Interest of RT-PCR in the Diagnosis and Follow-Up of Patients with Chronic Myeloid Leukemia (A Study in Western Algeria)
Download
Abstract
Background: This prospective study evaluated the implementation of RT-PCR for the molecular monitoring of chronic myeloid leukemia (CML) patients at Sidi Bel Abbès University Hospital.
Methods: We analyzed 31 consecutive CML patients (mean age 50 years, range 31–72). All patients carried the b3a2 fusion transcript. The Sokal risk distribution was as follows: high (6.45%), intermediate (51.61%), and low (41.93%). Molecular response was assessed by the *BCR-ABL1/ABL1* ratio (IS%) at diagnosis and during treatment follow-up.
Results: The median BCR-ABL1/ABL1 ratio declined to 3.45% (IQR 0.66–19) at 3 months (n=12), 0.37% (IQR 0.00028–23) at 6 months (n=29), and 0.01% (IQR 0.00021–12) at 18 months (n=14). MMR rates were significantly higher in patients achieving a ratio of ≤10% at 3 months (83.3% vs. 19.0%, p<0.001) and ≤1% at 6 months (69.0% vs. 28.0%, p<0.001). Only one patient (3.2%, 95% CI 0–9.3) without an early molecular response achieved MMR by 18 months.
Conclusions: Our findings confirm the clinical utility of RT-PCR for CML monitoring and emphasize the prognostic importance of early molecular response, particularly at the 3- and 6-month timepoints, in Algerian patients.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm characterized in >95% of cases by the Philadelphia chromosome (Ph), resulting from the t (9;22) (q34;q11) translocation. This generates the BCR-ABL1 fusion gene, which produces a constitutively active tyrosine kinase that drives leukemogenesis [1, 2]. While most CML cases are Ph-positive, rare Ph-negative variants with molecular BCR-ABL1 rearrangements or atypical transcripts have been reported [1, 3].
The introduction of tyrosine kinase inhibitors (TKIs), particularly imatinib, revolutionized CML treatment. Current ELN 2020 [4] and NCCN 2022 [5] guidelines emphasize molecular monitoring as the standard of care, with treatment response assessed through serial BCR-ABL1 quantification. However, real-world implementation faces challenges in resource-limited settings, where access to standardized molecular monitoring remains inconsistent [6].
In Algeria, while national TKI availability has improved since 2010, molecular diagnostic capacity remains concentrated in a few centers [7]. Our study at Sidi Bel Abbès University Hospital represents the first comprehensive evaluation of RT-PCR implementation for CML management in Western Algeria, where infrastructure limitations have previously restricted molecular monitoring, population-specific response data are lacking, and optimal monitoring intervals require local validation.
We report diagnostic and monitoring outcomes using internationally standardized methods, providing crucial data for optimizing CML care in similar resource-constrained settings.
Materials and Methods
Patients
Cohort Description and Representativeness
- Sampling and Inclusion/Exclusion Criteria
• Study Population: The study included 31 patients (both sexes were recruited) with chronic myeloid leukemia (CML), primarily recruited from the western region of Algeria (Hematology Department of Sidi Bel Abbès University Hospital).
• Patient Selection:
o Patient Types: The majority of patients (28/31, 90.3%) were in the chronic phase (CP) at diagnosis, while 3 patients (9.7%) were in the accelerated phase (AP). No patients in blast crisis (BC) were included.
o Inclusion Criteria: Patients with a confirmed CML diagnosis (presence of the *BCR-ABL1* transcript via qualitative RT-PCR).
o Exclusion Criteria: Based on the absence of molecular confirmation or incomplete follow-up.
Methods
A complete blood count, blood smear, and bone marrow aspirate were performed for each patient. Multiplex RT-PCR was conducted for suspected cases to confirm the CML diagnosis and determine the isotype of the BCR-ABL1 fusion transcript. All diagnosed patients were regularly evaluated according to ELN recommendations: every three months during the first year and every 3–6 months thereafter.
Molecular analysis was performed by quantifying the BCR-ABL1 fusion transcript from blood samples using RT-PCR at the Hematology Department of CHU Sidi Bel Abbès. A GeneXpert® system from Cepheid, containing RT reagents, was used for the analysis.
Conforming to ELN guidelines, a minimum threshold of 10,000 ABL1 copies was applied to validate measurements, ensuring adequate sensitivity down to MR4.5.
Sample Collection and Processing
Peripheral blood samples were collected from each patient in two 5 mL EDTA tubes for subsequent RNA extraction and complete blood count (NFS). To ensure RNA integrity, samples were processed promptly after collection. Total RNA was automatically extracted using the GeneXpert® system (Cepheid) following the manufacturer’s instructions. Extracted RNA was stored at -80°C until further analysis.
RNA Quality Control and Quantification
RNA quality was assessed by measuring the A260/ A280 ratio using a Nanodrop spectrophotometer. The quality and quantity of RNA were further verified during the RT-PCR process by the adequate amplification of the endogenous control gene (ABL1) and the Probe Check Control (PCC), which is integrated into the GeneXpert® assay to validate the technical integrity of each reaction.
RT-PCR Analysis and Standardization
The quantification of the *BCR-ABL1* transcript was performed on the GeneXpert® system using the Xpert® BCR-ABL Ultra assay. To ensure accurate and standardized results, all measurements were calibrated to the International Scale (IS). Each run included technical replicates (n=2) and IS-calibrated standards provided in the Ipsogen BCR-ABL1 Mbcr IS-MMR kit. Furthermore, the laboratory participates in the External Quality Assessment (EQA) program led by the European LeukemiaNet (ELN), ensuring ongoing proficiency and adherence to international standards. A minimum threshold of 10,000 ABL1 copies was required to validate each measurement, guaranteeing adequate sensitivity down to MR4.5, in accordance with ELN guidelines
Statistical Analysis
Data were entered into a database using SPSS software, following prior coding of variables. Statistical analyses were conducted using SPSS. Graphical representations of Major Molecular Response (MMR) at 12 and 18 months, based on early responses at 3 and 6 months, were created using Origin software.
Results
All patients in our study underwent qualitative multiplex RT-PCR. The cohort consisted of 10 men (32.3%) and 21 women (67.7%), with a mean age of 49.71 years (range: 31–72).
Women had higher MMR rates than men (80% vs 45% at 18 months, 95% CI: [62.5%, 97.5%] vs [16.1%, 74.9%], p = 0.018), although this analysis is limited by the small sample size.
Among them, 28 patients were in the chronic phase (90.3%) and 3 were in the acceleration phase (9.7%). The BCR-ABL1 fusion transcript was successfully identified in all 31 cases. Specifically, all patients presented the major transcript type b3a2 (Mb3a2).
Hemogram results at the time of diagnosis showed pathological findings in 100% of cases. These findings are summarized in Table 1.
| Variables | CML Patients | |
| Number of patients | N | 31 |
| Leucocytes (mm 3 ) | Median | 147600 |
| [Interval] | [23600-790000] | |
| Hemoglobin (g/dl) | Median | 10 |
| [Interval] | [6,9-14] | |
| platelets (10 9 /L) | Median | 519000 |
| [Interval] | [37000-1255000] | |
| Myelosis | Number | 31 |
| % | 100 | |
| Splenomegaly | Present (n/%) | 54,83 |
| Palpable spleen (cm) | [Interval] | [0-20] |
| sokal Score | Low (n/%) | 41,93 |
| Intermediate (n/%) | 51,61 | |
| High (n/%) | 6,45 |
Type of BCR-ABL1 Transcript in Diagnosed Patients
The molecular study using multiplex RT-PCR detected the BCR-ABL1 fusion transcript in all patients, with all showing the major transcript type b3a2 (100%).
Molecular Evaluation Results
Molecular evaluations were performed on 31 patients at various follow-up timepoints:
• Optimal response to treatment (n=10,32.3%): Defined by a BCR-ABL1 level of ≤10% at three months,≤1% at six months, and ≤0.1% at twelve months.
• Suboptimal response (n=9, 29%): Defined by a BCR-ABL1 level of >10% at three months, 1–10% at six months, and 0.1–1% at twelve months.
• Therapeutic failure (n=9, 29%): Defined by a BCR- ABL1 level of >10% at six months, >1% at twelve months, or by loss of molecular response at any time.
• Missing: 3 patients (9.7%) in accelerated phase not classified
Results of Quantification and Kinetics of the BCR- ABL1 Transcript:
Quantification results are expressed as percentages on the international scale.
At Diagnosis
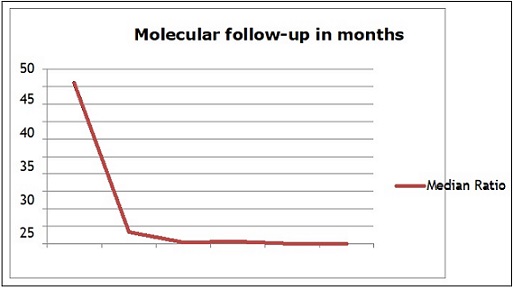
The median BCR-ABL1/ABL1 ratio was 46% (range: 12–100). During molecular follow-up, a significant decline in the BCR-ABL1/ABL1 ratio was observed. It decreased from a median of 46 IS at diagnosis to 3.45 IS after 3 months of follow-up (p<0.0001), to 0.067 IS after 12 months of treatment (p<0.0001), and finally to 0.01 IS at 18 months (p<0.0001) (Table 2; Table 3, Figure 1).
| Variable | OR | 95% CI | p-value |
| BCR-ABL1 3m (≥10% vs <10%) | 3.42 | [1.45 - 8.07] | 0.005 * |
| Age (per year) | 1.03 | [0.98 - 1.08] | 0.214 |
| Sokal (high vs low) | 2.11 | [0.92 - 4.85] | 0.078 |
| Sex (F vs M) | 0.87 | [0.41 - 1.85] | 0.719 |
| Parameter | Diagnosis (%IS) | 3 Months (%IS) | 9 Months (%IS) | 12 Months (%IS) | 18 Months (%IS) |
| Patients (n) | 31 | 12 | 6 | 28 | 14 |
| Median [IQR] | 46 [12-100] | 3.45 [0.66-19] | 0.66 [0.077-43] | 0.067 [0.0002-18] | 0.01 [0.00021-12] |
Figure 1. Kinetics of BCR-ABL1 Transcript Decline Over Time .
Determinants of Major Molecular Response (MMR):
• Sex: A slight advantage was observed in females regarding MMR rates, but the difference was not statistically significant (p=0.55).
• Age: Age did not significantly influence MMR rates (p=0.98).
• Type of Major Transcript: The b3a2 transcript type appeared to be associated with better molecular responses.
• Sokal Prognostic Score: A low Sokal score appeared to negatively impact MMR rates (p=0.74). Patients with a high Sokal score were five times more likely to fail to achieve MMR.
• Early Molecular Responses at 3 and 6 Months: MMR rates were significantly higher in patients with low transcript levels (≤10%) at 3 months (83.33% vs. 19%; p<0.0001) or (≤1%) at 6 months (68.96% vs. 28%; p<0.0001).
Among patients who did not achieve a 1 log10 reduction in BCR-ABL1 at 3 months (2/11, 16.67%), only one (3.22%) achieved MMR at 18 months (p<0.0001), while another patient failed to achieve it. Conversely, patients with BCR-ABL1 levels >10% at 6 months were unable to achieve MMR within the first 18 months. However, among the 20 patients with BCR-ABL1 levels between 1–10% at 6 months, 9 (31.04%) achieved MMR (p<0.0001).
Multivariate Analysis of MMR
In the multivariate analysis, we included all factors that were statistically significant or had a significance threshold of 20% in the univariate analysis.
A total of four variables were studied to identify key predictive factors for MMR: sex (p=0.30), Sokal prognostic score (p=0.87), and BCR-ABL transcript levels at 3 and 6 months (p<10⁻³).
In the logistic regression model, only the level of BCR- ABL transcript expression at 3 months was significantly associated with MMR (p=0.005).
Discussion
Our study represents the first comprehensive evaluation of CML molecular monitoring by RT-PCR in Western Algeria, demonstrating both the feasibility and challenges of implementing molecular diagnostics in a resource-limited setting. While our results validate the utility of standardized BCR-ABL1 testing, they also reveal important regional specificities and methodological limitations that warrant discussion. Transcript Detection and Geographic Heterogeneity
The exclusive detection of the b3a2 transcript (100%) in our cohort shows marked disparities with regional and international data. Unlike other Algerian studies reporting a 54% frequency of b3a2 [8], our series also differs from Tunisian observations showing 63.6% b3a2 [9]. Furthermore, it contrasts with global trends where certain countries like Syria (57.1% b2a2) and Mexico (48% b2a2) [10, 11] exhibit a predominance of the b2a2 transcript. These interpopulation variations may reflect genetic or environmental specificities unique to each region, although our limited sample size precludes definitive conclusions about these phenotypic differences.
This variability, potentially influenced by genetic or environmental factors, underscores the importance of regional studies. The observed false-negative case (due to low post-treatment transcript levels) confirms the sensitivity limitations of the method, as reported in a Korean study comparing conventional and multiplex RT-PCR [12].
Molecular Response Kinetics and Predictive Factors
Sequential monitoring of patients revealed several notable findings. We observed a highly significant decline in BCR-ABL1 levels over time (p<0.0001), with major molecular response (MMR) rates reaching 32.1% at 12 months and 57.1% at 18 months of treatment. These data are comparable to those reported in Egyptian studies [13] and by the GAT-LMC group [7]. In-depth analysis of response curves identified three distinct kinetic profiles (see Figure 1), likely reflecting the heterogeneity of leukemic stem cell depletion mechanisms under treatment [14]. These profiles include rapid responders, intermediate responders, and slow responders, suggesting variable dynamics of residual disease elimination among patients.
Key predictors of MMR included
1. Female sex (80% vs. 45% MMR at 18 months; p=0.018), consistent with data from Branford et al. [15] and Lin et al. [16].
2. Early molecular response (≤10% at 3 months: 83.3% MMR vs. 19%; p<0.0001), validating ELN thresholds [4].
Limitations and Perspectives
1. Single-center bias: Uncertain applicability to other Algerian regions.
2. Technical constraints: Our study has certain methodological limitations. First, the lack of analysis of atypical BCR-ABL1 transcript subtypes (such as e1a3, e6a2, or e13a3) is a significant limitation, as these variants could influence therapeutic response and prognosis, as demonstrated by previous studies in diverse populations. Second, we did not perform epigenetic profiling, although recent work, particularly by Hazarika et al. (2023) [17], has highlighted the crucial role of epigenetic modifications (such as DDIT3 and MGMT modulation) in tyrosine kinase inhibitor resistance and CML progression. These analytical gaps may explain some of the interindividual variations observed in molecular responses and limit our comprehensive understanding of the mechanisms underlying treatment failures. Future integration of these refined molecular parameters could optimize patient stratification and early adaptation of therapeutic strategies.
Unmeasured Factors
Our study did not include an evaluation of treatment adherence or drug interactions, although these factors significantly influence response to tyrosine kinase inhibitors (TKIs). As shown by Alhawamdeh et al. (2025) [18], pharmacological interactions can alter treatment efficacy. The absence of pharmacokinetic data and screening for co-medications limits our ability to identify certain treatment failures, underscoring the need to incorporate these parameters into future monitoring protocols.
Clinical Implications
Despite these limitations, our results confirm the applicability of ELN therapeutic targets in the Algerian context and advocate for optimized molecular monitoring. They particularly emphasize the need to decentralize molecular testing to improve diagnostic and follow-up access, as well as to integrate emerging biomarkers like c-Myc inhibition (Zehtabcheh et al., 2024) [19] to refine patient stratification. These findings call for multicenter studies to validate kinetic response thresholds and adapt international recommendations to regional specificities, while accounting for local technical and economic constraints.
In conclusion, at the end of our study, we conclude that the use of RT-PCR is essential for diagnosis as well as for molecular monitoring, supporting the implementation of a tailored molecular follow-up approach. Molecular monitoring increasingly relies on more sensitive measurements to evaluate treatment efficacy and monitor response.
Finally, RT-PCR, due to its sensitivity and standardization, provides a powerful tool for molecular follow-up. Adherence to this monitoring is crucial, particularly for the early identification of resistant patients.
Acknowledgments
We sincerely thank all the patients who participated in this study for their invaluable contribution.
Competing Interests
No competing interests were disclosed.
References
- The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia Arber DA , Orazi A, Hasserjian R, Thiele J, Borowitz MJ , Le Beau MM , Bloomfield CD , Cazzola M, Vardiman JW . Blood.2016;127(20). CrossRef
- A minute chromosome in human chronic granulocytic leukemia Nowell PC , Hungerford DA. . Science.1960;132:1497-501.
- Neutrophilic-chronic myeloid leukemia: a distinct disease with a specific molecular marker (BCR/ABL with C3/A2 junction) Pane F., Frigeri F., Sindona M., Luciano L., Ferrara F., Cimino R., Meloni G., Saglio G., Salvatore F., Rotoli B.. Blood.1996;88(7). CrossRef
- European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2020 update Baccarani M, Castagnetti F, Gugliotta G, Rosti G. Blood.2020;136(10):1201-14.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Chronic Myeloid Leukemia. Version 3.2022. Plymouth Meeting, PA: NCCN; 2022 .
- Evaluation of the Imatinib Treatment of Patients with Chronic Myeloid Leukemia (CML). A Retrospective Study from Algerian Working Group on CML (GAT-LMC) Djouadi K, Bouchakour A, Taoussi S, Abad M, Ouchenane Z, Sidi Mansour N, et al . Blood.2016;128(22):5454. CrossRef
- Real-world management of chronic myeloid leukemia in Algeria: Results from the Algerian CML Registry (GAT-LMC) Djouadi K, Abadi DEB , Hamladji RM , et al . Leuk Res.2019;85:106234.
- La RT-PCR quantitative en temps réel : application au diagnostic et à l'étude de la maladie résiduelle dans les leucémies myéloïdes chroniques [thèse]. Alger: Université d'Alger Harieche F. 2008.
- Analysis of the clinico-hematological relevance of the breakpoint location within M-BCR in chronic myeloid leukemia Bennour A, Ouahchi I, Achour B, Zaier M, Youssef YB , Khelif A, Saad A, Sennana H. Medical Oncology (Northwood, London, England).2013;30(1). CrossRef
- Frequency of BCR-ABL Transcript Types in Syrian CML Patients Farhat-Maghribi S, Habbal W, Monem F. Journal of Oncology.2016;2016. CrossRef
- BCR/ABL p210, p190 and p230 fusion genes in 250 Mexican patients with chronic myeloid leukaemia (CML) Arana-Trejo R. M., Ruíz Sánchez E., Ignacio-Ibarra G., Báez de la Fuente E., Garces O., Gómez Morales E., Castro Granados M., Ovilla Martínez R., Rubio-Borja M. E., Solís Anaya L., Herrera P., Delgado Llamas J., Kofman S.. Clinical and Laboratory Haematology.2002;24(3). CrossRef
- Comprehensive analysis of BCR-ABL transcript types in Korean CML patients using a newly developed multiplex RT-PCR Goh H, Hwang J, Kim S, Lee Y, Kim Y, Kim D. Translational Research: The Journal of Laboratory and Clinical Medicine.2006;148(5). CrossRef
- Kinetics of BCR-ABL Transcripts in Imatinib Mesylate treated Chronic Phase CML (CPCML), A Predictor of Response and Progression Free Survival Mahmoud HK , El Nahas Y, Abdel Moaty M, Abdel Fattah R, El Emary M, El Metnawy W. International journal of biomedical science: IJBS.2009;5(3).
- Dynamics of chronic myeloid leukemia response to long-term targeted therapy reveal treatment effects on leukemic stem cells Tang M, Gonen M, Quintas-Cardama A, Cortes J, Kantarjian H, Field C, Hughes TP , Branford S, Michor F. Blood.2011;118(6). CrossRef
- Implications of BCR-ABL1 kinase domain-mediated resistance in chronic myeloid leukemia Soverini S, Branford S, Nicolini FE , Talpaz , Deininger MWN , Martinelli G, Müller MC , Radich JP , Shah NP . Leukemia Research.2014;38(1). CrossRef
- Gender and BCR-ABL transcript type are correlated with molecular response to imatinib treatment in patients with chronic myeloid leukemia Lin H, Sjaarda J, Dyck J, Stringer R, Hillis C, Harvey M, Carter R, Ainsworth P, Leber B, Pare G, Sadikovic B. European Journal of Haematology.2016;96(4). CrossRef
- Epigenetic Modulation of DDIT3 and MGMT Expression Acts Synergistically with Resistance to Imatinib towards CML Disease Progression: A Hospital based Study Hazarika G, Kalita MJ , Kalita S, Das PP , Dutta K, Lahkar L, Rajkonwar A, Idris MG , Khamo V, Kusre G, Medhi S. Asian Pacific journal of cancer prevention: APJCP.2023;24(12). CrossRef
- Resveratrol-Induced Modulation of Key Genes and DNA Fragmentation in Chronic Myeloid Leukemia Cells Alhawamdeh L, Almajali B, Atoom AM , Saad HKM , Madi R, Al-Jamal HAN . Asian Pacific journal of cancer prevention: APJCP.2025;26(3). CrossRef
- Anti-Leukemic Effects of Small Molecule Inhibitor of c-Myc (10058-F4) on Chronic Myeloid Leukemia Cells Zehtabcheh S, Sheikh-Zeineddini N, Yousefi A, Bashash D. Asian Pacific journal of cancer prevention: APJCP.2024;25(6). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2025
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times