Epidemiology and treatment outcomes of testicular germ cell tumor at tertiary care center in Patna, India: A retrospective analysis
Download
Abstract
Background: Malignant testicular neoplasm constitutes about 1% of all cancers in male, but malignant germ cell tumors are most common tumors in adolescents and young adult males. In this study we report our experience of testicular germ cell tumors presenting at All India Institute of Medical Sciences, Patna, a tertiary referral centre, with respect to epidemiology, histopathology, management and outcome.
Methods: This study was conducted in department of Radiotherapy, All India Institute of Medical Sciences, Patna from August 2014 to September 2019. It was single institution based retrospective study in which data was retrieved from the recorded files & analysed. The study focused on epidemiology and survival outcomes.
Results: In this study we analysed, 38 histologically confirmed cases of testicular germ cell tumor. Seminoma and nonseminoma were 50% (19) and 50% (19) respectively. The median age was 31 years. The most common affected age group was 31 to 40 years. Metastasis was present in 50% of patients at presentation. At diagnosis the stage III, II and I were found in 50%, 28.9% and 21.1% respectively. Patients in good risk, intermediate risk and high risk were in 65.8%, 13.2% and 21.1% respectively. The median recurrence free survival (RFS) and overall survival (OS) was 52 months and 71 months respectively.
Conclusion: Most of the cases presented with advanced stage and majority of them had undergone high inguinal orchidectomy. The high nodal burden disease at presentation was associated with partial response to standard chemotherapy. It seems that there is the need of alternative chemotherapy regimen especially in nonseminomatous germ cell tumors. Patients presenting with disease confined to locoregional lymph nodes or local disease showed good prognosis.
Introduction
Malignant testicular neoplasm constitutes about 1% of all cancers in male [1], but malignant germ cell tumors (GCT) are most common tumors in adolescents and young adult males [2]. It has been seen that the incidence of testicular GCT has doubled in past 40 years [3]. India has the lowest incidence of testicular carcinoma that is about 1.7% [4]. Age standardized incidence in highest in New Zealand (7.8) followed by UK (6.3) and Australia (6.1) per 100000 men. Maldescended testis and cryptorchidism is the most common factor associated with testicular carcinoma, which increases the risk by 2-4 times [5]. GCTs are divided into seminoma and nonseminoma as they present with distinct epidemiology, natural history, which ultimately guides to management strategies. The Western nations reported earlier stage at diagnosis while most of the Indian studies have reported advanced stage at presentation [6]. In this we report our experience of testicular germ cell tumors presenting at All India Institute of Medical Sciences, Patna, a tertiary referral centre, focussing on epidemiology, histopathology, management, outcomes and prognosis.
Aim
Aim of the study to evaluate epidemiology, treatment outcome, survival and prognosis.
Materials and Methods
This study was conducted in department of Radiotherapy, All India Institute of Medical Sciences, Patna from August 2014 to September 2019. It was single institution based retrospective study in which data was retrieved from the recorded files & analysed after approval from Institutional Ethics Committee. The study focused on demographic profile as well as clinical presentation with respect to age, presenting complaints, histological types & tumours markers, surgical procedures, systemic chemotherapy, toxicity and disease free and overall survival outcomes. Data retrieved included details of clinical presentation, examination findings, radiological details, per operative findings, post operative histopathological report, adjuvant treatment details, and tumor markers AFP (alpha fetoprotein), βHCG (β Human chorionic gonadotropin), LDH (lactate dehydrogenase).
Statistical analysis
Statistical evaluation was done using SPSS version 25. Chi-square test and Kaplan Meier survival curves were plotted for recurrence free and overall survival. Recurrence free survival was defined as time from diagnosis till recurrence whereas overall survival was defined as time from diagnosis to death.
Results
In this study we analyzed 38 patients (n=38), histologically confirmed cases of germ cell tumor, of which seminoma and nonseminoma were 50% (19) and 50% (19) respectively. The median age of presentation was 31 years (1 – 61). The most common affected age group was 31 to 40 (31.6%) of the cases followed by age group of 21 to 30 years (28.9%) and least number of cases (7.9%) were seen in age group of > 50 years. Eastern Cooperative Oncology Group Performance Status (ECOG PS) was 0 in 31.6% of patients, ECOG PS 1 in 39.5%,ECOG PS 2 in 15.8% and ECOG PS 3 in 13.2%. Testicular swelling was the most common symptom and seen in 71.1% of patients. Other presenting symptoms were abdominal pain, abdominal pain with lump, hemoptysis, neck node and sacral mass seen in 5.3%, 5.3%, 5.3%, 7.9% and 5.3% respectively. Metastasis was observed in 50% of patients at initial work up, most common site of metastasis was lung (26.3%), liver in 7.9%, inguinal lymph nodes in 7.9% and supraclavicular lymph nodes in 7.9%. Post operative staging revealed that 50% of patients were in stage III followed by stage II and stage I in 28.9% and 21.1% respectively. Seminoma presented most commonly in stage II (21.1%) and non seminoma in stage III (34.2%). The median tumor size was 6 (3.2 - 12.3) cm. Upfront surgery was performed in 78.9% of cases and all consisted of high inguinal orchidectomy, 21.1% of cases were not able to undergo upfront surgery and these cases were considered for high inguinal orchidectomy after neoadjuvant chemotherapy. As per International Germ Cell Consensus Classification (IGCCCG) classification risk group we found in this study that good risk, intermediate risk and high risk were in 65.8%, 13.2% and 21.1% respectively. Among the good risk patients, 28% having metastatic disease and 72% with local disease at presentation while 87.5% of high risk patients presented with metastatic disease and 12.5% with local disease. In this study serum tumor marker groups were S0, S1, S2 and S3 in 2.6%, 60.5%, 26.3% and 10.5% respectively. We found that in seminoma the median value of AFP was 4 ng/ml (1.3 – 56), βHCG 638 mIU/ml (246 – 1200), LDH 486 U/L (212 – 1563) and in nonseminoma AFP was 380 ng/ml (0.5 – 1500), βHCG 316 mIU/ml (1.0 – 69399), LDH 558 U/L (312 – 3261).
The median volume of locoregional disease was 65 cc in seminoma and 120 cc in nonseminoma, these were measured on the basis of radiological parameters of largest locoregional lymph node from CT scan (Table 1) Response to chemotherapy after surgical intervention, showed that response evaluation criteria in solid tumors 1.1 (RECIST) of target lesion complete response (CR) in 15.8%, partial response (PR) in 63.2 % and radiological response could not be assessed in 21.1% of cases, similarly RECIST of non target lesion or of metastatic site were showing CR in 21.1%, PR in 21.1% and stable disease in 5.3% while RECIST could not be evaluated in 50% of cases.
| Seminoma | Nonseminoma | |||||||
| Mean | Max | Min | Median | Mean | Max | Min | Median | |
| Age (years) | 37 | 61 | 25 | 36 | 24 | 60 | 1 | 24 |
| Volume of locoregional disease | 288.43 | 1728.00 | .00 | 65.36 | 203.78 | 864.00 | .00 | 120.00 |
| Pre chemo AFP | 10.8 | 56.0 | 1.3 | 4.0 | 474.8 | 1500.0 | .5 | 380.0 |
| Pre chemo HCG | 640.7 | 1200.0 | 246.0 | 638.0 | 4953.2 | 69399.0 | 1.0 | 316.0 |
| Pre chemo LDH | 575 | 1563 | 212 | 486 | 891 | 3261 | 312 | 558 |
| Delay (days) | 7 | 24 | 0 | 5 | 7 | 20 | 0 | 9 |
| RFS (months) | 47 | 120 | 13 | 36 | 28 | 52 | 12 | 28 |
| OS (months) | 49 | 126 | 13 | 38 | 30 | 71 | 16 | 28 |
* Pre chemo – pre chemotherapy; AFP- alpha fetoprotein; HCG – β Human chorionic gonadotropin; LDH – lactate dehydrogenase; RFS – recurrence free survival; OS – overall survival; volume of locoregional disease in cc
Systemic chemotherapy was indicated in 94.7% and not indicated in only 5.3% of patients. Pre-chemotherapy pulmonary function test (PFT) was done in 65.8% while status of PFT was not known in 23.7% and not performed in 10.5% of cases. Neoadjuvant chemotherapy was considered in 21.1% of cases and these are the patients not able to undergo upfront surgery. Adjuvant chemotherapy was not taken or defaulted by 18.4% of patients and 81.6% of patients completed their scheduled chemotherapy. Neutropenia was the most common complication followed by diarrhoea and pulmonary toxicity was least common. Grade III diarrhoea was seen in 8.7% of patients, grade III mucositis in 2.6%, grade III neutropenia in 13.1%, febrile neutropenia in 5.7%, anaemia in 4.3% and pulmonary toxicity in 3.9% of patients respectively. Grade I or II complication of was seen in 22.3% of cases. Toxicities of the chemotherapy led to the delay in treatment schedule, 39.5% patients took their chemotherapy on schedule, 34.2% of patients were in delay in group of 1-10 days, 21.1% were in delay group of 11-20 days and 5.3% were in delay group of 21-30 days. The median number chemotherapy cycles were 4 (4-6). Baseline epidemiological characteristics are given in Table 2 on the basis of (histological category) seminoma and non seminoma.
| Seminoma | Non-Seminoma | |||||
| Count | Table N % | Count | Table N % | Chi- Square test; p value | ||
| Age group | 1 - 10 years | 0 | 0 | 4 | 10.5 | 0.029 |
| 11 - 20 years | 0 | 0 | 4 | 10.5 | ||
| 21 - 30 years | 5 | 13.2 | 6 | 15.8 | ||
| 31 - 40 years | 9 | 23.7 | 3 | 7.9 | ||
| 41 - 50 years | 3 | 7.9 | 1 | 2.6 | ||
| > 50 years | 2 | 5.3 | 1 | 2.6 | ||
| ECOG PS | ECOG_0 | 8 | 21.1 | 4 | 10.5 | 0.221 |
| ECOG_1 | 6 | 15.8 | 9 | 23.7 | ||
| ECOG_2 | 4 | 10.5 | 2 | 5.3 | ||
| ECOG_3 | 1 | 2.6 | 4 | 10.5 | ||
| Presenting complains | Abdominal pain | 2 | 5.3 | 0 | 0 | 0.025 |
| Abdominal pain and lump | 0 | 0 | 2 | 5.3 | ||
| Hemoptysis | 0 | 0 | 2 | 5.3 | ||
| Neck node | 0 | 0 | 3 | 7.9 | ||
| Sacral mass | 0 | 0 | 2 | 5.3 | ||
| Testicular swelling | 17 | 44.7 | 10 | 26.3 | ||
| Metastasis | No metastasis | 13 | 34.2 | 6 | 15.8 | 0.097 |
| Inguinal lymph nodes | 2 | 5.3 | 1 | 2.6 | ||
| Liver | 1 | 2.6 | 2 | 5.3 | ||
| Lung | 3 | 7.9 | 7 | 18.4 | ||
| Supraclavicular lymph nodes | 0 | 0 | 3 | 7.9 | ||
| Metastatic disease | Mets | 6 | 15.8 | 13 | 34.2 | 0.023 |
| Local disease | 13 | 34.2 | 6 | 15.8 | ||
| RECIST non target lesion | Complete response | 2 | 5.3 | 6 | 15.8 | 0.048 |
| Partial response | 2 | 5.3 | 6 | 15.8 | ||
| Stable disease | 2 | 5.3 | 0 | 0 | ||
| Not applicable | 0 | 0 | 1 | 2.6 | ||
| Non metastatic disease | 13 | 34.2 | 6 | 15.8 | ||
| RECIST target lesion | Complete response | 3 | 7.9 | 3 | 7.9 | 0.717 |
| Partial response | 11 | 28.9 | 13 | 34.2 | ||
| Stable disease | 0 | 0 | 0 | 0 | ||
| Response can’t be assessed (complete surgery) | 5 | 13.2 | 3 | 7.9 | ||
| S group | S0 | 0 | 0 | 1 | 2.6 | 0.107 |
| S1 | 14 | 36.8 | 9 | 23.7 | ||
| S2 | 5 | 13.2 | 5 | 13.2 | ||
| S3 | 0 | 0 | 4 | 10.5 | ||
| Risk group | Good | 17 | 44.7 | 8 | 21.1 | 0.003 |
| Intermediate | 2 | 5.3 | 3 | 7.9 | ||
| High | 0 | 0 | 8 | 21.1 | ||
| Surgical details | Biopsy | 2 | 5.3 | 4 | 10.5 | 0.607 |
| Left high inguinal orchidectomy | 7 | 18.4 | 5 | 13.2 | ||
| Right high inguinal orchidectom | 10 | 26.3 | 10 | 26.3 | ||
| Surgery was upfront | Yes | 16 | 42.1 | 14 | 36.8 | 0.426 |
| No | 3 | 7.9 | 5 | 13.2 | ||
| Stage | Stage I | 5 | 13.2 | 3 | 7.9 | |
| Stage II | 8 | 21.1 | 3 | 7.9 | ||
| Stage III | 6 | 15.8 | 13 | 34.2 | ||
| Indication of chemotherapy | Yes | 18 | 47.4 | 18 | 47.4 | 1 |
| No | 1 | 2.6 | 1 | 2.6 | ||
| Pre chemotherapy PFT | Yes | 12 | 31.6 | 13 | 34.2 | 0.361 |
| No | 1 | 2.6 | 3 | 7.9 | ||
| Not known | 6 | 15.8 | 3 | 7.9 | ||
| G-CSF use | Yes | 13 | 34.2 | 17 | 44.7 | 0.111 |
| No | 6 | 15.8 | 2 | 5.3 | ||
| Delay in days (group) | Not applicable | 6 | 15.8 | 3 | 7.9 | 0.122 |
| 1 - 10 days | 3 | 7.9 | 10 | 26.3 | ||
| 11 - 20 days | 5 | 13.2 | 3 | 7.9 | ||
| 21 - 30 days | 2 | 5.3 | 0 | 0 | ||
| No delay | 3 | 7.9 | 3 | 7.9 | ||
| Total number of cycles | 0 | 6 | 15.8 | 3 | 7.9 | 0.167 |
| 1 | 0 | 0 | 1 | 2.6 | ||
| 3 | 3 | 7.9 | 0 | 0 | ||
| 4 | 4 | 10.5 | 4 | 10.5 | ||
| 6 | 6 | 15.8 | 11 | 28.9 | ||
| Died (event) | Yes | 7 | 18.4 | 8 | 21.1 | 0.746 |
| No | 12 | 31.6 | 11 | 28.9 | ||
| Recurrence | Yes | 8 | 21.1 | 7 | 18.4 | 0.746 |
| No | 11 | 28.9 | 12 | 31.6 |
In this study on Kaplan Meier survival analysis we found that median duration of recurrence free survival (RFS) was 52 months (95% CI; 26.7 – 76.3 months) (Figure 1), duration of median overall survival (OS) was 71 months (95% CI; 35.1 – 106.8) months (Figure 2),the OS rates at 1, 3 and 5 years were 100%, 71.4% and 50.1% respectively.
Figure 1: Recurrence Free Survival (RFS) in month.
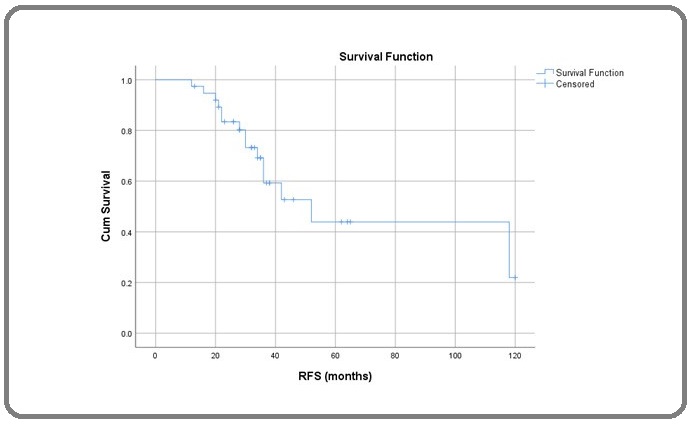
Figure 2: Overall Survival (OS) in month.
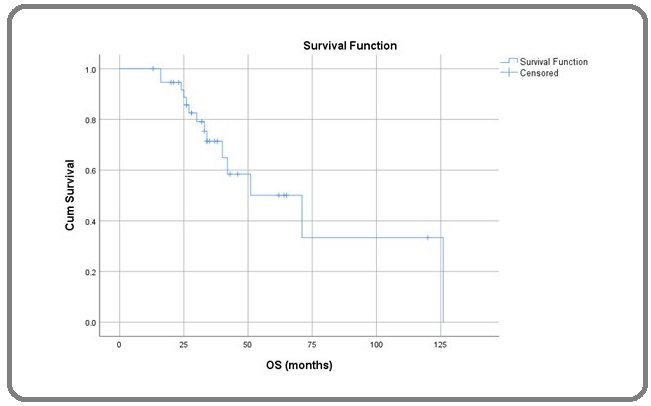
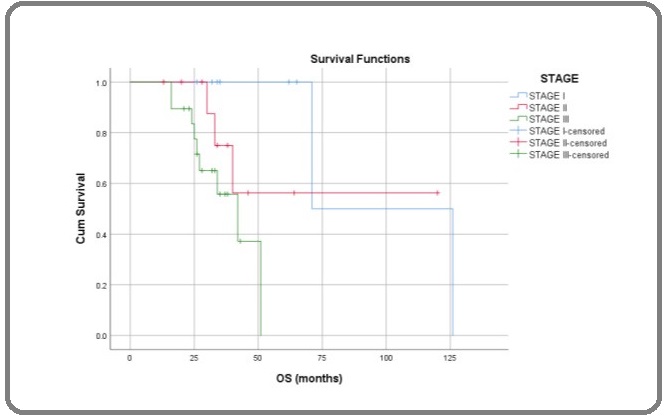
RFS rates at 1, 3 and 5 years were 97.4%, 69.2% and 43.9%. There was statistically significant differences in OS while comparing according to IGCCCG risk group (Log Rank; p= 0.017), metastatic versus local disease (Log Rank; p=0.008) stage of the disease (Log Rank; p=0.02) (Figure 3) and upfront surgery (Log Rank; p=0.001) but there was no statistical significant difference while comparing histological types (Log Rank; p=0.108).
Figure 3: Overall Survival in Months of Male GCT Comparing to Stage; Log Rank (Mantel-Cox) p = 0.02.
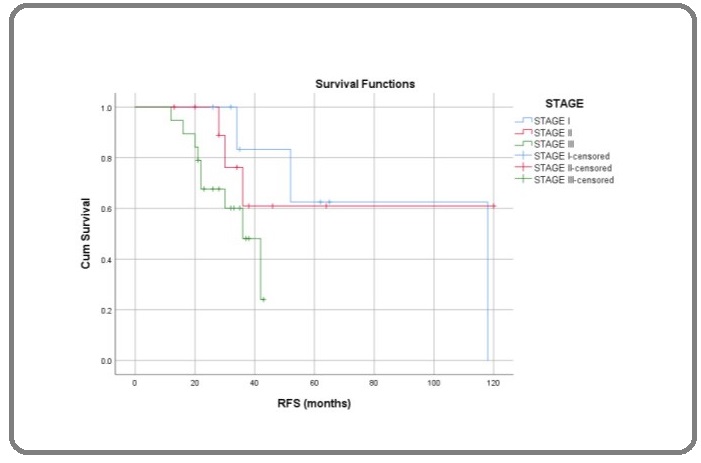
Similarly there was statistical significant difference in RFS as in risk group (Log Rank; p=0.05), metastatic disease (Log Rank; p=0.03) and upfront surgery (Log Rank; p=0.002) but there was no statistical significant difference in stage (Log Rank; p=0.12) (Figure 4), histological type (Log Rank; p=0.22).
Figure 4: Recurrence Free Survival in Months of Male GCT Comparing to Stage; Log Rank (Mantel-Cox) p = 0.102.
Discussion
There is a lack of recent data from India on epidemiology of germ cell tumor (GCT) except for few small series [7]. There is a recent publication on epidemiological study on GCT by Joshi et. al.[8]. In our study the median age of patients with seminoma and nonseminoma were 36 (25-61) and 24 (1-60) years respectively. Joshi et. al. found similar age distribution among the seminomatous and non seminomatous in their study as median age in seminoma and nonseminoma 39 and 28 years respectively [8]. Similar findings regarding the median age at diagnosis was noted by A. A. Ghazarian et. al. as median age at diagnosis were 36 years and 28 years for seminoma and nonseminoma respectively [9]. In this study 50% of the cases were seminomatous and 50 % of cases were non seminomatous GCT. ECOG performance status of patients in our study we found that seminomatous GCT presented most commonly in ECOG PS 0 and 1 while nonseminomatous GCT presented in ECOG 1, 2 and 3. Clinical presentation of seminomatous GCT were as testicular mass in 89.4% and 52.6% of the nonseminomatous GCT presented with testicular swelling and rest with symptoms of distant disease as supraclavicular metastasis, hemoptysis or abdominal lump. In this study seminomatous GCT with localized disease and metastatic disease were 34.2 % and 15.8% respectively and nonseminomatous GCT with localized and metastatic disease were 15.8% and 34.2 respectively. This can also be represented as 31.5% of seminomatous and 68.4% of nonseminomatous presented with metastatic disease. Most of the cases of nonseminomatous GCT presented with metastases. Epidemiological study by Joshi et. al. found similar outcome in their study with 64% of metastatic presentation in nonseminomatous GCT and 22.2% of metastatic disease in seminomatous GCT. We found in our study that the largest median tumor size was 6 cm (3.2-12) for seminoma and 6 cm (4-12.3) nonseminoma. For seminoma and non-seminoma, the largest median tumor size was 5.6 cm among black men and 5.7 cm among American populations as shown by A. A. Ghazarian et. al. in their study [9]. In this study we found that median pre-chemotherapy value of AFP, HCG and LDH in seminomatous GCT were 4 ng/ml, 638 mIU/ml and 212 U/L respectively. The median pre-chemotherapy value of AFP, βHCG and LDH in nonseminomatous GCT were 380 ng/ml, 316 mIU/ml and 558 U/L respectively. We found in our study that tumor markers S group as S0, S1, S2 and S3 were 0%, 73.6%, 21% and 5.2% respectively in seminomatous GCT while in nonseminomatous GCT S0, S1, S2 and S3 were 5.2%, 47.3%, 26.3% and 21% respectively. The serum tumor markers S group results were different from the findings of the study by Joshi et. al. [8]. Assessment of response to chemotherapy according to RECIST 1.1 we found that seminomatous GCT were showing CR, PR and SD in 15.7%, 57.8% and 0% respectively while response could not be assessed in 26.3%. In case of nonseminomatous GCT CR, PR and SD were 15.7%, 68.4% and 0% while response could not be assessed in 15.7%. The overall CR, PR and SD were 15.8%, 63.2% and 0% respectively and assessment could not be done in 21.1%. This response rate is very low in compared to reports from other studies showing response upto 95% with systemic chemotherapy [10]. This may be due to delayed presentation with high nodal burden disease and most cases associated with poor risk factors. Classifying the cases on the basis of risk factor we found that good risk, intermediate risk and high risk cases were 65.8%, 13.2% and 21.1% respectively. It was found in this study that Good risk patients having metastatic disease in 28% and 72% with local disease while 87.5% of high risk presented with metastatic disease and 12.5% with local disease. Cost et. al. In their study found that 60% of the high risk patients presented with metastases [11]. We found in our study that upfront surgery (high inguinal orchidectomy) was done in 78.9% of cases and, 21.1% of cases were not able to undergo upfront surgery, Saju S V et. al. in their study observed that 91% of their patients underwent upfront surgery and only 9% considered for biopsy [12] this difference may be attributable to delay presentation at tertiary care center. All patients in our study received first line chemotherapy as bleomycin, etoposide and cisplatin (BEP) based regimen. In this study Neutropenia was the most common complication followed by diarrhoea and pulmonary toxicity was least common. Grade III or IV diarrhoea, mucositis, neutropenia and febrile neutropenia was seen in 8.7%, 2.6%, 13.1%, and 5.7%. Anaemia in 4.3% and pulmonary toxicity in 3.9% of patients while 22.3% of patients developed grade I or II chemotherapy related complications. Joshi et. al. in their study reported grade III or IV non hematological toxicity in 18% of cases, grade III or IV hematological toxicity in 18% of cases and febrile neutropenia in 20% of cases. This difference may be due to prophylactic use of granulocyte colony stimulating factors (GCSF) in 78.9% in our study but prophylactic GCSF was not used in study by Joshi et. al. [8]. In this study Kaplan Meier survival analysis RFS rates at 1, 3 and 5 years were 97.4%, 69.2% and 43.9% respectively with median duration of RFS of 52 months (95% CI; 26.7 – 76.3 months). The median overall survival (OS) was 71 months (95% CI; 35.1 – 106.8). The OS rates at 1, 3 and 5 years were 100%, 71.4% and 50.1% respectively. Saju S V et. al. in their study observed 3 year RFS and OS 73.5% and 80.3% respectively, this findings were almost consistent with our study [12].
In conclusion, testicular germ cell tumor is predominantly a disease of young adults and it presents as a testicular mass in almost all cases for which consultation to physician was sought. Most of the cases presented with advanced stage and majority of them have undergone high inguinal orchidectomy which is the standard surgical procedure for testicular germ cell tumor. The high nodal burden disease presentation at our center and most of the patient showed partial response to standard chemotherapy it seems that there is the need of alternative chemotherapy regimen in expectation to good response especially in nonseminomatous germ cell tumors to achieve complete response. Patients presented with disease confined to locoregional lymph nodes or local disease having good prognosis as compared to metastatic disease which was reflected in both recurrence free survival and overall survival. Overall survival in our study was not matching to the data of western studies the reason for this may be advanced disease at presentation, bulky retroperitoneal nodal disease, treatment abandonment, failure to maintain dose intensity and lost to proper follow up.
Acknowledgments
We acknowledge the help extended by the Department of Oncosurgery, AIIMS, Patna.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Classification, epidemiology and therapies for testicular germ cell tumours Vasdev Nikhil, Moon Andrew, Thorpe Andrew C.. The International Journal of Developmental Biology.2013;57(2-3-4). CrossRef
- Cancer statistics, 2013 Siegel Rebecca, Naishadham Deepa, Jemal Ahmedin. CA: A Cancer Journal for Clinicians.2013;63(1). CrossRef
- Increasing Incidence of Testicular Cancer Worldwide: A Review HUYGHE ERIC, MATSUDA TOMOHIRO, THONNEAU PATRICK. Journal of Urology.2003;170(1). CrossRef
- Global incidence and outcome of testicular cancer Van Hemelrijck Mieke, Shanmugalingam Thurkaa, Soultati Aspasia, Chowdhury Simon, Rudman Sarah. Clinical Epidemiology.2013. CrossRef
- Risk factors for prostate and testicular cancer Boyle P., Zaridze D.G.. European Journal of Cancer.1993;29(7). CrossRef
- Epidemiology and Diagnosis of Testis Cancer Stevenson Scott M., Lowrance William T.. Urologic Clinics of North America.2015;42(3). CrossRef
- Germ cell tumours of the testis: Clinicalfeatures, treatment outcome and prognostic factors Bhutani M, Kumar L, Seth A, Thulkar S, Vijayaraghavan M, Kochupillai V. Natl Med J India.2002;15:18-21.
- Epidemiology of male seminomatous and nonseminomatous germ cell tumors and response to first-line chemotherapy from a tertiary cancer center in India Prabhash K, Joshi A, Zanwar S, Shetty N, Patil V, Noronha V, Bakshi G, Prakash G, Menon S. Indian Journal of Cancer.2016;53(2). CrossRef
- Recent trends in the incidence of testicular germ cell tumors in the United States Ghazarian A. A., Trabert B., Devesa S. S., McGlynn K. A.. Andrology.2014;3(1). CrossRef
- Equivalence of Three or Four Cycles of Bleomycin, Etoposide, and Cisplatin Chemotherapy and of a 3- or 5-Day Schedule in Good-Prognosis Germ Cell Cancer: A Randomized Study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council de Wit Ronald, Roberts J. Trevor, Wilkinson Peter M., de Mulder Pieter H.M., Mead Graham M., Fosså Sophie D., Cook Pat, de Prijck Linda, Stenning Sally, Collette Laurence. Journal of Clinical Oncology.2001;19(6). CrossRef
- Risk Stratification of Pubertal Children and Postpubertal Adolescents with Clinical Stage I Testicular Nonseminomatous Germ Cell Tumors Cost Nicholas G., Lubahn Jessica D., Adibi Mehrad, Romman Adam, Wickiser Jonathan E., Raj Ganesh V., Sagalowsky Arthur I., Margulis Vitaly. Journal of Urology.2014;191(5S). CrossRef
- Factors that impact the outcomes in testicular germ cell tumors in low–middle-income countries Saju S. V., Radhakrishnan Venkatraman, Ganesan Trivadi S., Dhanushkodi Manikandan, Raja Anand, Selvaluxmy Ganesarajah, Sagar Tenali Gnana. Medical Oncology.2019;36(3). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2020
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times