crowley JJ , Lucia MS , Goodman PJ , Minasian LM , et al . JAMA.2011;306(14). CrossRef
- Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial Omenn G. S., Goodman G. E., Thornquist M. D., Balmes J., Cullen M. R., Glass A., Keogh J. P., et al . Journal of the National Cancer Institute.1996;88(21). CrossRef
- Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance Albanes D., Heinonen O. P., Taylor P. R., Virtamo J., Edwards B. K., Rautalahti M., Hartman A. M., et al . Journal of the National Cancer Institute.1996;88(21). CrossRef
- Grand View Research, “Personalized Vitamins Market Size, Share & Trends Analysis Report By Application (Wellness Supplements, Disease-Based Supplements), By Dosage Form (Tablets, Capsules), By Distribution Channel, By Age Group, By Region, And Segment Forecasts, 2025 - 2030.” Accessed: Jan. 19, 2025. [Online]. Available: https://www.grandviewresearch.com/industry-analysis/personalized-vitamins-market-report#.
- “The 2011 Oxford CEBM Level of Evidence.” Oxford Centre for Evidence-Based Medicine Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, Moschetti L, et al . Accessed: Jan. 15, 2025. [Online]. Available: http://www.cebm.net/index.aspx?o=5653..
- Association between β-carotene supplementation and risk of cancer: a meta-analysis of randomized controlled trials Zhang Y, Yang J, Na X, Zhao A. Nutrition Reviews.2023;81(9). CrossRef
- The Effect of Multivitamins, Minerals, and Q10 on Precancerous Lesions of the Cervix Gilani MM , Mosavi AS , Akhavan S, Zamani M, Mohsenpour M, Mohsenpour F, Dehghan AP , Farhadi D, Torkzaban F. International Journal of Women’s Health and Reproduction Sciences.2021;9(4).
- Plasma coenzyme Q10 levels and postmenopausal breast cancer risk: the multiethnic cohort study Chai W, Cooney RV , Franke AA , Shvetsov YB , Caberto CP , Wilkens LR , Le Marchand L, et al . Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology.2010;19(9). CrossRef
- Reduced coenzyme Q(10) in female smokers and its association with lipid profile in a young healthy adult population Al-Bazi MM , Elshal MF , Khoja SM . Archives of medical science: AMS.2011;7(6). CrossRef
- Ethanol-induced oxidative stress: basic knowledge Comporti M, Signorini C, Leoncini S, Gardi C, Ciccoli L, Giardini A, Vecchio D, Arezzini B. Genes & Nutrition.2010;5(2). CrossRef
- Alcohol depletes coenzyme-Q(10) associated with increased TNF-alpha secretion to induce cytotoxicity in HepG2 cells Vidyashankar S, Nandakumar KS , Patki PS . Toxicology.2012;302(1). CrossRef
- Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals Vollset SE , Clarke R, Lewington S, Ebbing M, Halsey J, Lonn E, Armitage J, et al . Lancet (London, England).2013;381(9871). CrossRef
- Folic acid intake and folate status and colorectal cancer risk: A systematic review and meta-analysis Moazzen S, Dolatkhah R, Tabrizi JS , Shaarbafi J, Alizadeh BZ , Bock GH , Dastgiri S. Clinical Nutrition (Edinburgh, Scotland).2018;37(6 Pt A). CrossRef
- The association between dietary folate intake and risk of colorectal cancer incidence: A systematic review and dose‒response meta-analysis of cohort studies Khalighi Sikaroudi M, Soltani S, Kolahdouz-Mohammadi R, Imanifard R, Abdollahi S, Shahinfar H, Mohammadi Farsani G. Heliyon.2024;10(13). CrossRef
- Selenium for preventing cancer Vinceti M, Filippini T, Del Giovane C, Dennert G, Zwahlen M, Brinkman M, Zeegers MP , et al . The Cochrane Database of Systematic Reviews.2018;1(1). CrossRef
- The association between vitamin C and breast cancer, prostate cancer and colorectal cancer: A systematic review and meta-analysis Arshadi M, Ghazal N, Ghavidel F, Beygi Z, Nasiri Z, Zarepour P, Abdollahi S, Azizi H, Khodamoradi F. Clinical nutrition ESPEN.2025;65. CrossRef
- Efficacy of Vitamin C Supplements in Prevention of Cancer: A Meta-Analysis of Randomized Controlled Trials Lee B, Oh S, Myung S. Korean Journal of Family Medicine.2015;36(6). CrossRef
- Vitamin C Intake and Cancers: An Umbrella Review Chen Z, Huang Y, Cao D, Qiu S, Chen B, Li J, Bao Y, et al . Frontiers in Nutrition.2021;8. CrossRef
- Vitamin E and Multiple Health Outcomes: An Umbrella Review of Meta-Analyses Xiong Z, Liu L, Jian Z, Ma Y, Li H, Jin X, Liao B, Wang K. Nutrients.2023;15(15). CrossRef
- Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial Lee I, Cook NR , Gaziano JM , Gordon D, Ridker PM , Manson JE , Hennekens CH , Buring JE . JAMA.2005;294(1). CrossRef
- Long-Term Zinc Supplementation Improves Liver Function and Decreases the Risk of Developing Hepatocellular Carcinoma Hosui A, Kimura E, Abe S, Tanimoto T, Onishi K, Kusumoto Y, Sueyoshi Y, et al . Nutrients.2018;10(12). CrossRef
- Oral Zinc Supplementation Decreases the Risk of HCC Development in Patients With HCV Eradicated by DAA Hosui A, Tanimoto T, Okahara T, Ashida M, Ohnishi K, Wakahara Y, Kusumoto Y, et al . Hepatology Communications.2021;5(12). CrossRef
- Circulating essential metals and lung cancer: Risk assessment and potential molecular effects Bai Y, Wang G, Fu W, Lu Y, Wei W, Chen W, Wu X, et al . Environment International.2019;127. CrossRef
- “Zinc and breast cancer risk,” Hered Cancer Clin Pract K. Kaczmarek , et al . vol. 10.2012;no. Suppl 4:p. A6. CrossRef
- Mitochondrial dysfunction route as a possible biomarker and therapy target for human cancer Al-Faze R, Ahmed HA , El-Atawy MA , Zagloul H, Alshammari EM , Jaremko M, Emwas A, Nabil GM , Hanna DH . Biomedical Journal.2025;48(1). CrossRef
- Mitochondrial Metabolism: A New Dimension of Personalized Oncology Behnam B, Taghizadeh-Hesary F. Cancers.2023;15(16). CrossRef
- Beta-carotene breakdown products may impair mitochondrial functions--potential side effects of high-dose beta-carotene supplementation Siems W, Wiswedel I, Salerno C, Crifò C, Augustin W, Schild L, Langhans C, Sommerburg O. The Journal of Nutritional Biochemistry.2005;16(7). CrossRef
- Carotenoid metabolism: New insights and synthetic approaches Stra A, Almarwaey LO , Alagoz Y, Moreno JC , Al-Babili S. Frontiers in Plant Science.2022;13. CrossRef
- Oxidative stress and inflammation: elucidating mechanisms of smoking-attributable pathology for therapeutic targeting Addissouky TA , El Sayed IET , Ali MMA , Wang Y, El Baz A, Elarabany N, Khalil AA . Bulletin of the National Research Centre.2024;48(1). CrossRef
- Alcohol-Induced Oxidative Stress and the Role of Antioxidants in Alcohol Use Disorder: A Systematic Review Tsermpini EE , Plemenitaš Ilješ A, Dolžan V. Antioxidants (Basel, Switzerland).2022;11(7). CrossRef
- Enrichment of coenzyme Q10 in plasma and blood cells: defense against oxidative damage Niklowitz P, Sonnenschein A, Janetzky B, Andler W, Menke T. International Journal of Biological Sciences.2007;3(4). CrossRef
- Oxidative dna damage of lymphocytes in peripheral blood and ascites in cancer patients Wang J., Xing S. S., Guo S. B., Jin W., Zhang W.. Current Oncology (Toronto, Ont.).2012;19(Suppl 2). CrossRef
- "Reinforcement" by Tumor Microenvironment: The Seventh "R" of Radiobiology Taghizadeh-Hesary F. International Journal of Radiation Oncology, Biology, Physics.2024;119(3). CrossRef
- Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL , Schneck J, Gabrilovich DI . Nature Medicine.2007;13(7). CrossRef
- Oxidative stress by tumor-derived macrophages suppresses the expression of CD3 zeta chain of T-cell receptor complex and antigen-specific T-cell responses Otsuji M., Kimura Y., Aoe T., Okamoto Y., Saito T.. Proceedings of the National Academy of Sciences of the United States of America.1996;93(23). CrossRef
- Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2-dependent Notch signaling Paul MK , Bisht B, Darmawan DO , Chiou R, Ha VL , Wallace WD , Chon AT , et al . Cell Stem Cell.2014;15(2). CrossRef
- AMPK Maintains Cellular Metabolic Homeostasis through Regulation of Mitochondrial Reactive Oxygen Species Rabinovitch RC , Samborska B, Faubert B, Ma EH , Gravel S, Andrzejewski S, Raissi TC , et al . Cell Reports.2017;21(1). CrossRef
- Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators St-Pierre J, Drori S, Uldry M, Silvaggi JM , Rhee J, Jäger S, Handschin C, et al . Cell.2006;127(2). CrossRef
- Reactive Oxygen Species: Involvement in T Cell Signaling and Metabolism Franchina DG , Dostert C, Brenner D. Trends in Immunology.2018;39(6). CrossRef
- A Review on Natural Antioxidants for Their Role in the Treatment of Parkinson's Disease Mittal P, Dhankhar S, Chauhan S, Garg N, Bhattacharya T, Ali M, Chaudhary AA , et al . Pharmaceuticals (Basel, Switzerland).2023;16(7). CrossRef
- Zinc Gluconate Induces Potentially Cancer Chemopreventive Activity in Barrett's Esophagus: A Phase 1 Pilot Study Valenzano M. C., Rybakovsky E., Chen V., Leroy K., Lander J., Richardson E., Yalamanchili S., et al . Digestive Diseases and Sciences.2021;66(4). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2025
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times