TWIST1 Expression and Tumor Infiltrating Lymphocytes: Prognostic Value in Colorectal Adenocarcinoma
Download
Abstract
Background: Colorectal cancer remains a major health concern due to its high incidence and mortality. However, reports on the prognostic value for colorectal cancer were still limited. TWIST1 overexpression is associated with poor clinical prognosis in colorectal adenocarcinoma. Tumor-infiltrating lymphocytes (TILs) reflect the body’s immune response to cancer. This study aims to investigate these relationships between TWIST1 with histological grade, TILs grade, and depth of invasion in colorectal adenocarcinoma.
Methods: This cross-sectional study assessed TWIST1 expression in paraffin-embedded samples from an Indonesian population with colorectal adenocarcinoma. Immunohistochemical staining with a rabbit polyclonal TWIST1 antibody was performed. TWIST1 expression was evaluated under microscope. Statistical analysis was performed to determine association between TWIST1 with histological grade, TILs grade, and depth of invasion in colorectal adenocarcinoma.
Results: A total of 100 samples examined. Of 53 samples with low grade TILs, 39 (73.6%) showed strong TWIST1 expression. In intermediate grade TILs, 14 (50%) from 28 samples showed strong TWIST1 expression. In the high grade TILs, 8 (42.1%) from 19 samples showed strong TWIST1 expression. In low grade histological adenocarcinoma, 29 (47.5%) from 61 samples showed strong TWIST1 expression. In high grade adenocarcinoma, 32 (82.1%) from 39 samples showed strong TWIST1 expression. Statistical analysis using Chi square test shown a significant association between TWIST1 expression with both histological grade (p=0.001) and TILs grade (p=0.020), whereas no significant association was found with depth of invasion (p=0.976).
Conclusions: There is a significant positive correlation between TWIST1 expression with histological grade and TILs grade. Although, there is no significant correlation between TWIST1 expression with depth of invasion. Therefore, TWIST1 expression might be potential as a prognostic indicator in colorectal adenocarcinoma.
Introduction
Colorectal cancer is the third most common cancer worldwide, affecting the colon and/or rectum. More than 90% of colorectal cancers are adenocarcinoma. In developing countries, the incidence of colorectal carcinoma is increasing. According to Globocan 2022, there were 19,976,499 new cases of colorectal cancer with 904,019 deaths [1, 2]. In Indonesia, colorectal cancer ranks as the fourth most common malignancy after breast cancer, lung cancer, and cervical cancer [2].
The global incidence of new colorectal cancer cases is projected to increase to 3.2 million by 2040, in accordance with aging population and rising life expectancy [3]. Colorectal cancer generally affects men more frequently than women and typically occurs in individuals over 50 years of age. However, a shift has been observed, with an increasing number of cases reported in younger individuals. Multiple factors contribute to the development of colorectal cancer, including both modifiable and non-modifiable risk factors [3, 4]. This situation emphasize the urgency for a more comprehensive approach through prevention, screening programs, and therapeutic management to decrease the growing cases of colorectal cancer in the future.
Colorectal cancer is a malignant neoplasm that develops from normal colon epithelium, progressing through an adenoma before transforming into carcinoma [5]. Histopathological grading serves as an important prognostic indicator in colorectal cancer, maintaining its predictive value in conjunction with TNM stage. The TNM classification of colorectal cancer is determined through histopathological examination of the primary tumor (pT) to assess the depth of tumor invasion and regional lymph node involvement (pN), combined with clinical and radiological findings to establish the presence of distant metastasis (pM). Based on the World Health Organization (WHO) classification, colorectal carcinoma categorized into two grades: low grade and high grade. Colorectal cancer develops through multiple pathways, i.e. chromosomal instability (CIN), microsatellite instability (MSI), and CPG island methylator phenotype (CIMP). Among these, CIN is the predominant mechanism, frequently characterized by recurrent mutation in key genes such adenomatous polyposis coli (APC), KRAS (Kirsten rat sarcoma viral oncogene homolog), and tumor protein p53 (TP53) [5-7].
TWIST1 is a basic helix loop helix (bHLH) transcription factors that play a pivotal role in mesodermal tissue development by regulating various biological processes. TWIST1 is located on human chromosome 7p21 [8]. This gene is essential in modulating mesenchymal tissue during organogenesis. In cancer, overexpression of TWIST1 can induce aggressiveness of tumor. TWIST1 contributes to cancer initiation, progression, and metastasis through classic cellular signalling pathways, including P13K/AKT, TGF-β, NOTCH, and NF-ƙβ. It is recognized as a key inducer of epithelial mesenchymal transition (EMT), where increased EMT is associated with poor clinical prognosis.
TILs are important components of the tumor microenvironment, including dendritic cell, CD8+ T lymphocytes, CD4+ T lymphocytes, neutrophils, B cell, and macrophages. TILs can play critical role in controlling cancer growth through cytotoxic mechanisms and cytokine secretion [9-11]. In colorectal cancer, patient with high grade TILs often have more favourable prognosis, while low grade is linked with worse outcomes [12].
The study aims to investigate the association between TWIST1 expression, histopathological grade, TILs grade, and depth of invasion in colorectal adenocarcinoma.
Materials and Methods
Study Design and Data Collection
The study used an analytical observational approach with a cross-sectional design. A total of 100 paraffin- embedded tissue blocks from colon and/or rectal tumor primary colorectal adenocarcinoma were included. The sample size was determined based on a Lemeshow’s formula for comparing two proportions (α=0.05, power=0.95) and the power calculation using G*Power targeting a power of 0.80 and an alpha of 0.05 to detect a moderate effect size, ensuring sufficient statistical power to identify its associations. These samples were collected between January 2022 to June 2024 at the Anatomical Pathology Laboratory of Dr. Wahidin Sudirohusodo Hospital and Hasanuddin University Hospital based on inclusion criteria.
Histopathological Examination
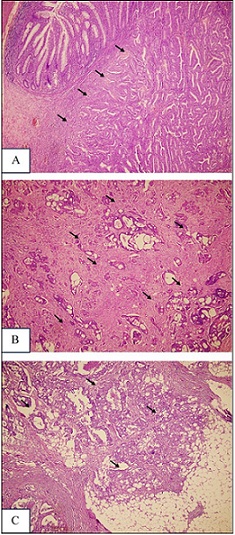
All the samples were stained with hematoxylin and eosin (H&E), and the diagnosis of colorectal adenocarcinoma was independently verified by experienced anatomical pathologists at Dr. Wahidin Sudirohusodo Hospital and Hasanuddin University Hospital. Depth of invasion was determined according to the TNM system. In this classification, pT1 refers to tumor spread into the submucosa, pT2 refers to invasion of the muscularis propria, pT3 refers to extension into the subserosa or non-peritonealized pericolic/ perirectal tissue, and pT4 refers to either perforation of the visceral peritoneum or invasion of neighbouring organs. Subclassification of a pT4a indicates peritoneal perforation, while pT4b is used when adjacent organs are directly involved (Figure 1) [5].
Figure 1. Depth of Invasion in Colorectal Adenocarcinoma (A). pT1, (B). pT2, (C). pT3 (H and E, 40x Magnification).
TILs were defined as lymphocytes within the tumor-associated stroma and assessed across the entire tumor section rather than limited hotspots. TILs were scored as a continuous percentage following the International TILs Working Group (ITWG) recommendations. For categorical analyses, percentages were classified according to ITWG guidelines as low (0–10%), intermediate (11–50%), and high (≥51%) (Figure 2) [13, 14].
Figure 2. Grading TILs in Colorectal Adenocarcinoma.(A). Low Grade (Score 1), (B). Intermediate Grade (Score 2), (C). High Grade (Score 3) (H and E, 100x Magnification).
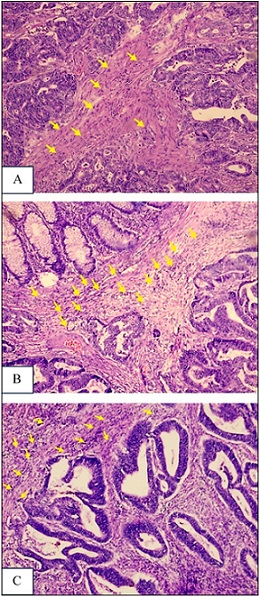
Discrepancies between the two pathologists assessments were resolved by calculating the average of their scores, ensuring consistent evaluation.
TWIST1 Immunohistochemistry (IHC) Examination
In Immunohistochemical analysis, Formalin-fixed In Immunohistochemical analysis, Formalin-fixed paraffin-embedded (FFPE) blocks were sectioned at a 3–4 µm thickness and subsequently deparaffinized. Staining was performed using a rabbit polyclonal TWIST1 antibody (catalog number E-AB-645429, Elabsience, Wuhan, China) at a dilution of 1:300. TWIST1 localization was observed in the cytoplasm of tumor cells (Figure 3).
Figure 3. TWIST1 Intensity in Colorectal Adenocarcinoma. (A). Strong (+3), (B). Intermediate (+2), (C). Low (+1), D. Negative (0) (IHC, 400x Magnification).
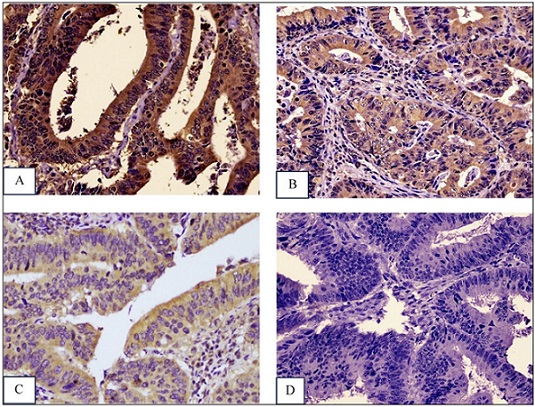
TWIST1 expression was evaluated under a light microscope at 400× magnification. The staining results were reviewed independently by two pathologists who were blinded to clinical information. For scoring, the total immunostaining score (TIS) was obtained by multiplying the intensity score (0 = no staining, 1 = weak, 2 = moderate, 3 = strong) with the proportion score (1 = 1–10%, 2 = 11–50%, 3 = 51–80%, 4 = 81–100%), giving a total range of 1–12. TWIST1 expression was based on the obtained TIS value, where improved scores of ≤4 were defined as weak expression and >4 were defined as strong expression [15, 16].
Statistical analysis data
Statistical analysis was performed using SPSS version 25. Data were summarized descriptively in frequency distributions and tables presenting the clinicopathological characteristics. Statistical analysis was carried out using the Chi-square test, Mann-Whitney U test, and Kruskal–Wallis test, with p <0.05 considered statistically significant.
Results
This study analyzed 100 paraffin block samples of colorectal adenocarcinoma. The majority of patients were over 50 years of age with a slight male predominance. Rectal tumor were the most frequent among others based on its topographical site. However, low grade colorectal adenocarcinoma represented the most frequent histopathological subtype. Most tumors demonstrated low grade TILs, while strong TWIST1 expression was identified in 61% of samples (Table 1).
| Characteristic | Sample Quantity (%) |
| Age | |
| ≤ 50 years | 28 (28) |
| > 50 years | 72 (72) |
| Gender | |
| Male | 56 (56) |
| Woman | 44 (44) |
| Tumor Location | |
| Proximal | 29 (29) |
| Distal | 29 (29) |
| Rectum | 36 (36) |
| Rectosigmoid | 6 (6) |
| Histopathological Grade | |
| Low | 61 (61) |
| High | 39 (39) |
| Grade TILs | |
| Low | 53 (53) |
| Intermediate | 28 (28) |
| High | 19 (19) |
| Depth of invasion (pT) | |
| pT1 | 17 (17) |
| pT2 | 41 (41) |
| pT3 | 42 (42) |
| Twist-1 Expression | |
| Strong | 61 (61) |
| Weak | 39 (39) |
| Total | 100 |
A strong correlation was identified between TWIST1 expression and histological grade (p=0.001), with high grade tumor exhibiting a higher frequency of strong expression. A significant correlation was also observed between TWIST1 expression and TILs grade (p=0.020) and (p=0.021), whereas tumor with lower lymphocytic infiltration displayed strong TWIST1 expression, highlighting an inverse relationship between TWIST1 activity and immune infiltration. Furthermore, Spearman’s correlation analysis further confirmed this trend, showing a significant negative correlation between TWIST1 expression and TILs grade (r= –0.291, p=0.003), suggesting that higher TWIST1 levels are associated with reduced TILs and a less immunoresponsive tumor microenvironment.
Conversely, no substantial correlations were identified with age, sex, or depth of invasion. Regarding depth of invasion (pT), no statistically significant association with TWIST1 expression was observed (p=0.976). However, given this relatively small number of cases within each pT category (pT1= 17, pT2= 41, pT3= 42), the study may have been underpowered to detect a true difference. Consequently, these results should be interpreted with caution (Table 2).
| Characteristic | Sample Size | Twist-1 Expression | Twist-1 | P Value b | ||
| Total Immunostaining Score (TIS) | ||||||
| Strong | Weak | P Value a | Mean±SD | |||
| n (%) | n (%) | |||||
| Age | 0.971 | 0.971 | ||||
| ≤50 years | 28 | 17 (60.7) | 11 (28) | 4.75±2.20 | ||
| >50 years | 72 | 44 (61.1) | 28 (38.9) | 5.18±2.07 | ||
| Gender | 0.729 | 0.73 | ||||
| Male | 56 | 35 (62.5) | 21 (37.5) | 5.11±2.04 | ||
| Woman | 44 | 26 (59.1) | 18 (40.9) | 5.00 ±2.22 | ||
| Tumor Location | 0.684 | 0.687 | ||||
| Proximal | 29 | 16 (55.2) | 13 (44.8) | 4.66±2.00 | ||
| Distal | 29 | 20 (69.0) | 9 (31.0) | 5.66±2.07 | ||
| Rectum | 36 | 22 (61.1) | 14 (38.9) | 5.03±2.22 | ||
| Rectosigmoid | 6 | 3 (50) | 3 (50) | 4.33±1.86 | ||
| Histopathological Grade | 0.001 * | 0.001 * | ||||
| Low | 61 | 29 (47.5) | 32 (52.5) | 4.51±2.10 | ||
| High | 39 | 32 (82.1) | 7 (17.9) | 5.92±1.84 | ||
| Grading TILs | 0.020 * | 0.021 * | ||||
| Low | 53 | 39 (73.6) | 14 (26.4) | 5.60±2.08 | ||
| Intermediate | 28 | 14 (50.0) | 14 (50.0) | 4.61±1.83 | ||
| High | 19 | 8 (42.1) | 11 (57.9) | 4.21±2.22 | ||
| pT | 0.976 | 0.976 c | ||||
| pT1 | 17 | 10 (58.8) | 7 (41.2) | 5.00±2.39 | ||
| pT2 | 41 | 25 (61.0) | 16 (39.0) | 5.07±1.92 | ||
| pT3 | 42 | 26 (61.9) | 16 (38.1) | 5.07±2.20 |
aChi Square Test, b Mann- Whitney Test, cKruskal Wallis Test, *p value <0.05
Discussion
In this study, it was found that TWIST1 expression is strong in high-grade adenocarcinoma (p=0.001) compared to low-grade adenocarcinoma. Tawfik et al [15] also reported the same result. Other study also reported that strong TWIST1 expression was especially observed in tumors that experienced poor differentiation [17].
In colorectal adenocarcinoma, TWIST1 induces EMT through the TGF-β, Wnt/β-catenin, and MAPK pathways, thereby increasing tumor aggressiveness [18, 19]. The tumor microenvironment comprising fibroblasts, endothelial cells, and diverse immune cells secretes cytokines and chemokines that regulate EMT during tumor progression. TWIST1 also regulates differentiation, with upregulation promoting EMT via downregulation of E-cadherin and upregulation of N-cadherin, weakening cell-cell adhesion and enhancing invasiveness. Additional roles include promoting angiogenesis, invadopodia formation, vasculogenic mimicry, and chromosomal instability [8], [20-22]. TWIST1 effects are mediated by interactions with transcription factors (Runx2, Sox9, MyoD), signalling pathways (TGF-β, BMP, Wnt, Notch, PI3K/AKT), and regulation by non-coding RNAs (miRNA, lncRNA, circRNA) [23, 24]. These changes likely contribute to the histopathological grade differences observed in our cohort, consistent with its role in EMT and tumor progression.
Strong TWIST1 expression was more common in low grade TILs, while weak TWIST1 expression was more common in high grade TILs. Spearman correlation showed a significant inverse relationship between TWIST1 expression and TILs grade (r= – 0.291, p=0.003), and categorical analyses showed a significant association between TWIST1 expression and TILs grade (p=0.020). Wang et al. [25] reported that TWIST1 expression is positively associated with tumor immunoregulation and immune checkpoint activity. PD-1/PDL1 demonstrated that TWIST1 expression promotes PD-1 upregulation, leading to T-cell exhaustion and limiting effective lymphocyte infiltration. Consequently, tumors with high TWIST1 expression can ultimately evade the effects of antitumor immunity and have fewer infiltrating lymphocytes.
Although our study did not directly assess PD-1/PD- L1 or other immune checkpoint pathways, our findings suggest that TWIST1-mediated EMT could affect immune suppression in colorectal tumors. While this remains a hypothesis, it is biologically reasonable and supports future research on the relationship between TWIST1 and immune checkpoint signalling in larger, well-defined groups [26].
The TNM staging system remains a cornerstone in colorectal cancer prognosis, evaluating primary tumor size, lymph node involvement, and presence of metastases [27-29]. We found no significant association between TWIST1 expression and depth of invasion (p=0.976). However, the small sample sizes for individual pT subgroups (pT1= 17, pT2= 41, pT3= 42) suggest limited statistical power. Therefore, a Type II error cannot be ruled out, and these results should be interpreted cautiously. This limitation highlights the need for larger cohorts to better understand the relationship between TWIST1 expression and tumor invasion depth. Differences from prior studies may also reflect tumor heterogeneity and microenvironmental factors influencing EMT and invasion.
Several limitations must be considered. First, only a single immunohistochemical assay was used, and specific TIL subtypes (e.g., CD8+, CD4+) were not characterized due to resource constraints. Second, the TWIST1 immunostaining cut-off, while based on literature, requires validation in larger populations.
In conclusions, there is a significant positive correlation between TWIST1 expression with histological grade and TILs grade. Although, there is no significant correlation between TWIST1 expression with depth of invasion. Therefore, TWIST1 expression might be potential as a prognostic indicator and must be validated trough a larger prospective studies.
Acknowledgements
General
The authors sincerely thank for supervisors, colleagues, and staff of Anatomical Pathology Laboratory at Hasanuddin University hospital and Dr. Wahidin Sudirohusodo Hospital for their invaluable guidance and support of this research.
Study Approval
Ethical approval for this study was granted by Research Ethics Committee of the Faculty of Medicine, Hasanuddin University, Makassar, Indonesia.
Ethical Declaration
The Ethics Committee of the Faculty Medicine granted approval the conduct of this research (Protocol #UH24090727 – Registry No. 805/UN4.6.4.5.31/ PP36/2024).
Funding Statement
This research received some funding/ support from Immuno-Oncology, Thematic Research Group Hasanuddin University, No15523/UN4.1/KEP/2024.
Authors Contribution
All authors contributed equally in this study.
Data Availability
The datasets used and analysed in this research are available from the corresponding, upon reasonable request.
Study Registration
We declare that this work is original and has not been submitted for publication or evaluation to any other journal.
Conflict of Interest
The authors declare no conflict of interest.
References
- Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL , Soerjomataram I, Jemal A. CA: a cancer journal for clinicians.2024;74(3). CrossRef
- Cancer statistics for the year 2020: An overview Ferlay J, Colombet M, Soerjomataram I, Parkin DM , Piñeros M, Znaor A, Bray F. International Journal of Cancer.2021. CrossRef
- Global colorectal cancer burden in 2020 and projections to 2040 Xi Y, Xu P. Translational Oncology.2021;14(10). CrossRef
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries Sung H, Ferlay J, Siegel RL , Laversanne M, Soerjomataram I, Jemal A, Bray F. CA: a cancer journal for clinicians.2021;71(3). CrossRef
- WHO Classification of Tumours Editorial Board, “WHO Calssification of Tumour 5th Edition.” International Agency for Research on Cancer, pp. 157–188, 2019. [Online]. Available: https://tumourclassification.iarc.who.int/chapters/31 .
- The significance of microsatellite instability in colorectal cancer after controlling for clinicopathological factors Kang S, Na Y, Joung SY , Lee SI , Oh SC , Min BW . Medicine (United States).2018;97(9). CrossRef
- Kumar, Robbins & Cotran Pathologic Basis of Diseases, Tenth Edit. 2021 .
- Targeting Twist expression with small molecules Pei H, Li Y, Liu M, Chen Y. MedChemComm.2017;8(2). CrossRef
- Tumor-infiltrating lymphocyte: features and prognosis of lymphocytes infiltration on colorectal cancer Qin M, Chen G, Hou J, Wang L, Wang Q, Wang L, Jiang D, et al . Bioengineered.2022. CrossRef
- Tumor-Infiltrating Lymphocytes in Colorectal Cancer: The Fundamental Indication and Application on Immunotherapy Bai Z, Zhou Y, Ye Z, Xiong J, Lan H, Wang F. Frontiers in Immunology.2022;12. CrossRef
- Tumor-infiltrating lymphocytes in cancer immunotherapy: from chemotactic recruitment to translational modeling Kraja FP , Jurisic VB , Hromić-Jahjefendić A, Rossopoulou N, Katsila T, Mirjacic Martinovic K, De Las Rivas J, Diaconu CC , Szöőr Á. Frontiers in Immunology.2025;16. CrossRef
- Programmed death-ligand 1 expression and tumor-infiltrating lymphocytes in colorectal adenocarcinoma Miskad UA , Hamzah N, Cangara MH , Nelwan BJ , Masadah R, Wahid S. Minerva Medica.2020;111(4). CrossRef
- A new method for evaluating tumor-infiltrating lymphocytes (TILs) in colorectal cancer using hematoxylin and eosin (H-E)-stained tumor sections Iseki Y, Shibutani M, Maeda K, Nagahara H, Fukuoka T, Matsutani S, Kashiwagi S, Tanaka H, Hirakawa K, Ohira M. PLOS ONE.2018;13(4). CrossRef
- Assessment of Tumor-infiltrating Lymphocytes Using International TILs Working Group (ITWG) System Is a Strong Predictor of Overall Survival in Colorectal Carcinoma: A Study of 1034 Patients Fuchs TL , Sioson L, Sheen A, Jafari-Nejad K, Renaud CJ , Andrici J, Ahadi M, Chou A, Gill AJ . The American Journal of Surgical Pathology.2020;44(4). CrossRef
- Immunohistochemical Expression of TWIST1 in Colorectal Adenocarcinoma Tawfik HM , Kamel MF , El-Hussieny M, Thabet DM . Minia Journal of Medical Research.2020;31(2). CrossRef
- Expression of trefoil factors and TWIST1 in colorectal cancer and their correlation with metastatic potential and prognosis Yusup A , Huji B , Fang C , Wang F , Dadihan T , Wang HJ , Upur H . ResearchGate.2025. CrossRef
- Twist1 is a potential prognostic marker for colorectal cancer and associated with chemoresistance Zhu D, Chen X, Zhang W, Wang J, Ouyang M, Zhong Q, Liu C. American Journal of Cancer Research.2015;5(6). CrossRef
- New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer Dongre A, Weinberg RA . Nature Reviews. Molecular Cell Biology.2019;20(2). CrossRef
- FGFR2 as A Prognostic and Predictive Marker in Colorectal Adenocarcinoma Based on TILs Grade Rianti AM , Cangara MH , Yamin A, Dahlan H, Ilyasa MR , Miskad UA . The Indonesian Biomedical Journal.2025;17(2). CrossRef
- Multiple biological functions of Twist1 in various cancers Zhao Z, Rahman MA , Chen ZG , Shin DM . Oncotarget.2017;8(12). CrossRef
- Tumor Immune Microenvironment during Epithelial–Mesenchymal Transition Taki M, Abiko K, Ukita M, Murakami R, Yamanoi K, Yamaguchi K, Hamanishi J, Baba T, Matsumura N, Mandai M. Clinical Cancer Research.2021;27(17). CrossRef
- Influence of the Twist gene on the invasion and metastasis of colon cancer Wang D, Rai B, Qi F, Liu T, Wang J, Wang X, Ma B. Oncology Reports.2018;39(1). CrossRef
- Emerging biological functions of Twist1 in cell differentiation Tu M, Ge B, Li J, Pan Y, Zhao B, Han J, Wu J, et al . Developmental Dynamics.;254(1):8-25. CrossRef
- Deregulation of TWIST1expression by promoter methylation in gastrointestinal cancers Alfahed A. Saudi Journal of Biological Sciences.2024;31(8). CrossRef
- A comprehensive exploration of twist1 to identify a biomarker for tumor immunity and prognosis in pan-cancer Wang Y, Li C, Jiang T, Yin Y, Wang Y, Zhao H, Yu L. Medicine.2024;103(15). CrossRef
- The Potential of PD-1 and PD-L1 as Prognostic and Predictive Biomarkers in Colorectal Adenocarcinoma Based on TILs Grading Rasyid NR , Miskad UA , Cangara MH , Wahid S, Achmad D, Tawali S, Mardiati M. Curr Oncol.2025. CrossRef
- AJCC Cancer Staging Manual. Springer International Publishing Gress DM , et al . 2017. CrossRef
- Prediction of colorectal tumor grade and invasion depth through narrow-band imaging scoring Maeyama Y, Mitsuyama K, Noda T, Nagata S, Nagata T, Yoshioka S, Yoshida H, et al . World Journal of Gastroenterology.2018;24(42). CrossRef
- Endoscopic Submucosal Dissection of Deeply Invasive Colorectal Cancers Using the Pocket-Creation Method: Analysis of Vertical Margins Morikawa T, Hayashi Y, Fukuda H, Ishii H, Nomura T, Ikeda E, Kitamura M, et al . Frontiers in Gastroenterology.2022;1. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2025
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times