The Association of Chronic Oral Mucositis with Quality of Life in Head and Neck Cancer Patients Undergoing Radiotherapy
Download
Abstract
Introduction: Oral mucositis is a common and debilitating side effect of radiotherapy in patients with head and neck cancer, significantly impacting their quality of life. This study aimed to investigate the prevalence of chronic oral mucositis and its association with the quality of life in patients undergoing head and neck radiotherapy.
Materials and Methods: This descriptive-analytical study was conducted on patients with head and neck cancer receiving radiotherapy at Radiotherapy department of Vasei hospital, Sabzevar. Demographic data (age, gender, comorbidities, tobacco use, and medications) and clinical data (cancer type, radiation dose, and radiotherapy duration) were collected. Quality of life was assessed using the WHOQOL-BREF questionnaire, and the severity of oral mucositis was evaluated based on WHO criteria. Data were analyzed using descriptive and analytical statistical methods, including chi-square, independent t-tests, and Mann-Whitney tests.
Results: Oral mucositis was observed in 90% of patients undergoing head and neck radiotherapy, with its severity significantly correlated with radiation dose (p < 0.001), radiotherapy duration (p = 0.02), and the presence of comorbidities (p = 0.03). Patients with chronic mucositis reported significantly different quality of life scores across physical, psychological, and social domains, with notable reductions in mental (p < 0.001), social (p < 0.001), and environmental health (p < 0.001). Additionally, tobacco use (CI: 1.3–6.1, p < 0.001) and a history of chemotherapy (CI: 1.1–5.2, p = 0.02) were associated with increased symptom severity. A significant correlation was observed between younger patient age and the occurrence of dysphagia and chronic mucositis (p < 0.001).
Conclusion: Chronic oral mucositis is a prevalent complication in head and neck radiotherapy patients, adversely affecting their quality of life. Effective management through preventive and therapeutic strategies can enhance patient outcomes. Future studies with larger sample sizes and a focus on novel therapeutic interventions for mucositis are recommended.
Introduction
Head and neck cancers, primarily comprising squamous cell carcinomas of the oral cavity, pharynx, and larynx, are among the most common malignancies worldwide. Radiotherapy is a cornerstone treatment for these cancers, used either alone or in combination with chemotherapy and surgery to target tumor cells. However, this therapeutic approach is associated with numerous side effects, one of the most common and challenging being oral mucositis. Oral mucositis refers to the inflammation and painful ulceration of the oral mucosa, resulting from direct damage by ionizing radiation to healthy tissues and the production of reactive oxygen species and inflammatory cytokines [1]. Studies have shown that oral mucositis occurs in 80% to 100% of head and neck cancer patients undergoing radiotherapy, with 60% to 80% of cases reaching high severities (Grade 3 or 4 based on WHO criteria) [2, 3].
Oral mucositis has a significant negative impact on patients’ quality of life, including severe pain, difficulty swallowing (dysphagia), weight loss, an increased risk of oral infections, and impairment of daily functions such as eating and speaking [4]. This complication affects not only physical health but also the psychological and social well-being of patients, leading to reduced quality of life, treatment interruptions, and increased healthcare costs. For instance, a study by Elting et al. (2007) demonstrated that severe mucositis in head and neck cancer patients could increase treatment costs by up to $6,000, imposing a substantial economic burden on both patients and healthcare systems [5].
Several factors influence the severity of oral mucositis, including radiation dose, duration of radiotherapy, use of concurrent chemotherapy, patient age, underlying diseases, and lifestyle habits such as tobacco use [2]. Furthermore, recent research, such as the study by San Valentin et al. (2023), has utilized omics approaches to identify genes and biological pathways associated with mucositis, which could improve the prediction and management of this condition [6].
Sonis et al. (2004) proposed a five-stage model for the pathophysiology of mucositis, involving initial damage, inflammatory signaling, apoptosis, ulceration, and healing. Apoptosis, or programmed cell death, is a natural process by which damaged cells are safely eliminated from the body. This model highlights the key roles of reactive oxygen species (ROS) and inflammatory mediators in the progression of mucositis [1].
Numerous efforts have been made to prevent and treat oral mucositis. The MASCC/ISOO guidelines recommend the use of agents such as benzydamine, palifermin, and chlorhexidine to reduce mucositis severity [7]. Additionally, recent studies have indicated that natural interventions like thyme honey, turmeric, and zinc and glutamine supplements can mitigate the severity of mucositis, pain, and weight loss [8, 9]. However, heterogeneity in treatment protocols and a lack of standardized practices across different centers pose significant challenges in managing this condition. For example, a study by Bergamaschi et al. (2024) across 25 radiotherapy centers in Italy found considerable variation in mucositis management protocols, with 14.5% of patients discontinuing treatment due to the severity of side effects [10]. Although intensity-modulated radiotherapy (IMRT) is widely considered to reduce mucositis severity compared to conventional techniques, recent evidence suggests that the difference may not be statistically significant. For instance, Kruser et al. found comparable rates of acute mucositis across IMRT, 3D-CRT, and tomotherapy cohorts, indicating that other clinical factors may have greater influence on mucosal outcomes [11].
Despite recent advances, chronic oral mucositis remains an unresolved clinical problem requiring further research. This study aims to investigate the prevalence of chronic oral mucositis in head and neck cancer patients undergoing radiotherapy and assess its association with quality of life. By focusing on related factors such as radiation dose, underlying diseases, and tobacco use, this research seeks to provide local data from the Radiotherapy Department of Vasei Hospital in Sabzevar to contribute to improved prevention and treatment strategies. The findings of this study could assist clinicians and health policymakers in designing targeted interventions to reduce the burden of this complication and enhance treatment outcomes.
Materials and Methods
Study Design and Setting
This research was a descriptive-analytical study conducted to investigate the frequency of chronic oral mucositis and its association with quality of life in head and neck cancer patients undergoing radiotherapy. The cross-sectional study was carried out in the Radiotherapy Department of Vasei Hospital in Sabzevar between 2016 and 2022.
Study Population and Sampling
The study population consisted of all head and neck cancer patients receiving radiotherapy at the Radiotherapy Department of Vasei Hospital in Sabzevar. Inclusion criteria were: (1) confirmed diagnosis of head and neck cancer (e.g., squamous cell carcinoma), (2) completion of radiotherapy treatment, and (3) willingness to participate and provide informed consent. Exclusion criteria included:
(1) presence of pre-existing severe oral conditions (e.g., active oral infections), (2) discontinuation of radiotherapy before completion, and (3) unwillingness to continue participation. A total of 50 eligible patients were enrolled using a census sampling method. All patients who met the inclusion criteria and completed their treatment during the study period were included in the final analysis.
Data Collection
Data were collected through face-to-face interviews and reviews of patients’ medical records. Demographic information, including age, gender, tobacco use, substance abuse, and underlying diseases (e.g., hypertension, heart disease, diabetes), was recorded. Clinical data, such as cancer type (e.g., squamous cell carcinoma), received radiation dose, duration of radiotherapy, and history of chemotherapy (including the types of chemotherapeutic agents used, such as 5-fluorouracil or platinum-based drugs), were also extracted. Radiotherapy was delivered using conventional fractionation (2 Gy per fraction, once daily). Among patients receiving chemotherapy, cisplatin was administered in 38% of cases. Oral mucositis was assessed using WHO criteria by two radiation oncologists; however, inter-rater reliability was not formally measured and is acknowledged as a methodological limitation.
Assessment Tools
Chronic oral mucositis was defined as persistent symptoms (e.g., pain, dysphagia, xerostomia) lasting more than 3 months after completion of radiotherapy. The severity of oral mucositis is usually assessed using a five-grade classification system (Grade 0 to 4), based on histopathologic and clinical criteria as described by Sunavala-Dossabhoy et al., which aligns with standard WHO grading practices [12]. No formal grading system (e.g., WHO or CTCAE) was applied in this study. Quality of life was evaluated using the WHOQOL-BREF questionnaire. This 26-item questionnaire examines four domains: physical health, psychological health, social relationships, and environmental health, and has been used in similar studies due to its high validity and reliability [13]. The questionnaires were administered by a trained researcher, and necessary explanations were provided to patients when needed.
Data Analysis
Data were analyzed using SPSS software (version 22). Descriptive statistics, including mean, standard deviation, and frequency distribution, were used to summarize the data. Tables were used to present the distribution of variables by gender, age, underlying diseases, and treatment type. The chi-square test was used to analyze the association between qualitative variables (e.g., gender and mucositis occurrence). For quantitative variables, the independent t-test was used if the data were normally distributed (based on the Kolmogorov-Smirnov test); otherwise, the non-parametric Mann-Whitney test was employed. A statistical significance level (p-value) of less than 0.05 was considered.
Ethical Considerations
This study was approved by the Ethics Committee of Sabzevar University of Medical Sciences with the code of IR.MEDSAB.REC.1402.091. Written informed consent was obtained from all participants, and patient information was kept confidential. Patients could withdraw from the study at any time without affecting their treatment process.
Results
A total of 50 patients with head and neck cancer, who underwent treatment at the Radiotherapy Department of Vasei Hospital in Sabzevar between 2016 and 2022, were included in this study.
Demographic and Clinical Characteristics
The majority of patients were male (68%), and approximately 84% were married. Half of the patients were illiterate, and 62% resided in urban areas. Tobacco use was reported in 26% of patients, while opium use was observed in 58%. Underlying medical conditions were present in 42% of patients, with hypertension (20%) and heart disease (12%) being the most prevalent. Table 1 shows demographic and clinical characteristics data of patients.
| Variable | Groups | n (%) |
| Gender | Male / Female | 34 (68) / 16 (32) |
| Marital Status | Married / Single | 42 (84) / 8 (16) |
| Literacy Level | Literate / Illiterate | 23 (46) / 27 (54) |
| Place of Residence | Urban / Rural | 31 (62) / 19 (38) |
| Tobacco Use | Yes / No | 13 (26) / 37 (74) |
| Substance Abuse | Yes / No | 29 (58) / 21 (42) |
| Underlying Disease | Yes / No | 21 (42) / 29 (58) |
| Cancer Type | Oropharynx / Larynx / Tongue / Lip | 23 (46) / 21 (42) / 4 (8) / 2 (4) |
| Chemotherapy | Yes / No | 40 (80) / 10 (20) |
Regarding cancer type, 46% of patients had oropharyngeal cancer, 42% had laryngeal cancer, 8% had tongue cancer, and 4% had lip cancer. Over 80% of patients received chemotherapy, with 5-FU being administered in 72% of cases. The mean age of the patients was 62 years, the total radiation dose was 62 Gy, the dose per session was 2 Gy, and the mean treatment duration was 6 weeks.
Incidence of Mucositis and Oral/oropharyngeal complications
Overall, 78% of patients experienced symptoms related to chronic oral mucositis. The most common symptoms were reduced salivary secretion, dysphagia, and a combination of both. Twenty-two percent of patients were asymptomatic. Chronic oral mucositis symptoms were observed in 10% of patients, while 90% showed no chronic symptoms.
Quality of Life Status
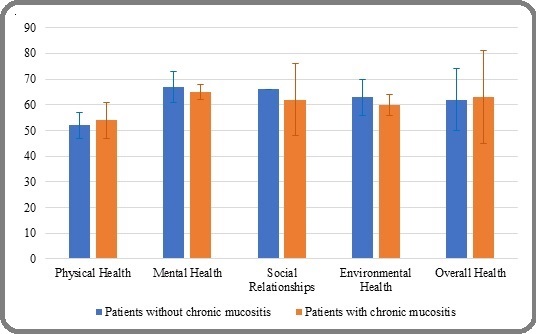
The mean quality of life scores across various domains were as follows: physical health (54), psychological health (67), social relationships (63), environmental health (63), and overall health (63). Statistical analysis revealed that patients with chronic oral mucositis symptoms had significantly lower scores in mental, social, environmental, and overall health domains compared to those without symptoms (p<0.001). However, physical health scores were slightly higher among symptomatic patients. Notably, the zero standard deviation observed in the “Social Relationships” domain reflects identical scores reported by all patients in that subgroup, as confirmed by the statistical output. Figure 1 illustrates the QoL score differences between groups.
Figure 1. Comparison of Mean Quality of Life Scores between Patients with and Without Chronic Mucositis Symptoms, p-value < 0.001.
Analytical Statistical Findings
Chi-square and Mann-Whitney tests indicated that chronic mucositis symptoms were significantly associated with several variables. Details are provided in Table 2.
| Variable | Groups | Chronic Oral Mucositis (%) | Dysphagia (Oral Mucositis-related) (%) | p-value (Chronic) | p-value (Dysphagia) |
| Gender | Male / Female | 68% / 32% | 75% / 25% | < 0.001 | 0.07 |
| Tobacco Use | Yes / No | 29% / 71% | 34% / 66% | < 0.001 | < 0.026 |
| Opium Use | Yes / No | 63% / 37% | 66% / 34% | 0.07 | < 0.019 |
| Cancer Type | Larynx / Oropharynx / | 47% / 43% / 6% / 4% | 48% / 47% / 5% / 0% | < 0.001 | 0.013 |
| Tongue / Lip | |||||
| Chemotherapy | Yes / No | 84% / 16% | 85% / 15% | < 0.01 | < 0.001 |
| Time Since Radiotherapy | >6 months / <6 months | 83% / 17% | 83% / 17% | < 0.001 | < 0.001 |
| Age (Mean ± SD) | With Symptoms / Without Symptoms | 61 ± 15 / 69 ± 9 | 61 ± 15 / 64 ± 15 | < 0.001 | < 0.001 |
| Total Radiation Dose (Gy) | With Symptoms / Without Symptoms | 62 ± 11 / 64 ± 5 | 63 ± 10 / 58 ± 11 | < 0.001 | < 0.001 |
| Dose per Session (Gy) | With Symptoms / Without Symptoms | 2 ± 0.07 / 2 ± 0 | 2 ± 0.07 / 2 ± 0 | < 0.001 | < 0.001 |
| Duration of Radiotherapy (wk) | With Symptoms / Without Symptoms | 6 ± 1 / 6 ± 0 | 6 ± 0 / 6 ± 0 | < 0.001 | 0.02 |
| Physical Health | With Symptoms / Without Symptoms | 54 ± 7 / 52 ± 5 | 52 ± 5 / 54 ± 7 | < 0.001 | < 0.001 |
| Mental Health | With Symptoms / Without Symptoms | 67 ± 6 / 65 ± 3 | 65 ± 3 / 67 ± 6 | < 0.001 | < 0.001 |
| Social Relationships | With Symptoms / Without Symptoms | 62 ± 14 / 66 ± 0 | 66 ± 0 / 62 ± 14 | < 0.001 | < 0.001 |
| Environmental Health | With Symptoms / Without Symptoms | 63 ± 7 / 60 ± 5 | 60 ± 4 / 63 ± 7 | < 0.001 | < 0.001 |
| Overall Health | With Symptoms / Without Symptoms | 63 ± 18 / 62 ± 12 | 63 ± 18 / 62 ± 12 | < 0.001 | < 0.001 |
Factors demonstrating a statistically significant association with chronic oral mucositis symptoms: Chronic symptoms were more prevalent in male patients compared to females (p < 0.001). Tobacco use was associated with increased swallowing difficulties and chronic symptoms (p < 0.001). Substance abuse showed a significant correlation with swallowing symptoms (p < 0.05). Cancer type (particularly laryngeal) was associated with higher symptom occurrence (p < 0.001). A history of chemotherapy was significantly associated with increased swallowing difficulties and chronic symptoms (p < 0.01).
More than six months post-radiotherapy was correlated with increased oral/oropharyngeal complications (p < 0.001). Symptomatic patients had a lower mean age (p < 0.001). Symptoms were associated with higher total radiation dose and lower dose per session (p < 0.001). Quality of life across all domains (physical, psychological, social, environmental, and overall) was significantly lower in patients with chronic symptoms (p < 0.001).
This study found a high prevalence of chronic oral mucositis in head and neck cancer patients, which was associated with a significant reduction in quality of life. Furthermore, significant correlations were observed between the occurrence of chronic symptoms and younger age, tobacco use, cancer type, chemotherapy, radiotherapy dosage, and time elapsed since treatment.
Discussion
This study was conducted to investigate the prevalence of chronic oral mucositis and its association with quality of life in patients undergoing head and neck radiotherapy. The results indicated that 90% of the patients experienced oral mucositis. This high prevalence is consistent with findings from previous studies. For example, Trotti et al. (2003) reported that oral mucositis occurs in over 80% of patients undergoing head and neck radiotherapy [14]. A recent study by Iovoli et al. (2023) also demonstrated that 70% of patients undergoing IMRT develop severe mucositis, which aligns with our results [2]. Additionally, Maria et al. (2017) reported that radiation-induced mucositis occurs in 100% of patients undergoing accelerated fractionation radiotherapy, a technique in which radiation doses are delivered more frequently over a shorter overall treatment period to intensify tumor control, highlighting the high severity of this complication in specific treatment protocols [15].
The severity of mucositis in this study was significantly associated with higher radiation doses (over 60 Gy) and longer radiotherapy duration (over 6 weeks) (P < 0.05). This finding is consistent with the study by Elting et al. (2007), which showed that higher radiation doses and longer treatment courses are associated with an increased risk of severe mucositis [5]. According to QUANTEC recommendations, the ideal maximum dose (Dmax) to the oral cavity should be kept below 50 Gy to reduce the risk of severe mucositis. However, in many clinical settings, this constraint may be exceeded due to tumor proximity or target volume requirements. Recent findings by Liu et al. (2025) further emphasize the importance of dose optimization and predictive modeling to mitigate mucosal toxicity [16]. This discrepancy may be due to differences in treatment protocols, types of radiotherapy equipment, or study population characteristics. For instance, the current study, conducted in the Radiotherapy Department of Vasei Hospital in Sabzevar, may differ from other centers in terms of equipment and protocols. This may also limit generalizability to other settings with different protocols or patient populations.
Tobacco and substance use in this study were associated with more severe mucositis and dysphagia (P < 0.05). This result aligns with the findings of Lalla et al. (2022), which demonstrated that smoking and substance use increase mucosal inflammation and delay tissue repair [7]. Additionally, patients with underlying conditions such as hypertension and heart disease experienced more severe mucositis (P < 0.05). This finding is supported by the study of Lio et al. (2025), which indicated that underlying conditions such as hypertension and cardiovascular diseases are associated with increased radiotherapy complications, including mucositis [16]. This association may be due to reduced tissue repair capacity in patients with underlying conditions, highlighting the need for special attention in managing these patients.
One of the key findings of this study was the negative impact of chronic mucositis on patients’ quality of life. Patients with chronic mucositis reported significantly lower scores in psychological, social, and environmental health domains of the WHOQOL-BREF questionnaire (p < 0.01). Interestingly, physical health scores were slightly higher among symptomatic patients. This result is consistent with the study by Epstein et al., which demonstrated that severe mucositis substantially reduces patients’ quality of life, particularly in physical functions such as swallowing and nutrition [17]. Additionally, a study by Elting et al. (2008) reported that mucositis occurs in nearly all head and neck radiotherapy patients and has significant negative effects on quality of life and functional status, often to the extent that opioid analgesics provide insufficient pain relief [3]. These findings underscore the importance of early prevention and management of mucositis. The observation of higher physical domain scores in patients with chronic mucositis may reflect survivor bias, as these individuals were more likely to be alive and available for follow-up at ≥6 months post-treatment. This pattern has been noted in other survivorship studies and warrants cautious interpretation.
In this study, the use of concurrent chemotherapy (e.g., 5-fluorouracil and platinum-based agents) was associated with increased mucositis severity (p < 0.05). This finding aligns with the study by Pulito et al. (2020), which reported that the combination of chemotherapy and radiotherapy increases the risk of severe mucositis, particularly in patients receiving specific agents such as 5-FU [18]. This highlights the need for targeted preventive strategies in patients undergoing combined modality therapy.
Although this study did not specifically evaluate mucositis management approaches, a review of the literature indicates that various methods have been proposed for the prevention and treatment of this condition. For example, the MASCC/ISOO guidelines recommend the use of benzydamine for the prevention of radiation-induced mucositis in head and neck cancer patients [7]. Furthermore, a study demonstrated that the use of honey or propolis can reduce mucositis severity [19]. These natural approaches, due to their accessibility and low side effects, represent attractive options for mucositis management. Additionally, a study by Bjordal et al. (2023) showed that low-level laser therapy may be effective in reducing mucositis severity and improving patients’ quality of life [20]. These findings suggest the potential of novel approaches to alleviate the burden of this condition, though further research is needed to confirm their efficacy.
The association between younger age and chronic mucositis symptoms in our study contrasts with some evidence suggesting delayed mucosal repair in older patients [21]. This discrepancy may be influenced by unmeasured confounders such as baseline performance status, HPV-related tumor biology, or behavioral factors including tobacco and opium use. Further studies with stratified analysis are needed to clarify these associations. Severe oral mucositis imposes substantial economic burdens. As reported by Elting et al., the estimated cost per patient can exceed $6,000 due to hospitalization, nutritional support, and treatment delays. These findings highlight the importance of preventive strategies and the potential cost-effectiveness of advanced radiotherapy techniques.
The observed decline in social and environmental QoL domains among patients with chronic mucositis underscores the need for structured survivorship support. Nurses are well-positioned to screen for persistent symptoms using targeted questions or brief QoL instruments during routine follow-up. Evidence-based interventions such as oral care bundles including saline rinses, topical analgesics, and hydration protocols can be nurse-led and integrated into outpatient care. Timely referrals to speech therapy and nutrition counseling further enhance recovery and reduce isolation, especially in head and neck cancer survivors.
This study had several limitations that should be considered. The absence of qualitative data (e.g., patient narratives) limits deeper insight into the lived experience of chronic mucositis. No formal power calculation was performed prior to sampling, which may affect the statistical robustness of subgroup analyses. The sample size (50 patients) was relatively small, which may limit the generalizability of the results. Additionally, the study was conducted in the radiotherapy department of Vasei Hospital in Sabzevar, which may differ from other regions in terms of equipment, treatment protocols, or demographic characteristics. Our study included a predominantly male population (68%), which may affect generalizability. Gender-related differences in symptom reporting such as underreporting of pain among women have been documented and may influence observed prevalence rates. Future research should incorporate gender-sensitive tools and strive for balanced representation to better capture the full spectrum of survivorship experiences. The cross-sectional design allowed for assessment of chronic mucositis prevalence but did not capture its incidence or symptom trajectory over time; future longitudinal studies are recommended to evaluate progression and recovery. Moreover, the lack of direct evaluation of mucositis prevention and treatment methods in this study restricted the analysis of their effectiveness. The absence of formal mucositis grading limits direct comparison with studies reporting severity distributions (e.g., Grade 3/4 rates). Additionally, the observed chronic prevalence (10%) may reflect differences in assessment methods, follow-up duration, or patient characteristics compared to other studies [22-24]. Finally, the high prevalence of opium use (58%) among participants may have influenced mucosal outcomes through mucoactive or immunomodulatory effects, warranting further investigation.
In conclusion, this study demonstrated that oral mucositis is a common complication in patients undergoing head and neck radiotherapy, with 90% of patients experiencing it. The severity of mucositis was significantly associated with higher radiation doses (over 60 Gy), longer radiotherapy duration (over 6 weeks), tobacco use, and the presence of underlying conditions such as hypertension and heart disease. Additionally, chronic mucositis had a significant negative impact on patients’ quality of life, particularly in psychological, social, and environmental domains, as evidenced by lower scores on the WHOQOL-BREF questionnaire among patients with chronic mucositis.
The findings of this study underscore the importance of early and effective management of oral mucositis. Preventive strategies such as maintaining oral hygiene, using mucosal protective agents (e.g., benzydamine or palifermin), and novel approaches like laser therapy can help reduce the severity of mucositis and improve patients’ quality of life. The results also highlight the need for special attention to patients with risk factors such as tobacco use, substance abuse, or underlying diseases, as these groups are at higher risk of experiencing severe mucositis and related complications like dysphagia.
For future research, it is recommended that studies with larger sample sizes and more diverse populations be conducted to enhance the generalizability of the findings. Investigating the efficacy of novel prevention and treatment methods, such as laser therapy, honey-based interventions, or new medications like palifermin, could contribute to improved mucositis management. Longitudinal studies to assess changes in quality of life over time and after treatment completion could provide a better understanding of the long-term effects of mucositis. Finally, exploring the impact of socioeconomic factors, such as education level and place of residence, on mucositis severity and quality of life could help identify at-risk groups. Survivors may benefit from simple supportive strategies such as sipping ice water to ease oral discomfort, maintaining good oral hygiene, and avoiding tobacco products to reduce mucosal irritation and promote healing. Future research should explore predictive biomarkers such as interleukin-6 (IL-6), identified through omics-based approaches, to stratify mucositis risk. Additionally, randomized trials investigating cryotherapy may offer promising avenues for prevention and symptom mitigation in head and neck cancer patients undergoing radiotherapy. Also, future research can explore nurse-driven peer support interventions, which may enhance psychological resilience, reduce isolation, and improve long-term quality of life. Survivor-led groups and structured mentoring programs could be integrated into nursing follow-up protocols to provide accessible, empathetic support.
Funding
This study was fully funded by the IR.MEDSAB. REC.1402.091Sabzevar University of Medical Sciences.
Clinical trial registration
Not applicable
Conflicts of interest/Competing interests
Authors declare that they have no conflicts of interest.
Availability of data and material
The data sets used and/or analyzed during the current study are available from the corresponding authors per reasonable request.
Authors’ contributions
Conceptualization: R.M., and R.Gh, Methodology: R.M., and R.Gh, Software: Z.B., Validation: R.Gh, and R.M., Formal analysis: Z.B. and H.A Investigation: R.M., Data Curation: P.R., Writing - Original Draft: H.R.S, Writing - Review & Editing: H.R.S, R.Gh, and H.A., Visualization: R.Gh, Supervision: R.M. and Project administration: R.Gh.
Ethics approval
This study was registered and implemented at Sabzevar University of Medical Sciences with the ethical code IR.MEDSAB.REC.1402.091.
Consent to participate
Written informed consent was obtained from all participants, and patient information was kept confidential.
Consent for publication
Written informed consent was obtained from all participants, and the trial was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We sincerely thank all head and neck cancer patients who participated in this study with their patience and valuable cooperation. This study was registered and implemented at Sabzevar University of Medical Sciences with the ethical code IR.MEDSAB. REC.1402.091. We extend our thanks to the Clinical Research Development Unit of Vasei Hospital, affiliated with Sabzevar University of Medical Sciences for their kind support.
Declaration on generative AI and AI-assisted technologies in the writing process
We used AI in translation of this manuscript.
References
- Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients Sonis ST , Elting LS , Keefe D, Peterson DE , Schubert M, Hauer-Jensen M, Bekele BN , et al . Cancer.2004;100(9 Suppl). CrossRef
- Severe Oral Mucositis After Intensity-Modulated Radiation Therapy for Head and Neck Cancer Iovoli AJ , Turecki L, Qiu ML , Khan M, Smith K, Yu H, Ma SJ , Farrugia MK , Singh AK . JAMA network open.2023;6(10). CrossRef
- Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life Elting LS , Keefe DM , Sonis ST , Garden AS , Spijkervet F. K. L., Barasch A, Tishler RB , et al . Cancer.2008;113(10). CrossRef
- Oral complications in the treatment of cancer patients Mosel D. D., Bauer R. L., Lynch D. P., Hwang S. T.. Oral Diseases.2011;17(6). CrossRef
- Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies Elting LS , Cooksley CD , Chambers MS , Garden AS . International Journal of Radiation Oncology, Biology, Physics.2007;68(4). CrossRef
- Attempts to Understand Oral Mucositis in Head and Neck Cancer Patients through Omics Studies: A Narrative Review San Valentin EMD , Do K, Yeung SJ , Reyes-Gibby CC . International Journal of Molecular Sciences.2023;24(23). CrossRef
- MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy Lalla RV , Bowen J, Barasch A, Elting L, Epstein J, Keefe DM , McGuire DB , et al . Cancer.2014;120(10). CrossRef
- Efficacy of turmeric in the treatment of oral mucositis in patients with head and neck cancer after radiotherapy or chemoradiotherapy: a systematic review and meta-analysis Wu C, Wu H, Shih C, Yeh T, Ma W. Frontiers in Pharmacology.2024;15. CrossRef
- Effectiveness of natural-based products for radiation-induced oral mucositis therapy: A systematic review Pranadwista ZF , Nur'aeny N. Cancer Treatment and Research Communications.2023;36. CrossRef
- Management of radiation-induced oral mucositis in head and neck cancer patients: a real-life survey among 25 Italian radiation oncology centers Bergamaschi L, Vincini MG , Zaffaroni M, Pepa M, Angelicone I, Astone A, Bergamini C, et al . Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer.2023;32(1). CrossRef
- Acute hematologic and mucosal toxicities in head and neck cancer patients undergoing chemoradiotherapy: a comparison of 3D-CRT, IMRT, and helical tomotherapy Kruser TJ , Rice SR , Cleary KP , Geye HM , Tome WA , Harari PM , Kozak KR . Technology in Cancer Research & Treatment.2013;12(5). CrossRef
- Histopathologic grading of oral mucositis Sunavala-Dossabhoy G., Abreo F., Timiri Shanmugam P. S., Caldito G.. Oral Diseases.2015;21(3). CrossRef
- The World Health Organization Quality of Life Assessment (WHOQOL): development and general psychometric properties The-WHOQOL-Group . Social Science & Medicine (1982).1998;46(12). CrossRef
- Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review Trotti A, Bellm LA , Epstein JB , Frame D, Fuchs HJ , Gwede CK , Komaroff E, Nalysnyk L, Zilberberg MD . Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2003;66(3). CrossRef
- Radiation-Induced Oral Mucositis Maria OM , Eliopoulos N, Muanza T. Frontiers in Oncology.2017;7. CrossRef
- The Trajectory of Oral Mucositis in Head and Neck Cancer Patients Undergoing Radiotherapy and its Influencing Factors Liu M, An R, Wu Z, Dai L, Zeng Q, Chen W. Ear, Nose, & Throat Journal.2025;104(5). CrossRef
- Quality of life and oral function following radiotherapy for head and neck cancer Epstein J. B., Emerton S., Kolbinson D. A., Le N. D., Phillips N., Stevenson-Moore P., Osoba D.. Head & Neck.1999;21(1). CrossRef
- Oral mucositis: the hidden side of cancer therapy Pulito C, Cristaudo A, Porta CL , Zapperi S, Blandino G, Morrone A, Strano S. Journal of experimental & clinical cancer research: CR.2020;39(1). CrossRef
- Honey and a mixture of honey, beeswax, and olive oil-propolis extract in treatment of chemotherapy-induced oral mucositis: a randomized controlled pilot study Abdulrhman M, Elbarbary NS , Ahmed Amin D, Saeid Ebrahim R. Pediatric Hematology and Oncology.2012;29(3). CrossRef
- A systematic review with meta-analysis of the effect of low-level laser therapy (LLLT) in cancer therapy-induced oral mucositis Bjordal JM , Bensadoun R, Tunèr J, Frigo L, Gjerde K, Lopes-Martins RA . Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer.2011;19(8). CrossRef
- Mucosal wound healing: the roles of age and sex Engeland CG , Bosch JA , Cacioppo JT , Marucha PT . Archives of Surgery (Chicago, Ill.: 1960).2006;141(12). CrossRef
- Prevalence of oral mucositis, dry mouth, and dysphagia in advanced cancer patients Mercadante S, Aielli F, Adile C, Ferrera P, Valle A, Fusco F, Caruselli A, et al . Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer.2015;23(11). CrossRef
- Comparison of Dose- Volume Histograms of Three Whole Breast Radiotherapy Regimes: Conventional, Normal Hypofractionation and FAST-FORWARD Hypofractionation Nabizadeh-Javan AH , Porouhan P, Jalambadani Z, Javadinia SA , Chaman R, Dhawan G, et al . Asian Pacific Journal of Cancer Prevention.2025;26(11):4109-4115. CrossRef
- Burden of Oral Mucositis: A Systematic Review and Implications for Future Research Berger K, Schopohl D, Bollig A, Strobach D, Rieger C, Rublee D, Ostermann H. Oncology Research and Treatment.2018;41(6). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2026
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times