Hexokinase 2 (HK2) Expression Pattern Comparison in Prostate Neoplasms
Download
Abstract
Introduction: This study aims to assess the differences in HK2 expression patterns in Benign Prostatic Hyperplasia (BPH), Prostatic Intraepithelial Neoplasia (PIN), Low Grade Prostate Adenocarcinoma (LGPAC) and High Grade Prostate Adenocarcinoma (HGPAC).
Materials and Methods: A cross-sectional observational study was conducted on 80 samples of prostate tissue embedded in paraffin, diagnosed from January 2020 to December 2022. Samples were divided into categories: Benign Prostatic Hyperplasia (BPH, n=37), Prostatic Intraepithelial Neoplasia (PIN, n=10), Low Grade Prostate Adenocarcinoma (LGPAC, n=9), and High Grade Prostate Adenocarcinoma (HGPAC, n=24). Immunohistochemistry was employed to evaluate HK2 expression Intensity, Proportion, and the Immunoreactive Score (IR-Score) approach was utilized to assign a score.
Result: There were significant differences in HK2 expression patterns among the groups (p=0.001). Regarding intensity, Benign Prostatic Hyperplasia (BPH) predominantly showed weak to moderate expression (32.4% weak, 67.6% moderate), while Prostatic Intraepithelial Neoplasia (PIN) demonstrated 90.0% moderate and 10.0% strong expression. Low Grade Prostate Adenocarcinoma (LGPAC) was also mostly moderate (88.9%), with 11.1% showing strong intensity, whereas High Grade Prostate Adenocarcinoma (HGPAC) was predominantly strong (75.0%). The percentage of stained area differed significantly (p=0.001), with Benign Prostatic Hyperplasia (BPH) showing 29.7% in ≤10%, 10.0% in 10–50%, and 67.6% in >50%. Prostatic Intraepithelial Neoplasia (PIN) and Low Grade Prostate Adenocarcinoma (LGPAC) demonstrated 100.0% staining in 10–50%, while High Grade Prostate Adenocarcinoma (HGPAC) showed 25.0% in 10–50% and 75.0% in >50%. Immunoreactive Score (IR-Score) analysis further revealed significant differences(p=0.001), with weak scores in 32.4% and strong scores in 67.6% of Benign Prostatic Hyperplasia (BPH) cases, while all Prostatic Intraepithelial Neoplasia (PIN), Low Grade Prostate Adenocarcinoma (LGPAC), and High Grade Prostate Adenocarcinoma (HGPAC) cases showed strong scores.
Conclusion: HK2 expression rises progressively from benign to malignant prostate lesions, with high-grade prostate adenocarcinoma exhibiting the strongest intensity and the largest stained area. Elevated HK2 expression is reliably noted in malignant cases compared to benign lesions. These results suggest that HK2 overexpression correlates with increased malignancy, reinforcing its possible function as a prognostic biomarker in prostate cancer.
Introduction
According to GLOBOCAN 2020 data, prostate cancer is the second most common disease in men and the fourth most common cancer globally [1]. It is the primary cause of cancer-related mortality for men in Western nations, accounting for over 28,000 fatalities in the US in 2012 and 94,000 deaths in Europe in 2008 [1]. In Asia, the average incidence of prostate cancer is 7.2 per 100,000 men per year. Over the past eight years, 1,102 patients with prostate cancer, with an average age of 67.18 years, have been treated in three educational center hospitals in Indonesia: Jakarta, Surabaya, and Bandung [2, 3].
Histopathological diagnosis of prostate tumors is established by evaluating architectural, nuclear, cytoplasmic, and luminal glandular components [4-6]. However, the architectural similarity between prostate adenocarcinoma and multifocal Prostatic Intraepithelial Neoplasia (PIN), the presence of small malignant glandular foci among benign glands, sample limitations, basal cell evaluation, and morphological variations of malignant lesions resembling benign lesions can complicate diagnostic determination [6].
Hexokinase 2 (HK2) is a key enzyme in the glycolysis pathway, responsible for the conversion of glucose to glucose-6-phosphate [7]. Because HK2 can give cancer cells energy substrates, increased expression of this gene has been linked to fast tumor growth [8]. HK2 has been identified as a potential target for developing more effective therapeutic strategies in addressing prostate cancer [9].
According to recent study, HK2 expression correlates with Gleason scores and is noticeably higher in prostate cancer tissues than in normal prostate epithelium [10]. Furthermore, HK2 overexpression has been linked to resistance to conventional therapies, such as hormonal therapy and chemotherapy in prostate cancer [9, 11].
This study was conducted to examine HK2 expression in prostate neoplasms originating from prostate glandular epithelial cells using samples from Makassar. By understanding the HK2 expression pattern in prostate neoplasms, it is expected to help establish prognosis determination and therapeutic approaches in prostate neoplasm patients. Data on HK2 expression in prostate neoplasms in Southeast Asia, particularly in Makassar, Indonesia, are still limited, so this study makes an important contribution to the regional literature.
Aim of the Study
This study aims to examine HK2 expression in prostate neoplasms originating from prostate glandular epithelial cells in prostate neoplasms patients in Makassar, Indonesia, which has not been previously conducted.
Materials and Methods
This observational analytical study with a cross- sectional design was conducted at the Anatomical Pathology Laboratory of Hasanuddin University Hospital in Makassar. The study received approval from the institutional ethics committee and was carried out in alignment with the Declaration of Helsinki.
The study population included all paraffin block archives of prostate tissues sent to the Anatomical Pathology Laboratory of Dr. Wahidin Sudirohusodo Hospital and Hasanuddin University Hospital, which were histopathologically diagnosed as Benign Prostatic Hyperplasia (BPH), Prostatic Intraepithelial Neoplasia (PIN), Low Grade Prostate Adenocarcinoma (LGPAC), and High Grade Prostate Adenocarcinoma (HGPAC) with H&E staining from January 2020 to December 2022.
Consecutive sampling was employed to choose samples according to their order of arrival at the laboratories until the necessary sample size was achieved. A sample size calculation was conducted utilizing the formula for comparing proportions between two groups, leading to a minimum needed sample of 80 specimens.
Sample Size Formula:
n=((Z∝√(2P(1-P))+Zβ√(P1 (1-P1)+P_0 (1-P0))2)/ (P1-P0)2
n ≥ 80 samples (2 groups) Note:
n: number of samples
Zα: 95% confidence interval (1.96)
Zβ: 40% power value (0.4)
P1: proportion of PI3K expression in prostate carcinoma cases (0.4)
P0: proportion of HK2 expression in prostate carcinoma cases (0.4)
minimum sample :
n: (1,96)2 x 0,4 x (1-0,4) = 80 sample (0,1)2
Criteria for Inclusion: Prostate tumor samples identified by pathologists as Benign Prostatic Hyperplasia (BPH), Prostatic Intraepithelial Neoplasia (PIN), Low Grade Prostatic Adenocarcinoma (LGPAC), and High Grade Prostatic Adenocarcinoma (HGPAC) using H&E staining. Paraffin blocks derived from tissues prepared in accordance with immunohistochemistry protocols. Exclusion Criteria: Samples lacking complete details about patient identity, clinical information, and macroscopic descriptions. Compromised, inadequate, exhausted paraffin block specimens, or specimens that were unsuitable for immunohistochemistry staining reprocessing.
Immunohistochemistry Procedure
Sections of tissue (3-4 μm thick) were sliced from paraffin blocks and placed on poly-L-lysine slides. Following deparaffinization and rehydration, immunohistochemical staining was conducted utilizing the Cell Marque Detection Kit with HK2 Rabbit Monoclonal Primary Antibody (1:100 dilution). Positive HK2 expression was characterized by brown staining in the cytoplasm, observed through light microscopy.
HK2 Expression Evaluation
HK2 expression was assessed through a semi- quantitative immunoreactive scoring system: Intensity: 0 (absent staining), 1 (low), 2 (intermediate), 3 (high). Proportion of stained area: 1 (≤10% positive cells), 2 (>10% and ≤50% positive cells), 3 (>50% positive cells). Immunoreactive Score (IR-Score) Evaluation: Determined by intensity multiplied by proportion, classified as weak (0-6) or strong (9). Two pathologists evaluated all slides separately, and agreement was achieved for any cases with discrepancies.
Statistical Analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) version 26.0. Descriptive statistics were used for demographic characteristics. Chi-square test was used to compare categorical variables between groups. A p-value <0.05 was considered statistically significant.
Results
Sample Characteristics
A total of 80 prostate tissue samples were analyzed (Table 1), consisting of 37 cases of Benign Prostatic Hyperplasia (BPH) (46.3%), 10 cases of Prostatic Intraepithelial Neoplasia (PIN) (12.5%), 9 cases Low Grade Prostatic Adenocarcinoma (LGPAC) (11.3%), and 24 cases of High Grade Prostate Adenocarcinoma (HGPAC) (30.0%).
| Variable | Group | ||||
| BPH | PIN | LGPAC | HGPAC | Total | |
| Age | n (%) | n (%) | n (%) | n (%) | |
| < 70 years | 27 (73.0) | 4 (40.0) | 5 (55.6) | 13 (54.2) | 49 (61.3) |
| ≥ 70 years | 10 (27.0) | 6 (60.0) | 4 (44.4) | 11 (45.8) | 31 (38.8) |
| Mean ± SD: 68.11 ± 8.76 | |||||
| Total | |||||
| n (%) | 37 (100.0) | 10 (100.0) | 9 (100.0) | 24 (100.0) | 80 (100.0) |
*Source : Primary data 2024. Benign Prostatic Hyperplasia (BPH); Prostatic Intraepithelial Neoplasia (PIN); Low Grade Prostate Adenocarcinoma (LGPAC); High Grade Prostate Adenocarcinoma (HGPAC).
The mean patient age was 68.11 ± 8.76 years, with 49 patients (61.3%) aged <70 years and 31 patients (38.8%) aged ≥70 years. HK2 expression was evaluated using the Immunoreactive Score (IR-Score), calculated by multiplying staining intensity with the proportion of the stained area, and classified as negative, weak positive, or strong positive (Figure 1).
Figure 1. HK2 Expression in Prostate Neoplasms.(A) Benign Prostatic Hyperplasia (BPH) 100x, HK2 is weakly expressed; (B) Prostatic Intraepithelial Neoplasia (PIN) 100x, HK2 is strongly expressed; (C) Prostatic Intraepithelial Neoplasia (PIN) 400x, HK2 is strongly expressed; (D) Low Grade Prostatic Adenocarcinoma (LGPAC) 100x, HK2 is strongly expressed; (E) Low Grade Prostatic Adenocarcinoma (LGPAC) 400x, HK2 is strongly expressed; (F) High Grade Prostatic Adenocarcinoma (HGPAC) 100x, HK2 is strongly expressed; (G) High Grade Prostatic Adenocarcinoma (HGPAC) 400x, HK2 is strongly expressed .
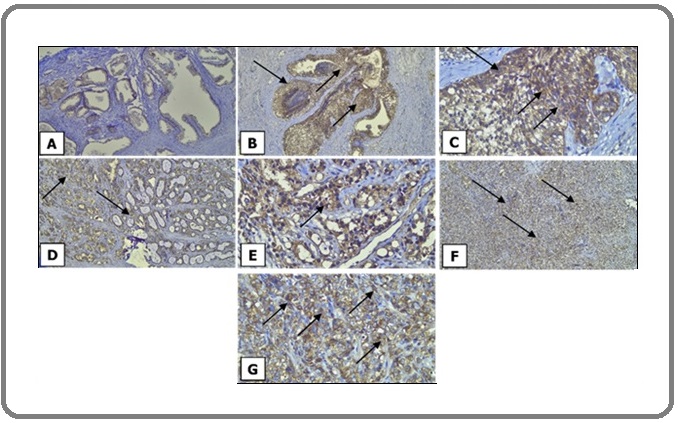
HK2 Expression in Prostate Tumors. Marked variations in HK2 expression were noted across groups (p<0.001) (Table 2).
| Variable | Group | X2 | |||||
| BPH | PIN | LGPAC | HGPAC | Total | p-value | ||
| Intensity | n (%) | n (%) | n (%) | n (%) | |||
| Weak | 12 (32.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 12 (15.0) | 83.781* | < 0.001** |
| Moderate | 25 (67.6) | 9 (90.0) | 8 (88.9) | 0 (0.0) | 42 (52.5) | ||
| Strong | 0 (0.0) | 1 (10.0) | 1 (11.1) | 24 (100.0) | 26 (32.5) | ||
| % Proportion | |||||||
| Stained area ≤10% | 11 (29.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 11 (13.8) | 61.828* | < 0.001** |
| Stained area 10% to 50% | 1 (2.7) | 10 (100.0) | 9 (100.0) | 6 (25.0) | 26 (32.5) | ||
| Stained area > 50% | 25 (67.6) | 0 (0.0) | 0 (0.0) | 18 (75.0) | 43 (53.8) | ||
| Immunoreactive Score (IR-Score) | |||||||
| Weak | 12 (32.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 12 (15.0) | 14.538* | < 0.001** |
| Strong | 25 (67.6) | 10 (100.0) | 9 (100.0) | 24 (100.0) | 68 (85.0) | ||
| Total | |||||||
| n (%) | 37 (100.0) | 10 (100.0) | 9 (100.0) | 24 (100.0) | 80 (100.0) |
* Fisher’s exact test, significant if p<0.05; **Chi-Square test, significant if p<0.05; Benign Prostatic Hyperplasia (BPH); Prostatic Intraepithelial Neoplasia (PIN); Low Grade Prostate Adenocarcinoma (LGPAC); High Grade Prostate Adenocarcinoma (HGPAC).
HK2 Expression Intensity in Prostate Neoplasms
Within the Benign Prostatic Hyperplasia (BPH) group, 12 instances (32.4%) exhibited weak intensity, while 25 instances (67.6%) displayed moderate intensity, and there were no instances of strong intensity. The PIN group displayed 9 instances (90.0%) with moderate intensity and 1 instance (10.0%) with strong intensity. The Low Grade Prostate Adenocarcinoma (LGPAC) group exhibited 8 cases (88.9%) of moderate intensity and 1 case (11.1%) of strong intensity. In contrast, the High Grade Prostate Adenocarcinoma (HGPAC) group was mainly robust, with 18 cases (75.0%) exhibiting strong intensity and 6 cases (25.0%) exhibiting moderate intensity.
HK2 Expression Proportion in Prostate Neoplasms
Within the Benign Prostatic Hyperplasia (BPH) group, 11 cases (29.7%) exhibited ≤10% stained area, 4 cases (10.8%) displayed 10–50%, and 22 cases (59.5%) demonstrated >50%. Every Prostatic Intraepithelial Neoplasia (PIN) and Low Grade Prostate Adenocarcinoma (LGPAC) case (100%) exhibited staining in the 10–50% range. In High Grade Prostate Adenocarcinoma (HGPAC), 6 cases (25.0%) exhibited 10–50% stained area, while 18 cases (75.0%) displayed >50%.
HK2 Expression IR Score in Prostate Neoplasms
Weak HK2 expression was noted exclusively in the Benign Prostatic Hyperplasia (BPH) group (12 cases, 32.4%), while strong expression was identified in 25 cases (67.6%) of BPH and in every case of Prostatic Intraepithelial Neoplasia (PIN) (100%), Low Grade Prostate Adenocarcinoma (LGPAC) (100%), and High Grade Prostate Adenocarcinoma (HGPAC) (100%). The median Immunoreactive Score (IR-Score) were 6.00 (range 1.00–6.00) for Benign Prostatic Hyperplasia (BPH), 4.00 (range 4.00–6.00) for Prostatic Intraepithelial Neoplasia (PIN), 4.00 (range 4.00–6.00) for Low Grade Prostate Adenocarcinoma (LGPAC), and 9.00 (range 6.00–9.00) for High Grade Prostate Adenocarcinoma (HGPAC).
Discussion
Sample Characteristics
In this study, 80 prostate samples were examined, comprising 37 Benign Prostatic Hyperplasia (BPH), 10 Prostatic Intraepithelial Neoplasia (PIN), 9 Low Grade Prostate Adenocarcinoma (LGPAC), and 24 HGPAC. The average age of patients was 68.1 years, aligning with worldwide epidemiological data indicating that prostate cancer primarily affects older men (Agell L, et al., 2011 [12]; IARC, 2020 [1]; DeVita VT, et al., 2018 [13]). Prostate cancer cases predominantly affect men over 65, with over two-thirds of instances found in this age group, mirroring demographic patterns observed in various Asian and Western studies (Bott SRJ & Ng KL, 2021 [14]; Kemenkes RI, 2018 [2]).
Histopathologically, Benign Prostatic Hyperplasia (BPH) remains the most frequent benign lesion, while adenocarcinoma is the most common malignant neoplasm of the prostate (Epstein JI, 2012 [15]; Humphrey PA, 2017 [16]; Moch H, et al., 2016 [17]). Prostatic Intraepithelial Neoplasia (PIN) represents an important precursor lesion with molecular and morphological changes that precede invasive carcinoma (Magers MJ, et al., 2015 [18]; Garg M, et al., 2013 [19]). Thus, the sample composition in this study reflects the natural spectrum of prostate pathology, ranging from benign hyperplasia to precursor lesions and invasive carcinoma.
HK2 Expression Intensity in Prostate Neoplasms
Marked variations in staining intensity were observed among diagnostic categories (p<0.001). Benign Prostatic Hyperplasia (BPH) displayed weak to moderate expressions of HK2, Prostatic Intraepithelial Neoplasia (PIN) typically showed moderate to strong expressions, while adenocarcinoma particularly High Grade Prostate Adenocarcinoma (HGPAC) demonstrated strong intensity in the majority of instances. This pattern corresponds with the idea that metabolic reprogramming, especially the activation of glycolysis through HK2, is a significant feature of malignant transformation (Chen J, et al., 2014 [7]; Ciscato F, et al., 2021 [20]; Patra KC, et al., 2013 [8]).
HK2 initiates the first irreversible stage of glycolysis by transforming glucose into glucose-6-phosphate, and its heightened expression promotes tumor cell growth, resistance to cell death, and adaptation to low oxygen environments (Rascio F, et al., 2021 [21]; Marimuthu S, et al., 2021 [22]). In prostate cancer, Sun X, et al. (2021) [10] showed that elevated HK2 expression is associated with higher Gleason grades and negative clinical characteristics, aligning with our finding that High Grade Prostate Adenocarcinoma (HGPAC) exhibited the most intense staining.
HK2 Expression Proportion in Prostate Neoplasms
The ratio of stained cells exhibited clear patterns. Benign Prostatic Hyperplasia (BPH) cases exhibited variability, varying from ≤10% to over 50%. In contrast, Prostatic Intraepithelial Neoplasia (PIN) exhibited a consistent staining percentage (100% of cases in 10–50%), whereas adenocarcinoma, particularly High Grade Prostate Adenocarcinoma (HGPAC), displayed wider staining, with over half of cases indicating >50% positivity.
This discovery highlights the importance of HK2 in fulfilling the metabolic needs of dividing tumor cells (Ciscato F, et al., 2021 [20]; Patra KC, et al., 2013 [8]). Prostatic Intraepithelial Neoplasia (PIN), as a precursor lesion, exhibited moderate expression, aligning with initial molecular alterations that occur before invasion (Epstein JI, 2018 [23]; Magers MJ, et al., 2015 [18]). The increase of HK2-positive regions in carcinoma corresponds with the Warburg effect, where cancer cells depend on aerobic glycolysis despite having abundant oxygen (Kumar V, et al., 2021 [24]; Rosai J, 2018 [25]).
HK2 Expression IR Score in Prostate Neoplasms
When intensity and proportion were integrated into the Immunoreactive Score (IR-Score), there was a gradual rise from benign to malignant lesions. Benign Prostatic Hyperplasia (BPH)recorded the lowest median score (6.00), Prostatic Intraepithelial Neoplasia (PIN) and Low Grade Prostate Adenocarcinoma (LGPAC) had intermediate scores (4.00), whereas High Grade Prostate Adenocarcinoma (HGPAC) achieved the highest score (9.00). Robust HK2 expression was consistently observed in all Prostatic Intraepithelial Neoplasia (PIN) and adenocarcinoma cases, whereas weak expression occurred solely in Benign Prostatic Hyperplasia (BPH). This lends credence to the hypothesis that the upregulation of HK2 is a critical factor in the development of prostate cancer (Patra KC, et al. (2013) [8]) demonstrated that HK2 is essential for the initiation and preservation of tumors in experimental models. Likewise, (Sun X and colleagues (2021)[10]) identified significant correlations between HK2 expression, Gleason grade, and unfavorable results. These results emphasize that HK2 expression indicates tumor aggressiveness and advancement.
Clinical and Biological Implications
These results are consistent with earlier work by (Sun et al. (2021) [10]), which showed that HK2 expression is markedly elevated in prostate cancer tissues with higher Gleason Grade Groups and correlates with negative clinicopathological characteristics. The association of HK2 expression with tumor grade in our cohort reinforces its possible value as a prognostic indicator that could assist in clinical decision-making. Our findings emphasize HK2 as a possible diagnostic and prognostic biomarker in prostate tumors. The gradual rise from benign to malignant lesions indicates its effectiveness in distinguishing uncertain cases, especially in small biopsies or lesions with similar histology (Egevad L, et al., 2021 [26]; Murgod PS, et al., 2021 [27]). Prostatic Intraepithelial Neoplasia (PIN) and early carcinoma exhibiting strong HK2 positivity may suggest a greater malignant potential, necessitating increased clinical monitoring (Amin MB & Tickoo SK, 2016 [28]; Cheng L, et al., 2020 [29]).
Elevated HK2 levels in High Grade Prostate Adenocarcinoma (HGPAC) bolster its prognostic significance, aligning with more aggressive clinical behavior (Epstein JI, 2018 [18]; Kryvenko ON & Epstein JI, 2016 [30]; Sehn JK, 2018 [6]). From a treatment perspective, HK2 has become a potential molecular target. The inhibition of HK2 has been demonstrated to disrupt tumor metabolism, increase cell sensitivity to chemotherapy and radiotherapy, and decrease tumor proliferation (Chen J, et al., 2014 [7]; Patra KC, et al., 2013 [8]; Wang Y, et al., 2019 [9]). Consequently, HK2 immunohistochemistry might support diagnosis, risk assessment, and inform upcoming personalized treatments. Specifically, in limited or unclear biopsy samples, HK2 staining can aid in differentiating between benign and malignant lesions, thus enhancing diagnostic precision.
Collectively, recent studies have highlighted multiple molecular and metabolic pathways contributing to prostate cancer progression (Samami et al. (2022) [31]).
demonstrated that circulating microRNAs hold diagnostic and prognostic potential, reflecting tumor biology and patient outcomes. (Wu et al. (2025) [32]) identified METCAM/MUC18 as a promising biomarker and therapeutic target associated with tumor aggressiveness and metastatic potential. Meanwhile, (Alhallaq and Sultan. (2025) [33]) emphasized the central role of glutamine and glutamate metabolic reprogramming in sustaining prostate cancer growth and therapeutic resistance. Together, these findings underscore the importance of integrating molecular (miRNA, METCAM/MUC18) and metabolic (HK2, glutamine/glutamate) markers to improve early detection, risk stratification, and targeted treatment strategies in prostate cancer.
The overexpression of HK2 in prostate carcinoma highlights its potential as a complementary biomarker in current risk stratification systems based on Gleason grade, PSA levels, and MRI findings. Increased HK2 expression, which reflects enhanced glycolytic activity, has been shown to correlate positively with Gleason scores and tumor aggressiveness (Kudryavtseva et al., (2016) [34]; Wang J et al., (2017) [35]). Integrating HK2 into existing prognostic models may therefore improve risk assessment and refine patient stratification, particularly among cases with similar histological grades.
Given its pivotal role in the Warburg effect, HK2 overexpression may also serve as a predictive marker for treatment response. Tumors with high HK2 activity demonstrate increased metabolic flexibility and may exhibit reduced sensitivity to androgen deprivation or radiation therapy, suggesting that HK2 inhibition could enhance therapeutic efficacy (Deng & Lu, (2015) [36]). Although this study did not assess outcomes such as recurrence, progression, or survival, future prospective studies are warranted to validate HK2’s potential role as a predictive and therapeutic biomarker in prostate cancer management.
Several previous studies have demonstrated that elevated HK2 expression is associated with increased tumor aggressiveness and poor prognosis in prostate and other cancers (Chen et al., (2014) [7]; Patra et al., (2013) [8]). High HK2 levels enhance glycolytic flux driven by PTEN and p53 loss, supporting tumor proliferation and therapy resistance (Deng & Lu, (2015) [36]). Therefore, future longitudinal studies are essential to validate HK2’s prognostic and predictive value and to explore its potential integration into clinical decision-making models for prostate cancer management.
Compared with other emerging biomarkers such as AMACR, ERG, and PTEN loss, HK2 provides a distinct metabolic perspective rather than representing purely genomic or structural alterations. AMACR and ERG are primarily diagnostic markers, while PTEN loss and ERG rearrangements are linked to tumor aggressiveness (Andarawi et al., (2025) [37]; Sayed et al., (2023) [38]. In contrast, HK2 reflects metabolic reprogramming that sustains tumor proliferation and survival, offering complementary biological and prognostic information to these conventional molecular markers (Kudryavtseva et al., (2016) [34]; Wang J et al., (2017) [35]; Patra et al., (2013) [8]).
Collectively, these findings suggest that HK2 might function not only as a diagnostic supplement but also as a prognostic and possibly therapeutic biomarker in prostate tumors. Validation in larger, clinically annotated cohorts is necessary to determine its role in standard practice.
Study Limitations
This study has several limitations. The cross-sectional nature of the design prevents causal conclusions, and the limited number of Prostatic Intraepithelial Neoplasia (PIN) and Low Grade Prostate Adenocarcinoma (LGPAC) cases might restrict statistical power. Being a single-center study limits its generalizability. Moreover, the absence of correlation with clinical outcomes and survival statistics hindered prognostic validation. Future multi-center prospective studies involving larger cohorts and extended follow-up are necessary to validate HK2 as a reliable diagnostic and prognostic biomarker in prostate cancer (Agell L, et al., (2011) [12]; IARC, 2020 [1]; Sehn JK, 2018 [6]).
In conclusion, this study shows that various forms of prostate tumors exhibit significant differences in HK2 expression levels. HK2 expression increases progressively from benign to malignant lesions, with high-grade adenocarcinoma showing the greatest intensity, the largest stained area, and the highest Immunoreactive Score (IR-Score). Consistently elevated HK2 expression was observed in malignant cases, while benign prostatic hyperplasia showed primarily weak to moderate expression. These findings support the notion that increased HK2 expression is associated with heightened malignancy and may result in worse prognosis, highlighting its potential role as a predictive and prognostic biomarker in prostate cancer. This is the first study from Makassar, Indonesia, analyzing HK2 expression in prostate neoplasms.
Furthermore, HK2 immunohistochemistry may aid in the histopathological diagnosis of prostate tumors and provide significant prognostic information. However, more extensive studies involving broader participant demographics and links to clinical outcomes are essential to establish its definitive clinical importance.
Declarations
Clinical trial registration
Not applicable.
Conlflicts of interest / Competing interests
The authors declare that they have no conflicts of interest.
Availability of data and material
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Authors’ contributions
J.L. contributed to data collection and primary drafting of the manuscript. M.H.C., U.A.M. contributed to the manuscript’s conception, study design, and final drafting, and supervised the research. M.H.C. and U.A.M. contributed to data interpretation and manuscript editing.
M.H.C. and U.A.M. contributed to histopathological evaluation and analysis. C.K. and B.J.N. supervised the research. S.T. contributed to statistical analysis and interpretation. A.Y. contributed to the Anatomical Pathology Laboratories at Dr. Wahidin Sudirohusodo Hospital. All authors reviewed and approved the final version of the manuscript.
Ethics approval: This study was approved by the Ethics and Research Committee of the Faculty of Medicine, Hasanuddin University, Makassar, Indonesia (Approval No. 1079/UN4.6.4.5.31-/PP36/2024).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Acknowledgments
We express our sincere gratitude to the patients and to the technical staff of the Anatomical Pathology Laboratories at Dr. Wahidin Sudirohusodo Hospital and Hasanuddin University Hospital for their valuable assistance. This study was conducted as part of the thesis requirements for Jamilah Lestari, MD.
Declaration on generative AI and AI-assisted technologies in the writing process
The authors affirm that no generative AI or AI- assisted technologies were used in the preparation of this manuscript; all writing, analysis, and revisions were performed by the authors.
Originality Declaration for Figures
All figures included in this manuscript are original and have been created by the authors specifically for the purposes of this study. No previously published or copyrighted images have been used. The authors confirm that all graphical elements, illustrations, and visual materials were generated from the data obtained in the course of this research or designed uniquely for this manuscript.
References
- International Agency for Research on Cancer. Prostate cancer factsheet. In: Globocan 2020, vol. 23. Lyon, France: IARC; 2020. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/27-Prostate-fact-sheet.pdf .
- Kementerian Kesehatan Republik Indonesia. Pedoman Nasional Pelayanan Kedokteran Tata Laksana Kanker Prostat. Jakarta: Kementerian Kesehatan RI; 2018. Available from: https://kemkes.go.id/id/pnpk-2018---tata-laksana-kanker-prostat .
- Tumours of the urinary tract. In: Clinical Equine Oncology Knottenbelt DC , Patterson-Kane JC , Snalune KL. . Eivesler.2015;:652–63. CrossRef
- DiFiore’s Atlas of Histology with Functional Correlations. 12th ed. Wolters Kluwer Health; 2013 Eroschenko VP . Available from: https://books.google.co.id/books?id=sH87M12QswcC..
- Anatomy of the prostate gland and surgical pathology of prostate cancer Hammerich KH , Ayala GE , Wheeler TM . Cambridge University Press.2008. CrossRef
- Prostate Cancer Pathology: Recent Updates and Controversies Sehn JK . Missouri Medicine.2018;115(2).
- Hexokinase 2 overexpression promotes the proliferation and survival of laryngeal squamous cell carcinoma Chen J., Zhang S., Li Y., Tang Z., Kong W.. Tumour Biol.2014;35(4). CrossRef
- Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer Patra K.C., Wang Q., Bhaskar P.T., Miller L., Wang Z., Wheaton W.. Cancer Cell.2013;24(2). CrossRef
- Branched-Chain Amino Acid Metabolic Reprogramming Orchestrates Drug Resistance to EGFR Tyrosine Kinase Inhibitors Wang Y., Zhang J., Ren S., Sun D., Huang H.-Y., Wang H.. Cell Rep.2019;28(2). CrossRef
- Expression and Clinical Significance of HKII and HIF-1α in Grade Groups of Prostate Cancer Sun X., Huang Q., Peng F., Wang J., Zhao W., Guo G.. Front Genet.2021;12(680928). CrossRef
- MUC5AC Expression in Various Tumor Types and Nonneoplastic Tissue - A Tissue Microarray Study on 10,399 Tissue Samples Rico S.D., Mahnken M., Buscheck F., Dum D., Luebke A.M., Kluth M.. Technol Cancer Res Treat.2021;20(15330338211043328). CrossRef
- PI3K signaling pathway is activated by PIK3CA mRNA overexpression and copy gain in prostate tumors, but PIK3CA, BRAF, KRAS and AKT1 mutations are infrequent events Agell L., Hernández S., Salido M., Muga S., Juanpere N., Arumí-Uria M.. Mod Pathol.2011;24(3). CrossRef
- Cancer: Principles & Practice of Oncology DeVita V.T., Lawrence T.S., Rosenberg S.A.. 11th ed. Wolters Kluwer Health.2018. 1. Available from: https://oncology.lwwhealthlibrary.com/book.aspx?bookid=2549.
- Prostate Cancer Bott S.R.J., Ng K.L.. Exon Publications.2021. CrossRef
- Diagnosis of limited adenocarcinoma of the prostate Epstein J.I.. Histopathology.2012;60(1). CrossRef
- Histopathology of Prostate Cancer Humphrey P.A.. Cold Spring Harb Perspect Med.2017;7(10). CrossRef
- WHO Classification of Tumours of the Urinary System and Male Genital Organs. 4th ed. Lyon: IARC Press; 2016 Moch H, H, Humphrey PA , Ulbright TM , Reuter VE . Available from: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-The-Urinary-System-And-Male-Genital-Organs-2016..
- Intraductal Carcinoma of the Prostate: Morphologic Features, Differential Diagnoses, Significance, and Reporting Practices Magers M, Kunju LP , Wu A. Archives of Pathology & Laboratory Medicine.2015;139(10). CrossRef
- Histopathological spectrum of 364 prostatic specimens including immunohistochemistry with special reference to grey zone lesions Garg M, Kaur G, Malhotra V, Garg R. Prostate International.2013;1(4). CrossRef
- Hexokinase 2 in Cancer: A Prima Donna Playing Multiple Characters Ciscato F, Ferrone L, Masgras I, Laquatra C, Rasola A. International Journal of Molecular Sciences.2021;22(9). CrossRef
- The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review Rascio F, Spadaccino F, Rocchetti MT , Castellano G, Stallone G, Netti GS , Ranieri E. Cancers.2021;13(16). CrossRef
- Mucins reprogram stemness, metabolism and promote chemoresistance during cancer progression Marimuthu S, Rauth S, Ganguly K, Zhang C, Lakshmanan I, Batra SK , Ponnusamy MP . Cancer and Metastasis Reviews.2021;40(2). CrossRef
- Prostate cancer grading: a decade after the 2005 modified system Epstein JI . Modern Pathology.2018;31. CrossRef
- Robbins & Cotran Pathologic Basis of Disease. 10th ed. Elsevier; 2021. Available from: https://shop.elsevier.com/books/robbins-and-cotran-pathologic-basis-of-disease/kumar/978-0-323-53113-9 Kumar V, Abbas AK , Aster JC , Perkins JA . .
- Rosai and Ackerman’s Surgical Pathology Hoda SA , Patel A. American Journal of Clinical Pathology.2018;149(6). CrossRef
- Benign mimics of prostate cancer Egevad L, Delahunt B, Furusato B, Tsuzuki T, Yaxley J, Samaratunga H. Pathology.2021;53(1). CrossRef
- Histomorphological Mimickers of benign prostatic lesions with prostatic adenocarcinoma Murgod PS , Doshi PR , Nisal AR , Nimbargi RC . Journal of Pathology of Nepal.2021;11(1). CrossRef
- Diagnostic Pathology: Genitourinary. In: Diagnostic Pathology: Genitourinary Amin MB , Tickoo SK . Elsevier.2016. CrossRef
- Urologic Surgical Pathology Cheng L, MacLennan GT , Bostwick DG . Elsevier.2020. CrossRef
- Prostate Cancer Grading: A Decade After the 2005 Modified Gleason Grading System Kryvenko ON , Epstein JI . Archives of Pathology & Laboratory Medicine.2016;140(10). CrossRef
- The Potential Diagnostic and Prognostic Value of Circulating MicroRNAs in the Assessment of Patients With Prostate Cancer: Rational and Progress Samami E, Pourali G, Arabpour M, Fanipakdel A, Shahidsales S, Javadinia SA , Hassanian SM , Mohammadparast S, Avan A. Frontiers in Oncology.2022;11. CrossRef
- METCAM/MUC18 is a Biomarker and Therapeutic Target for Prostate Cancer Wu G, Hsieh C, Fu Y, Chuang Y, Wei Y, Pong Y, Su Y, Tsai V, Wu J. Asian Pacific Journal of Cancer Prevention.2025;26(8). CrossRef
- Fueling Prostate Cancer: The Central Role of Glutamine/Glutamate Metabolic Reprogramming Alhallaq A, Sultan N. Asian Pacific Journal of Cancer Prevention.2025;26(9). CrossRef
- Expression of HK2 Gene Is Deregulated in Prostate Cancer Kudryavtseva AV , Fedorova MS , Zhavoronkov A, Moskalev AA , Zasedatelev AS , Zhuravlev YM , et al . Asian J Pharm.2016;10(3):S297–S302. Available from: https://www.researchgate.net/publication/317224006_Expression_of_HK2_gene_is_deregulated_in_Prostate_cancer.
- Increased expression of glycolytic enzymes in prostate cancer tissues and association with Gleason scores Wang J, Li J, Li X, Peng S, Li J, Yan W, Cui Y, Xiao H, Wen X. International Journal of Clinical and Experimental Pathology.2017;10(11).
- Targeting hexokinase 2 in castration-resistant prostate cancer Deng Y., Lu J.. Mol Cell Oncol.2015;2(3). CrossRef
- Exploring the efficacy of AMACR, ERG, and AR immunostains in prostatic adenocarcinoma and their association with novel grade groups Andarawi M.O., Otifi H., Hassan H., Yousif A.A., Mustafa S.A., Elsiddig S.A., Babker A.M., Ali E.I., Elhag O.O.. Eur J Histochem.2025;69(1). CrossRef
- Immunohistochemical expression of alpha-methyl-CoA racemase (AMACR) and ERG in prostatic adenocarcinoma and prostatic hyperplasia: a comparative study Sayed R.M.S., El Shorbagy G., Shibel P.E.E.. Asian Pac J Cancer Prev.2023;24(8). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2026
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times