The Effect of Curcumin in Combination Chemotherapy with 5-FU on non-Malignant Fibroblast Cells
Download
Abstract
Background: Curcumin has long been known to possess therapeutic properties and recent research have shown that it can inhibit malignant cell proliferation in vivo and in vitro. However, studies on combination therapy with curcumin and chemotherapeutic drugs are so limited.
Methods: With this regard, we studied the effect of curcumin on the toxicity of 5-Flouracil(5-FU) in the treatment of normal fibroblasts in vitro using L929 (nonmalignant fibroblast cell) cell line. Curcumin in the doses of 5 and 15 μM was used. First control groups treated to curcumin, alone while second control groups received the chemotherapeutic drug, separately. Experimental groups received curcumin in combination with 5-Flouracil. Cell viability was measured after 24, 48, and 72h through MTT method. Statistical differences were analyzed by ANOVA test.
Results: At all studied times in combination cases (curcumin+5-FU) with increasing concentration of curcumin, toxicity was decreased. Indeed, curcumin in combination with 5-Fu in low concentration inhibited the effect of 5-FU (p<0.05).
Conclusion: It can be concluded that curcumin in combination therapy with 5-FU may induce lower toxicity in normal cells and reduce possible side effects.
Introduction
Cancer considered as the major health problem in modern medicine which is related to the high rate of mortality after cardiovascular diseases in enormous countries [1]. Today, whether in industry [2][3][4] or medicine [1] applications, the use of nanotechnology has become an important tool to improve the efficiency and the health states. Indeed, there are numerous surveys which investigate the role of the tumor microenvironment in cancer development [5][6][7][8]. Fibroblasts in the tumor stroma are known with their crucial function in carcinogenesis especially in the initiation of epithelial tumor progression. Indeed these cells surrounding the cancerous tissue form a separate microenvironment [2]. Based on the previous report investigate the possible response of patient toward chemotherapeutics is an important issue in cancer-related treatments [9]. Indeed, The major element indefinite drug delivery strategies are precise drug delivery to the targeted organ with optimal concentration with minimal toxicity and maximum efficiency of the drug [10]. In this regards, Chemotherapy is a major treatment option in various cancers [11]. Though the most considerable effects of chemotherapy for cancer therapy, the toxicity impacts of chemotherapy exerted organ damages include liver and kidney injury, immunosuppressant, and etc [12]. Recently, investigated the novel, less toxic and, effective therapeutic strategies has been the main issues in advanced cancer treatments [13]. Certainly, Chemotherapy non-toxic agents could be an interesting attitude for decreased the cancer-related stigma [14]. Besides, new cytotoxic agents which definitely destroy the cancer cells and simultaneously elicit an antitumor immune response is promising topics in cancer therapy [15]. In this regards, the induction of Fibroblast apoptosis could be considered as a major function in normal and pathological scar development [16]. 5-Fluorouracil (5-FU) is an effective inhibitor of the growth of fibroblasts, which is the central cell mediators of scar development. Cytotoxic effects of 5-FU on cancer cells is recognized but there are little data about its impacts on the non-malignant cells [17]. The previous study shows the possible apoptosis-inducing effects of polyphenol agent; curcumin. This agent exerts the fibroblast apoptosis and may be played as an efficient therapeutic factor. Previously data indicated that curcumin promote the apoptosis in various cell lines. While the cell killing mechanism of curcumin is not fully determined and it seems that this influence is related to cell type and reactive oxygen species (ROS) [18][19].In this way, we investigate the combined effects of the 5-FU and curcumin on the non-Malignant Fibroblast in treating L929 cells.
Materials and Methods
Materials
Mouse fibroblast cell line (L929) was obtained from Pasteur Institute-Iran (national cell bank of Pasteur Institute) and curcumin form MERK Company. MTT [3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide], DMSO, RPMI 1640 (Roswell Park Memorial Institute), Fetal Bovine serum (FBS), phosphate-buffered saline (PBS) were purchased from Sigma-Aldrich (St. Louis, MO). Ferric reducing/antioxidant power (FRAP) photometric in vitro assay kit obtained from East Sage Holding Research Company.
Cell culture and treatment
L929 cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 U/ml penicillin and 100 mg/ml streptomycin in a humidified 95% air/5% CO2 incubator at 37°C. Cells were allowed attaching for 12 h before treatment. Curcumin (98% purity) was dissolved in DMSO (Dimethyl Sulfoxide), as a 10 mg/ml stock solution, and stored at -20°C in a light protected cover. Curcumin was diluted in complete medium exactly before experiments in a way that the final concentration of DMSO was not more than 1.5%. The doses of curcumin used were 5 and 15μM. For the 5-Flouracil group, final concentration was 10µg/ml. Toxicity and antioxidant capacity assayed at 24, 48 and 72h. Briefly, cells were incubated with 5-Flouracil with or without curcumin for 24, 48 and 72 h. For the first control group, different mentioned concentrations of curcumin were used for 24, 48 and 72 h. For the second control group, 5-Flouracil was added to the plates, separately without receiving curcumin. Negative control group contained cells without any treatment. DMSO concentration was the same in all groups. After a specific time, cell viability was assessed by MTT assay and anti-oxidant power by FRAP assay.
MTT assay
Cell viability was assessed after treatment by MTT colorimetric assay method. The MTT assay is based on the capacity of the mitochondrial enzyme, succinate-dehydrogenase of viable cells to transform the MTT salt into a blue colored formazan product and is proportional to the number of living cells present. The concentration of 5 mg/ml MTT was prepared by dissolving MTT in phosphate-buffered saline (PBS) solution and the solution was filtered through a 0.22μm filter. Briefly, about 20μl of MTT stock solution was added to all the wells and the plates were incubated at 37 °C in the CO2 incubator for 4 h. After that, culture medium quickly removed from each well and 150μl of DMSO was added to all the wells to dissolve the formazan crystals. The plates were agitated in room temperature for 10 min then the optical density (OD) of each well was measured at a test wavelength of 570 nm and a reference wavelength of 650 nm with a microplate spectrophotometer (OD570 - OD630).
Statistical analysis
All analyses were performed using SPSS 16.0 statistical software. Normality of data distribution was assessed using the Kolmogorov-Smirnov method. Comparison of cell viabilities was conducted using ANOVA and Tukey Post Hoc analyses. P-value of less than 0.05 was considered significant. Comparisons were conducted using ANOVA statistical test.
Results
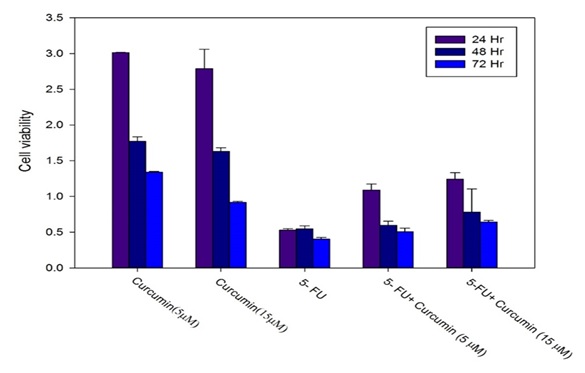
MTT analysis shows that curcumin exerted lower cytotoxic effects in compared to 5-FU in all times and concentrations in compared to 5-FU(P<0.05). At all times in combination cases (curcumin+5-FU) with increasing concentrations of curcumin, toxicity was decreased. 5-FU toxicity at all times was significant. Indeed, curcumin in combination with 5-Fu in low concentration inhibited the effect of 5-FU (p<0.05). The cellular viability in different treatments using MTT assay shown in Figure1 and Table 1.
| Groups | L-929 relative cell viability,24h | L-929 relative cell viability,48h | L-929 relative cell viability,72h |
| Curcumin (5 μM) | 3.013±0.003 | 1.771±0.064 | 1.340±0.009 |
| Curcumin (15 μM) | 2.788±0.271 | 1.630±0.052 | 0.917±0.013 |
| 5- fluoruouracil | 0.528±0.020 | 0.547±0.040 | 0.403±0.023 |
| 5- fluoruouracil + Curcumin (5 μM) | 1.089±0.085 | 0.596±0.056 | 0.505±0.051 |
| 5-fluoruouracil + Curcumin (15 μM) | 1.241±0.090 | 0.779±0.326 | 0.642±0.020 |
Figure 1: Cell viability was assessed by MTT assay in various incubation times at 24, 48 and 72h..
Discussion
Among all types of disorders including chronic disorders [20][21][22] cancer has furthermost significant Recently, the effectiveness of various chemotherapeutic on cancer cells were assessed [23][24][25][26]. Many chemotherapeutic agents are accompanied with several side effects.
So, in some surveys, herbal agents evaluated in cancer therapy [15][27][28]. which show acceptable cytotoxic effects.
Our survey evaluates the unique effects of curcumin in combination with 5-FU, to measure how curcumin reduce the cytotoxic effects of 5-Fu in non-Malignant Fibroblast cells- L929.
This survey shows that different doses of curcumin were able to reduce the cytotoxicity of 5-FU on fibroblasts. This is due to this fact that curcumin inhibits the regulation of cell death, and prevents the cytotoxicity of chemotherapeutic agent 5-FU.
In agreement with our results, Haryuna et al indicated that curcumin decreased the Noise-Exposed cochlear fibroblasts death, which was in accordance with our data [29]. In this regards, it could be suggested that curcumin potentially acts as a supportive agent in the prevention and management for fibroblasts damage within chemotherapy of cancer cells.
In a similar research study, curcumin used as an effectual adjuvant to cisplatin cancer treatment. This approach in head and neck cancer could facilitate cisplatin chemoresistance by modifying therapeutic targets and decrease the cisplatin-related ototoxic side effects [30].
An earlier study establishes the impact of curcumin on peroxynitrite-induced damage in rat spiral ganglion neurons. Curcumin revoked cytochrome c release, blocked activation of caspase-3, and changed the expression of the Bcl-2 family [31].
In addition, Curcumin considerably improved nonischemic wound healing in a dose-response versus controls by increased reepithelialization. Enhanced wound healing effects were related to substantial reductions in pro-inflammatory cytokines interleukin (IL)-1 and IL-6. Likewise, Curcumin decreased hypertrophic scarring [32].
In the current survey, with an initial examination of 5-Fu- in non-Malignant Fibroblast cells- L929 cells we examined the impacts of curcumin on the cytotoxicity of 5-Fu by taking different doses.
Overall curcumin as a protective agent against chemotherapy on fibroblast cells exerted the anti-death activity in a dose-dependent manner in all examined times.
However, our results, from both in vitro examination, offer the valuable perceptions into the advantages of curcumin on elevating the protective effect and decline the side effects of 5-FU chemotherapy, , which is valuable results. However, additional studies proposed to evaluate the effectiveness of curcumin on fibroblast cells.
In conclusions, the current research confirms that curcumin exerted dose-dependent effects even at low concentrations in non-Malignant Fibroblast cells- L929 cells. In other words, all significant effects of curcumin on the viability of the cells were considerable. However, the combined effects of curcumin and 5-Fu showed lower cytotoxic effects in compared to different concentrations of 5-Fu. Curcumin at low concentrations, at some time, can reduce the negative effect of the drug on these cells. Regarding the fact, that combination therapy induced toxicity in L929 cells, it can be concluded that such combination therapy may induce lower toxicity in normal cells and reduce possible side effects.
References
- Evaluation of synthesized platinum nanoparticles on the MCF-7 and HepG-2 cancer cell lines Mohammadi H, Abedi A, et al . International Nano Letters.2013;3(1):1-5.
- Recent research contributions concerning use of nanofluids in heat exchangers: A critical review Bahiraei Mehdi, Rahmani Reza, Yaghoobi Ali, Khodabandeh Erfan, Mashayekhi Ramin, Amani Mohammad. Applied Thermal Engineering.2018;133. CrossRef
- Application of nanofluid to improve the thermal performance of horizontal spiral coil utilized in solar ponds: Geometric study Khodabandeh Erfan, Safaei Mohammad Reza, Akbari Soheil, Akbari Omid Ali, Alrashed Abdullah A.A.A.. Renewable Energy.2018;122. CrossRef
- Numerical study of flow and heat transfer of water-Al2O3 nanofluid inside a channel with an inner cylinder using Eulerian–Lagrangian approach Ahmadi Ali Akbar, Khodabandeh Erfan, Moghadasi Hesam, Malekian Navid, Akbari Omid Ali, Bahiraei Mehdi. Journal of Thermal Analysis and Calorimetry.2017;132(1). CrossRef
- Cancer associated fibroblasts: An essential role in the tumor microenvironment Tao Leilei, Huang Guichun, Song Haizhu, Chen Yitian, Chen Longbang. Oncology Letters.2017;14(3). CrossRef
- Tumor microenvironment: a main actor in the metastasis process Spano Daniela, Zollo Massimo. Clinical & Experimental Metastasis.2012;29(4). CrossRef
- Tumor Microenvironment Complexity: Emerging Roles in Cancer Therapy: Figure 1. Swartz Melody A., Iida Noriho, Roberts Edward W., Sangaletti Sabina, Wong Melissa H., Yull Fiona E., Coussens Lisa M., DeClerck Yves A.. Cancer Research.2012;72(10). CrossRef
- Microenvironmental regulation of tumor progression and metastasis Quail Daniela F, Joyce Johanna A. Nature Medicine.2013;19(11). CrossRef
- Cytotoxic effects of the newly-developed chemotherapeutic agents 17-AAG in combination with oxaliplatin and capecitabine in colorectal cancer cell lines Kheradmand Fatemeh, Mohammadian Mahshid, Zeynali Shima, Azarbaijani AnahitaFathi, Khadem Ansari MohammadHassan. Research in Pharmaceutical Sciences.2017;12(6). CrossRef
- General Characteristics and Cytotoxic Effects of Nano-Poly (Butyl Cyanoacrylate) Containing Carboplatin on Ovarian Cancer Cells Kanaani Leila, Ebrahimi-Far Meysam, Kazemi S Maryam, Choupani Edris, Mazloumi Tabrizi Maral, ebrahimi shahmabadi hasan, Akbarzadeh Khiyavi Azim. Asian Pacific Journal of Cancer Prevention.2017;18(1). CrossRef
- Effects of Cisplatin-Loaded Niosomal Nanoparticleson BT-20 Human Breast Carcinoma Cells Kanaani Leila, javadi Iraj, Ebrahimi-Far Meysam, Ebrahimi shahmabadi Hasan, Akbarzadeh Khiyavi Azim, Mehrdiba Torkan. Asian Pacific Journal of Cancer Prevention.2017;18(2). CrossRef
- The effect of nanoliposomal and PE gylated nanoliposomal forms of 6-gingerol on breast cancer cells Khalili M, Akbarzadeh A, et al . Research Journal of Recent Sciences.2009;2277(2502).
- Preparation, characterization, and cytotoxic effects of liposomal nanoparticles containing cisplatin: anin vitrostudy Poy Donya, Akbarzadeh Azim, Ebrahimi Shahmabadi Hasan, Ebrahimifar Meysam, Farhangi Ali, Farahnak Zarabi Maryam, Akbari Azam, Saffari Zahra, Siami Fatemeh. Chemical Biology & Drug Design.2016;88(4). CrossRef
- Preparation of silibinin loaded pegylatedniosomal nanoparticles and investigation of its effect on MCF-10A human breastcancer cell line Sajjadiyan S Z, Ghadernejad H, Toofani Milani A, Mohammadian M, Abdolahpour S, Taslimi S, Moradi- Sardareh H, Afrisham R, Kooti W. Der Pharmacia Lettre.2016;8(16):70-75.
- Antitumor Immunostimulatory Effect of Sitosterol from Salvia atropatana on Tumor bearing mice Rostaminasab S, Noori S, Yaghmaei B, Dolatabad M R, Milani A T, Mohammadian M. Advances in Bioresearch.2015;6(5):133-140.8p.
- Curcumin-induced fibroblast apoptosis andin vitrowound contraction are regulated by antioxidants and heme oxygenase: implications for scar formation Scharstuhl A., Mutsaers H.A.M., Pennings S.W.C., Szarek W.A., Russel F.G.M., Wagener F.A.D.T.G.. Journal of Cellular and Molecular Medicine.2009;13(4). CrossRef
- Effects of 5-fluorouracil on proliferating fibroblasts in vivo Ophir Avinoam. Experimental Eye Research.1991;53(6). CrossRef
- Curcumin synergizes with 5-fluorouracil by impairing AMPK/ULK1-dependent autophagy, AKT activity and enhancing apoptosis in colon cancer cells with tumor growth inhibition in xenograft mice Zhang Pan, Lai Ze-Lin, Chen Hui-Fen, Zhang Min, Wang An, Jia Tao, Sun Wen-Qin, Zhu Xi-Min, Chen Xiao-Feng, Zhao Zheng, Zhang Jun. Journal of Experimental & Clinical Cancer Research.2017;36(1). CrossRef
- [Effect of curcumin on growth and function of fibroblast in human hyperplastic scar] Kang JY, Huang H, Zhu FQ. Zhongguo Zhong Xi Yi Jie He Za Zhi.2009 Dec;29(12):1100-1103.
- Association between Plasma Cholesteryl Ester Transfer Protein activity and Lipid profiles in Metabolic Syndrome in an Iranian Population Goodarzi MT , Mohammadian M , Borzouei Sh , et al . Int Res J Biological Sci.2014;3:89-90.
- Comparison of Biochemical Factors and Liver Enzymes in type 2 Diabetes Patients and Healthy Individuals Ebrahimi Far M, Mazdapour M, Kaki A, Mohammadi P, et al . Bull. Env. Pharmacol. Life Sci.2015;4:1-4.
- Evaluation of Serum Iron, Zinc and Their Relationships with Glycemic Control Status in Iranian Elderly Women with Type 1 Diabetes Mellitus Mahshid Mohammadian , Attabak Toofani Milani , Mohammad Reza Hassas , Siamak Rashidi , Elmira Roshani Asl , Sadegh Rostaminasab , Mohadeseh Nemati , Farid Javandust , Narmin Mokarizadeh , Farhad fathi younesi . Journal of Pharmacy and Pharmacology.2015;3(9). CrossRef
- A molecular basis for the synergy between 17‑allylamino‑17‑demethoxy geldanamycin with Capecitabine and Irinotecan in human colorectal cancer cells through VEFG and MMP-9 gene expression Zeynali-Moghaddam Shima, Mohammadian Mahshid, Kheradmand Fatemeh, Fathi-Azarbayjani Anahita, Rasmi Yousef, Esna-Ashari Omid, Malekinejad Hassan. Gene.2019;684. CrossRef
- Pegylation of nanoliposomal paclitaxel enhances its efficacy in breast cancer Esfahani MKM , et al. . Tropical Journal of Pharmaceutical Research.2014;13(8):1195-1198.
- To evaluate the effect of formulation of Nanoarchaeosomal 6-gingerol on the growth of breast cancer MCF-7 cell line Ahmadi L, Chiani M, et al . New Cellular and Molecular Biotechnology Journal.2015;5(19):47-52.
- Advantages of paclitaxel-loaded nano niosomes to nanoliposomal formulation: an in vitro study Zarei M, Norouzian D, Chiani M, Ebrahimi H, Mohammadi M, Akbarzadeh A. Int J Life Sci Bt & Pharm Res.2013;2:335-342.
- Evaluation the cytotoxicity of nanoliposomal artemisinin on breast cancer cell line Dadgar N, Alavi E, Moftakhari Esfahani M, et al . New Cellular and Molecular Biotechnology Journal.2014;4:99-103.
- Making paclitaxel nanoliposomal and evaluating its effect on the MCF-7 Maedeh Koohi Moftakhari Esfahani , Seyed Ebrahim Alavi , Amir Heidarinasab , Mohsen Chiani . New Cellular and Molecular Biotechnology Journal.2013;9:67-71.
- Curcumin Reduces the Noise-Exposed Cochlear Fibroblasts Apoptosis Haryuna Tengku, Riawan Wibi, Nasution Ardyansyah, Ma'at Suprapto, Harahap Juliandi, Adriztina Indri. International Archives of Otorhinolaryngology.2016;20(04). CrossRef
- Molecular targets for anticancer redox chemotherapy and cisplatin-induced ototoxicity: the role of curcumin on pSTAT3 and Nrf-2 signalling Fetoni A R, Paciello F, Mezzogori D, Rolesi R, Eramo S L M, Paludetti G, Troiani D. British Journal of Cancer.2015;113(10). CrossRef
- Curcumin attenuates peroxynitrite-induced neurotoxicity in spiral ganglion neurons Liu Wenwen, Fan Zhaomin, Han Yuechen, Lu Sumei, Zhang Daogong, Bai Xiaohui, Xu Wei, Li Jianfeng, Wang Haibo. NeuroToxicology.2011;32(1). CrossRef
- Intravenous curcumin efficacy on healing and scar formation in rabbit ear wounds under nonischemic, ischemic, and ischemia-reperfusion conditions Jia Shengxian, Xie Ping, Hong Seok Jong, Galiano Robert, Singer Adam, Clark Richard A. F., Mustoe Thomas A.. Wound Repair and Regeneration.2014;22(6). CrossRef
- Preparation, Characterization and Cytotoxicity of Silibinin- Containing Nanoniosomes in T47D Human Breast Carcinoma Cells Amiri B, Ebrahimi-Far M, Saffari Z, Akbarzadeh A, Soleimani E, Chiani M. Asian Pac J Cancer Prev.2016;17(8):3835-3838.
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times