In Vitro Cytotoxicity of Zingiber officinale -Mediated Gold Nanoparticles against Melanoma Cell Lines
Download
Abstract
Aim: The present work investigates the cytotoxic activity of a candidate compound against A375 human melanoma cells. Cell viability was assessed using the MTT assay, while scanning electron microscopy (SEM) provided ultrastructural details and Fourier-transform infrared spectroscopy (FTIR) was applied to evaluate alterations in functional groups.
Materials and Methods: A375 cells were seeded in 96-well plates at 1 × 10⁴ cells/well, treated with serial dilutions of the compound for 48 h, and analyzed for viability through formazan formation. Cell morphology was preserved with ethanol-acetic acid fixation for SEM observation, and FTIR spectra were collected to examine compound-related molecular interactions.
Results: The treatment produced a dose-responsive reduction in viability, with an IC₅₀ value of 4.6 ± 0.3 µg/mL. At higher doses, SEM micrographs revealed morphological hallmarks consistent with apoptosis, including bleb formation and cellular shrinkage. FTIR signatures indicated shifts in protein and lipid regions, suggesting direct interaction with cellular macromolecules.
Conclusion: The compound displays significant in vitro cytotoxicity against A375 melanoma cells, with supporting evidence from morphological and spectral analyses. While the findings highlight potential bioactivity, they are preliminary and require confirmation through broader studies involving additional models and more specific mechanistic assays.
Introduction
Skin cancer has several forms, but melanoma stands out as the most lethal and treatment-resistant. It originates from melanocytes, the pigment-producing cells of the skin, and even with medical progress, it remains one of the cancers most difficult to control. According to recent global reports, more than 325,000 people were diagnosed with melanoma in 2020, and over 57,000 lives were lost in the same year [1]. These numbers underline why melanoma continues to be a serious health problem worldwide. Although immunotherapies, targeted drugs, and their combinations have extended survival for some patients, the disease still poses a daunting challenge. Melanoma cells carry a high mutational load, spread rapidly to distant organs, and often develop resistance to existing drugs [2, 3]. This combination of factors creates an urgent demand for treatment strategies that can overcome resistance while limiting side effects and toxicity. In recent years, nanotechnology has entered the field of oncology as a promising tool to address these gaps. Among the many nanomaterials explored, gold nanoparticles (AuNPs) have attracted special interest. They are well tolerated in biological systems, can be modified easily at the surface, and move efficiently across cellular barriers [4]. Beyond serving as carriers for therapeutic molecules, AuNPs themselves have shown anticancer activity, including triggering programmed cell death, interfering with angiogenesis, and disturbing tumor signaling pathways [5]. Traditional methods for synthesizing nanoparticles often rely on harsh chemicals. To counter this, researchers have turned to “green synthesis,” which uses natural products such as plant extracts to reduce and stabilize nanoparticles. This route is safer, eco-friendly, and often produces particles with higher biological compatibility [6]. Among the plants studied for such purposes, Zingiber officinale (commonly known as ginger) is especially compelling. Its bioactive molecules gingerols, shogaols and paradols have demonstrated the ability to slow tumor cell growth, encourage apoptosis, and block processes involved in invasion and metastasis [7]. The ZnO-Metformin-Folic Acid nanocomposite and Zingiber officinale-mediated gold nanoparticles both aim to improve targeted therapy with reduced toxicity, though the latter employs a green synthesis approach enriched with phytochemical benefits [8, 9]. Likewise, plant-based nanoemulsions and ginger-AuNPs underscore the therapeutic potential of natural nanocarriers against melanoma. In addition, insights into BRAF and NRAS mutations offer a molecular basis for patient stratification, while cancer registry findings highlight the pressing need for accessible and cost-effective nanotherapies [10, 11]. Using ginger extract to generate AuNPs therefore creates a two-in-one system: the particles gain stability from the plant material, while the phytochemicals add their own anticancer activity. Despite these advantages, the combined impact of ginger-mediated AuNPs on melanoma has not been systematically studied. This is a crucial gap, given that melanoma often resists current treatments. Addressing this gap, the present research evaluates the in-vitro toxicity of ginger-based gold nanoparticles against melanoma cell lines. Specifically, we examine their influence on cell viability, programmed cell death, and morphological changes. The significance of this work lies not only in its scientific contribution but also in its ecological dimension. By adopting a green synthesis approach, it aligns cancer therapy development with sustainable practices. Ultimately, this investigation aims to demonstrate how modern nanotechnology, when combined with traditional medicinal resources, could contribute to safer, more effective, and environmentally responsible strategies for tackling melanoma.
Materials and Methods
Green Synthesis of Gold Nanoparticles
Gold nanoparticles were synthesized using a green chemistry approach with Zingiber officinale (ginger) extract. Fresh ginger rhizomes were thoroughly washed, air-dried, and cut into small sections. Approximately 10 g of the chopped material was boiled in 100 mL of distilled water for 15 min, cooled, and filtered through Whatman No. 1 paper to obtain the extract. For nanoparticle preparation, 90 mL of aqueous chloroauric acid solution (1 mM HAuCl₄·3H₂O, SRL Chemicals, India) was mixed with 10 mL of ginger extract under continuous stirring at 60 °C for 20 min. The solution gradually changed from pale yellow to ruby-red, confirming nanoparticle formation. Reaction pH was adjusted to 7.2 using 0.1 M NaOH (Himedia, India). The colloidal suspension was centrifuged at 12,000 rpm for 15 min, and the pellet was washed three times with deionized water. The purified AuNPs were stored at 4 °C for further use.
FT-IR Spectroscopy
To identify phytochemicals involved in stabilization, FT-IR spectra were recorded using a Nicolet 5700 spectrometer (Thermo Scientific, USA). About 2 mg of dried AuNPs was ground with KBr powder (Himedia, India) and pressed into translucent pellets. Spectral acquisition was carried out in the 500-4000 cm⁻¹ window to highlight functional groups and vibrational bands present in the nanoparticle matrix [12].
X-ray Diffraction Analysis
The crystalline nature of the biosynthesized gold nanoparticles was examined using X-ray diffraction (XRD). The dried nanoparticle powder obtained from the Zingiber officinale extract was subjected to analysis with Cu Kα radiation (λ = 1.5406 Å). Scanning was carried out over the 2θ range of 10°-60° at a steady scan speed. The acquired diffraction profile was used to identify lattice structures and verify the presence of metallic gold phases [13].
Scanning Electron Microscopy
Surface morphology of the nanoparticles was inspected via SEM. A drop of colloidal AuNPs was spread onto a polycarbonate substrate and allowed to air-dry. To eliminate moisture, critical point drying with CO₂ was applied. For enhanced conductivity, a gold coating of ~10 nm thickness was deposited using a sputter coater (Himedia, India). Samples were visualized with a JEOL JSM5600LV microscope (JEOL, Japan) at 20 kV, producing high-resolution images of particle topography [14].
Cell Culture
A375 human melanoma cells were procured from the National Centre for Cell Sciences (NCCS, Pune, India). Cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Himedia, India) supplemented with 10% fetal bovine serum (FBS; Gibco, USA), 2 mM L-glutamine (SRL, India), 1.5 g/L sodium bicarbonate, 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, and 10 mM HEPES. To prevent contamination, 100 IU/ mL penicillin and 100 µg/mL streptomycin (Himedia, India) were added. Cultures were incubated at 37 °C in a humidified chamber with 5% CO₂ [15].
Morphological Examination
To investigate how the treatment influenced cellular structure, A375 melanoma cells were first seeded onto sterile glass coverslips (1 × 10⁵ cells per coverslip) and allowed to attach overnight under standard culture conditions. Once the experimental treatments were completed, the cells were gently rinsed with phosphate- buffered saline (PBS, pH 7.2; Himedia, India) to remove residual medium. Fixation was performed using freshly prepared 4% paraformaldehyde (SRL, India) for 15 minutes at room temperature, which helped preserve the fine details of cell morphology. Following another PBS wash, the cells were counterstained with 0.1% crystal violet solution (Himedia, India) to improve visualization. Microscopic observation was carried out using an inverted phase-contrast microscope (Nikon Eclipse, Japan) at both 10× and 40× objectives. Images were captured from three randomly selected fields per group to provide representative data. Distinctive apoptotic hallmarks such as cell shrinkage, membrane blebbing, rounding, and detachment were carefully recorded. These features were then compared between treated and untreated groups to determine the extent of nanoparticle-induced cell death.
Cytotoxicity by MTT Assay
To evaluate the inhibitory effect of ginger-mediated AuNPs, the MTT assay was employed. Cells were seeded in 96-well plates at 1 × 10⁴ cells/well and incubated until ~80% confluency. Following exposure to different nanoparticle concentrations for 48 h, the medium was replaced with 100 µL of MTT solution (0.5 mg/mL in PBS; Himedia, India). After 4 h at 37 °C, formazan crystals formed and were solubilized in dimethyl sulfoxide (DMSO, SRL, India). Optical density was measured at 570 nm using a microplate reader (Bio-Rad, USA). Cell viability was expressed as:
Cell viability(%)=( (OD of treated sample)/(OD of control)) x 100
Apoptosis Detection by AO/EtBr Staining
Apoptotic induction was assessed through dual staining with acridine orange (AO; SRL, India) and ethidium bromide (EtBr; Himedia, India). Cells (1 × 10⁵/ mL) were resuspended in PBS (pH 7.2; Himedia, India) and stained with 1 µL of AO-EtBr mixture (100 µg/mL each). After 2 min incubation, cells were washed twice with PBS and viewed immediately under a Nikon Eclipse fluorescence microscope at 400×. Green-stained nuclei indicated live cells, while orange-red staining marked apoptotic or necrotic populations [16].
Statistical Analysis
All experiments were performed in triplicate (n = 3). Results are presented as mean ± standard deviation (SD). Statistical significance between groups was determined using one-way ANOVA followed by Tukey’s post-hoc test (GraphPad Prism 9, USA). A threshold of p < 0.05 was considered statistically significant.
Results
FTIR Analysis
Fourier-transform infrared (FTIR) analysis was conducted to identify the biomolecular functional groups responsible for the reduction and stabilization of ginger- mediated gold nanoparticles (AuNPs) (Figure 1).
Figure 1. FT-IR Spectra of AuNPs Showing Functional Groups Responsible for Nanoparticle Stabilization and Reduction.
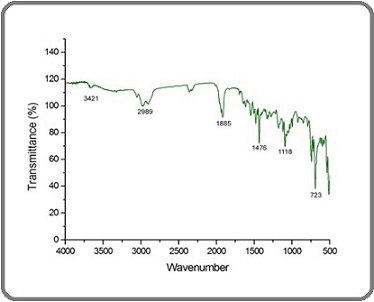
A strong peak observed at approximately 3421 cm⁻¹ indicates N-H stretching vibrations associated with aliphatic primary amines. These amine groups are commonly found in alkaloids and amino acids present in ginger and are believed to cap the nanoparticles, providing steric stabilization that prevents aggregation. Another prominent absorption around 1885 cm⁻¹ is attributed to C=O stretching vibrations of anhydrides or carbonyl groups, often found in aldehydes, ketones, or phenolic compounds abundant in ginger. These carbonyl moieties play a dual role by acting as electron donors, facilitating the reduction of Au³⁺ ions to metallic Au, and participating in surface binding with the nanoparticles. The band detected near 1476 cm⁻¹ corresponds to bending vibrations of alkane groups, reflecting hydrocarbon chains from essential oils or other hydrophobic constituents of ginger extract. This hydrophobic environment may also aid in stabilizing the nanoparticles by creating a protective barrier. Lastly, a distinct peak at 1118 cm⁻¹, assigned to C-O stretching of secondary alcohol groups, highlights the presence of hydroxyl-containing flavonoids and polysaccharides that further stabilize the nanoparticle surface through hydrogen bonding and electrostatic interactions.Collectively, FTIR spectra reveal that multiple phytochemicals amines from alkaloids, carbonyl groups from phenolics, alkane chains, and hydroxyl groups from alcohols and flavonoids work synergistically to reduce gold ions and form an organic corona around the particles. This corona not only prevents particle aggregation but also enhances biocompatibility and facilitates cellular uptake.Importantly, the protective organic layer formed by these biomolecules likely contributes to the cytotoxic effects observed in vitro. The capping agents can modulate nanoparticle surface charge and hydrophobicity, influencing interactions with cellular membranes and intracellular targets in cancer cells. Thus, FTIR findings provide a mechanistic link between the chemical composition of ginger extract, nanoparticle stability, and their enhanced biological activity, including the induction of apoptosis in oral cancer cells.
X- Ray Diffraction Analysis
In Figure 2 the XRD spectrum of Au nanoparticle shows multiple sharp diffraction peaks, with the most intense reflection appearing around 12-15° (2θ).
Figure 2. XRD Pattern of Gold Nanoparticles Synthesized Using Zingiber Officinale Extract. The sharp peaks confirm their crystalline nature.
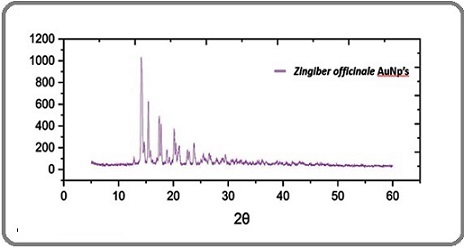
Several additional peaks of smaller intensities were observed in the 15-30° region, confirming the crystallinity of the Au nanoparticle. The narrowness and distinctness of these peaks suggest a well-ordered arrangement of atoms within the nanoparticles. The intensity variations also indicate that the particles exhibit preferential orientation and are not completely uniform in shape.
Surface morphology using Scanning Electron Microscopy
The structural and Morphological traits of the biosynthesized for exploring Au nanoparticles (AuNPs), scanning electron microscopy was used (SEM) (Figure 3).
Figure 3. SEM Images of AuNPs Displaying Rod-like Morphology and Aggregation Due to Plant Metabolite Influence.

Rod-shaped nanoparticles with an inclination to agglomerate were emphasized by the electron beam imaging. These aggregates created a homogeneous dispersion, indicating that the plant extract’s secondary metabolites aided in the nucleation and stability of the nanoparticles. Additional proteins may have inhibited the autonomous nucleation of reduced atoms by influencing the aggregation process, as seen by the particle clustering.
Cytotoxic Assay
The effect of ginger-derived gold nanoparticles on A375 melanoma cells was evaluated using the MTT assay. As the nanoparticle concentration increased across the tested range (0-100 µg/mL), cell viability showed a clear downward trend (Figure 4).
Figure 4. MTT Assay Results Showing the Cytotoxic Effect of AuNPs on A375 Cancer Cells in a Dose-dependent Manner.
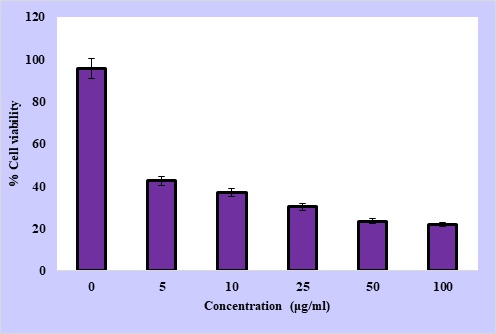
At very low doses, around 1-5 µg/mL, the reduction in survival was minimal, with most cells (85-90%) remaining metabolically active. Once the treatment reached intermediate levels (10-25 µg/mL), however, a sharper decline was evident, with viability falling to nearly two-thirds of the control. Exposure to higher concentrations (50-100 µg/mL) caused a striking loss of cell survival, leaving less than one-third of the population intact compared to untreated controls. From this data, the half-maximal inhibitory concentration (IC50) was estimated to be 4.6 ± 0.3 µg/mL, which indicates that substantial cytotoxicity is achieved at relatively low exposure levels. All experiments were performed in triplicate (n = 3), and the findings were statistically validated. One-way ANOVA followed by Tukey’s post hoc test confirmed that reductions in viability at doses of 10 µg/mL and above were highly significant (p < 0.05). Taken together, these results demonstrate not only that the nanoparticles exert strong anti-proliferative effects but also that their impact follows a well-defined dose-response pattern. This consistency strengthens their promise as candidates for melanoma therapy.
Cell Morphology Analysis
Using light microscopy, morphological alterations in A375 cells in response to AuNP treatment were investigated (Figure 5A).
Figure 5. A Bright-field Microscopy Images of Control A375 Cells Showing Intact Morphology with no Significant Structural Changes and 5B A375 Cells Treated with AuNPs at IC50 Concentration, Exhibiting Membrane Blebbing, Cell Shrinkage, and Detachment, Indicating Apoptosis.
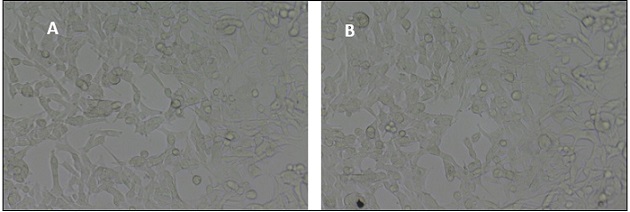
Control cells did not significantly alter their normal form. On the other hand, cells exposed to AuNPs at IC50 concentrations showed dose-dependent membrane blebbing, cellular shrinkage, and surface detachment (Figure 5B). These changes imply that AuNPs cause apoptotic cell death by interfering with the stability of cell membranes and cytoskeletal integrity.
Cell Death Analysis Using Fluorescent Microscopy
Apoptotic induction in A375 cells following treatment with ginger-mediated AuNPs was evaluated using acridine orange (AO) and ethidium bromide (EtBr) staining under fluorescence microscopy. Control cells exhibited uniform green fluorescence, indicating high viability, whereas treated cells showed a marked increase in orange to red fluorescence, reflecting early and late apoptotic events, respectively (Figure 6).
Figure 6. (A) Fluorescence Microscopy Image of Control A375 Cells Stained with AO/EtBr, Showing Uniform Green Fluorescence, Indicating Viable Cells and (B) AO/EtBr-stained A375 Cells Treated with AuNPs at IC50 Concentration, Displaying Orange/red Fluorescence, Signifying Nuclear Condensation and Apoptosis.
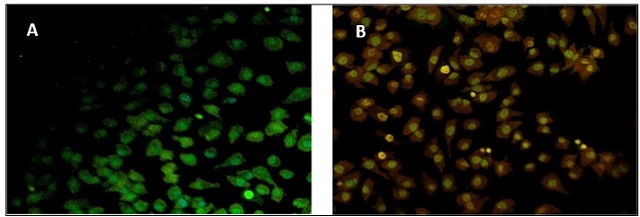
To provide quantitative insight, at least 200 cells per sample were counted across three independent experiments (n = 3). The analysis revealed that treatment at the IC50 concentration (4.6 ± 0.3 µg/ mL) resulted in approximately 58% early apoptotic cells, 22% late apoptotic/necrotic cells, and 20% viable cells (Figure 6, Table 1).
| Treatment (µg/mL) | Viable Cells (%) ± SD | Early Apoptotic Cells (%) ± SD | Late Apoptotic/Necrotic Cells (%) ± SD |
| 0 (Control) | 100 ± 2.1 | 0 ± 0 | 0 ± 0 |
| IC 50 (4.6 ± 0.3) | 20 ± 2 | 58 ± 3 | 22 ± 2.5 |
These data confirm that the nanoparticles trigger programmed cell death in a significant portion of the melanoma cell population and support their potential utility as anticancer agents.
Discussion
This research showcases the effective production and analysis of gold nanoparticles (AuNPs) through a bio-mediated process. The FTIR examination identified various functional groups, such as alcohols, anhydrides, alkanes, and primary amines, which enhanced the stability and reduction of AuNPs. The diffraction pattern of Zingiber officinale-derived AuNPs demonstrates their crystalline configuration, a feature often reported in biologically synthesized nanomaterials. The strong reflection around 12-15° aligns with the face-centered cubic (fcc) structure typical of elemental gold. Additional reflections in the higher-angle region reflect contributions from organic residues of the plant extract, which may act as stabilizing and capping agents. The clustering tendency visible in microscopy analyses corresponds with the peak intensities here, suggesting that the biomolecules from ginger extract influence crystal growth and orientation. Compared with chemical synthesis routes, the broader peaks seen in this spectrum are characteristic of reduced particle size and strain effects, common in green synthesis pathways [3, 16, 17]. These biomolecules serve as capping and stabilizing agents, playing a vital role in nanoparticle formation, as noted in earlier research [17]. SEM investigation revealed that Au nanoparticles primarily displayed a rod-like structure with a propensity for clustering. This finding is consistent with previous studies indicating that biological extracts impact nanoparticle dimensions and form due to the presence of secondary metabolites [18]. The aggregation of Au NPs indicates that plant extract biomolecules facilitate particle stabilization, a common occurrence in green nanoparticle synthesis. The cytotoxicity assay findings exhibited a strong inhibitory effect of AuNPs on A375 human skin cancer cells [19]. An IC50 value of 4.6 ± 0.3 µg/mL suggests that AuNPs have considerable cytotoxic properties, possibly due to their ability to trigger apoptosis [20]. These results align with prior studies, showing that AuNPs can induce cell death through oxidative stress and membrane disruption. Fluorescence microscopy analysis further validated the induction of apoptosis, as evidenced by nuclear condensation and a transition from green to orange/red fluorescence in AO/EtBr-stained cells. These findings underscore the potential of AuNPs as promising anticancer agents, necessitating further comprehensive mechanistic investigations [21].
In conclusion, in this study, gold nanoparticles (AuNPs) were successfully synthesized using environmentally friendly methods and thoroughly characterized. FTIR analysis identified functional groups important for nanoparticle stabilization. The XRD analysis confirms that AuNPs synthesized using Zingiber officinale extract possess a crystalline nature with reflections characteristic of gold’s fcc lattice. The presence of sharp yet variably intense peaks highlights both the nanoscale crystallite size and the influence of bio-organic compounds from the extract in modulating growth. These findings reinforce the role of plant-mediated synthesis in producing stable, crystalline nanoparticles suitable for further biological evaluation, while SEM imaging revealed their rod-like morphology. The cytotoxic effects of these biologically synthesized AuNPs were demonstrated in vitro on A375 human melanoma cells, with an IC50 value of 4.6 ± 0.3 µg/ mL, and fluorescence microscopy provided preliminary evidence of apoptosis induction. These findings highlight the potential of green-synthesized AuNPs as candidates for further investigation in anticancer research. Future studies should focus on elucidating the specific molecular mechanisms underlying their cytotoxic effects and exploring their efficacy across additional cancer models.
Acknowledgments
Statement of Transparency and Principals
• Author declares no conflict of interest
• Study was approved by Research Ethic Committee of author affiliated Institute.
• Study’s data is available upon a reasonable request.
• All authors have contributed to implementation of this research.
Originality Declaration for Figures
All figures included in this manuscript are original and have been created by the authors specifically for the purposes of this study. No previously published or copyrighted images have been used. The authors confirm that all graphical elements, illustrations, and visual materials were generated from the data obtained in the course of this research or designed uniquely for this manuscript.
Declaration on generative AI and AI-assisted technologies in the writing process
Not applicable.
References
- Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040 Arnold Melina, Singh Deependra, Laversanne Mathieu, Vignat Jerome, Vaccarella Salvatore, Meheus Filip, Cust Anne E., et al . JAMA dermatology.2022;158(5). CrossRef
- Zingiber officinale rhizome extracts mediated ni nanoparticles and its promising biomedical and environmental applications Hussain Tahir, Faisal Shah, Rizwan Muhammad, Almostafa Mervt M., Younis Nancy S., Yahya Galal. BMC Complementary Medicine and Therapies.2023;23(1). CrossRef
- The Antimicrobial Efficacy Against Selective Oral Microbes, Antioxidant Activity and Preliminary Phytochemical Screening of Zingiber officinale Ahmed Naveed, Karobari Mohmed Isaqali, Yousaf Anam, Mohamed Roshan Noor, Arshad Sohaib, Basheer Syed Nahid, Peeran Syed Wali, et al . Infection and Drug Resistance.2022;15. CrossRef
- A critical review of Ginger's (Zingiber officinale) antioxidant, anti-inflammatory, and immunomodulatory activities Ayustaningwarno Fitriyono, Anjani Gemala, Ayu Azzahra Mutiara, Fogliano Vincenzo. Frontiers in Nutrition.2024;11. CrossRef
- Lubguban, Arnold A., Arnold C. Alguno, Roberto M. Malaluan, and Gerard G. Dumancas. Carrageenan-Mediated Green Synthesis of Metal Oxide Nanoparticles for Various Applications. CRC Press 2025.
- Mustafa, Iswaibah, and Nyuk Ling Chin “Antioxidant Properties of Dried Ginger (Zingiber Officinale Roscoe) Var. Bentong.” Foods.2023;12(1):178.
- Zingiber officinale var. rubrum: Red Ginger’s Medicinal Uses Zhang Shiming, Kou Xuefang, Zhao Hui, Mak Kit-Kay, Balijepalli Madhu Katyayani, Pichika Mallikarjuna Rao. Molecules.2022;27(3). CrossRef
- In vitro Evaluation of Zinc Oxide-Metformin Folic Acid Nanocomposite as a Targeted Drug Delivery System for Cancer Therapy Ezzat Nourhan, Emadeldien Nagy, Ali Miar Khaled, Fahd Serene, Shebl Sarah, Elshishiny Malak, Gedamy Mohamed Ramadan, et al . Asian Pacific journal of cancer prevention: APJCP.2025;26(2). CrossRef
- Estimated Cancer Incidence in Northern Tunisia in 2023: Northern Tunisia Cancer Registry Khiari Houyem, Arfaoui Emna, Mahjoub Najet, Henchiri Soumaya, Sliti Alyssa, Hsairi Mohamed. Asian Pacific journal of cancer prevention: APJCP.2024;25(12). CrossRef
- BRAF and NRAS Mutations and the Association with Prognosis of Acral Lentiginous and Nodular Melanomas in Indonesia Anwar Sumadi Lukman, Ferronika Paranita, Cahyono Roby, Sugandhi William, Pradana Gizza Dandy. Asian Pacific journal of cancer prevention: APJCP.2024;25(10). CrossRef
- Plant Based Extract Oil-Based Nano emulsions: Impact on Human Melanoma Cell Line Ellithy Marwa Mohamed Ahmed, Abdrabo Reham Ahmed Mohamed. Asian Pacific journal of cancer prevention: APJCP.2024;25(5). CrossRef
- Apoptotic and Antioxidant Activity of Gold Nanoparticles Synthesized Using Marine Brown Seaweed: An In Vitro Study Rajeshkumar S., Parameswari R. P., Jayapriya J., Tharani M., Ali Huma, Aljarba Nada H., Alkahtani Saad, Alarifi Saud. BioMed Research International.2022;2022. CrossRef
- Sustainable Synthesis of Gold Nanoparticles for Drug Delivery and Cosmeceutical Applications: A Review Jayeoye, Titilope John, Eze Fredrick Nwude, Sudarshan Singh, Bhupendra G. Prajapati, Devesh U. Kapoor, Nongnuj Muangsin . .
- Gold nanoparticle enhanced TNFα antibody interface using saliva for predicting prognosis in OSCC Hema Shree K., Gayathri R., Veeraraghavan Vishnu Priya, Ramani Pratibha, Ramadoss Ramya, Yuwanati Monal. Archives of Oral Biology.2025;173. CrossRef
- Physicochemical Analysis of Green Synthesis Gold Nanoparticles of Edible Plant Extracts Chinnasamy Shalini, E Kowsaliya, Malakondaiah Suresh, B Ramesh Babu P., Jothinathan Mukesh Kumar Dharmalingam, Visuvasam Motcha Rakkini. Research Journal of Pharmacy and Technology.2025;18(4). CrossRef
- Evaluation of Anti Cholesterol and Antioxidant Potentiality of Aqueous Extracts of Citrus aurantifolia, Zingiber officinale and its Formulation - a Comparative In vitro Study ivasubramanian Priyanka R, Gayathri V, Vishnu Priya J, Selvaraj , S Kavitha . Journal of Pharmaceutical Research International.;:1873-82.
- Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth Shankar S. Shiv, Rai Akhilesh, Ahmad Absar, Sastry Murali. Journal of Colloid and Interface Science.2004;275(2). CrossRef
- Biosynthesis of gold and silver nanoparticles using Emblica Officinalis fruit extract, their phase transfer and transmetallation in an organic solution Ankamwar Balaprasad, Damle Chinmay, Ahmad Absar, Sastry Murali. Journal of Nanoscience and Nanotechnology.2005;5(10). CrossRef
- Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf Huang J, Li Q, Sun D, Lu Y, Su Y., Yang X, Hong J . Nanotechnology, 18(10), 105104..2025.
- Green Synthesis of Silver Nanoparticles Using Aqueous Solution of Ficus benghalensis Leaf Extract and Characterization of Their Antibacterial Activity Saxena Antariksh, Tripathi R. M., Zafar Fahmina, Singh Priti. Materials Letters.2012;67(1). CrossRef
- Green synthesis of silver nanoparticles using latex of Jatropha curcas Bar H, Bhui DK, Sahoo GP, Sarkar P, De SP, Misra A . Colloids and Surfaces A: Physicochemical and Engineering Aspects.2025;339(1):33-43. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2025
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times