Epidemiology, Pattern of Recurrence and Survival in Triple-negative Breast Cancer
Download
Abstract
Background: Breast cancer is the most common cancer in the world. Triple-negative breast cancer (TNBC) characterized by the absence of estrogen receptor (ER), progesterone receptor (PR), and Her2neu receptor. This study investigated the epidemiological characteristics and survival in non-metastatic TNBC.
Materials and methods: Data from medical records of patients with breast cancer between 20014 and 2018 were retrieved, and patients with TNBC were identified and analyzed for demographic and clinicopathological features. Survival analyses were performed using the Kaplan–Meier method for disease-free survival (DFS) and overall survival (OS).
Results: A total of 457 nonmetastatic breast cancer patients were registered at our institute from January 2014 to August 2018, of which 137 were triple-negative breast cancer (TNBC). This accounted for 29.9% of nonmetastatic breast cancer during this period. With the median age of 45 years at diagnosis, the most common presenting complaint was breast lump. The median duration of symptoms was 30 months. The most commonly affected age group was 41-50 years. The majority of the patients were in a locally advanced stage (69.3%) while 30.7% were in the early stage. 29.2% recurrence at 38 months of median follow up. Recurrence was statistically significantly correlating with age ≤ 35 (p= < 0.001), pathological stage (p= < 0.001), nodal status at diagnosis (p= < 0.001), perineural invasion (PNI) (p= < 0.001), number of positive lymph nodes (p= < 0.001). The mean DFS and OS were 43.6 and 46 months respectively. 3-year DFS and OS were 65.5% and 66.2 % respectively.
Conclusion: TNBCs are high-grade tumors mostly presented in locally advanced stages and most of the patients are young. TNBCs are clinically aggressive with high risk of metastasis to visceral organs. The survival of TNBCs in the Indian scenario is poor in comparison to Western populations, probably due to racial factors, socioeconomic factors and health care access facility.
Introduction
Breast cancer is the most common cancer diagnosed annually, as per GLOBOCAN 2018 data the incidence and mortality of breast cancer is 11.6% and 6.6% respectively [1]. Breast cancer is the leading cause of cancer-related death among women around the world. Breast cancer is the most frequently observed cancer (14% of the total cases) and it is the leading cause of cancer death (11·1% of the total cases) in India [2]. In India among the females breast cancer is the most common cancer with an incidence of 27.7%. In developing countries, about half the breast cancer cases and 60% of the deaths estimated to occur [3]. Breast cancer is one of the most complex diseases in terms of cellular origin, tumor pathology, molecular subtypes, gene mutations, metastatic pattern, disease progression, therapeutic response and clinical outcome [4][5]. Breast cancer can be subclassified into different subtypes on the basis immunohistochemical (IHC) protein overexpression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor type 2 (Her2neu) as luminal A (ER-positive; Her2neu-negative), luminal B (ER-positive; Her2neu-positive); Her2neu enriched (ER-negative; Her2neu-positive) and triple-negative or basal-like (ER-negative; Her2neu-negative) [6][7]. The triple-negative breast cancers (TNBC) are considered as most malignant subtypes as these subtypes are associated with increased tumor size, increased incidence of axillary lymph node involvement and poor prognosis as compared to other subtypes [8][9][10]. TNBC accounts for approximately 12% to 17% of all invasive breast cancers in Western populations. This study was aimed to investigate the epidemiological characteristics and survival in non-metastatic TNBC presented at a tertiary care center at Kolkata.
Materials and Methods
Data from the medical records of patients attending the department of Radiotherapy at the Institute of Post Graduate Medical Education and Research (IPGME&R), Kolkata were retrieved between January 2014 to August 2018 of non-metastatic TNBC were identified and analyzed after approval from Institutional Ethics Committee. Tumors with IHC of ER, PR with expression ≤1% and a score of 0 or +1 for Her2neu considered as TNBC. IHC for Her2neu having a score of +2 were considered for fluorescence in situ hybridization (FISH) and those with FISH negative for Her2neu also considered as Her2neu negative. IHC done on formalin-fixed paraffin-embedded sections by polymer horseradish peroxidase technique. Patients with TNBCs classified histopathologically according to WHO classification [11]. Histological grade of tumors was determined using Nottingham histological score [12]. All the patients were staged according to the American Joint Committee on Cancer (AJCC TNM) 7th edition. Patients with stage I, IIA and a subset of IIB (T2N1M0) considered as early breast cancer (EBC) while a subset of stage IIB (T3N0M0), IIIA, IIIB and IIIC as locally advanced breast cancer (LABC). The morphological parameters analyzed were tumor size, histological type, histological grade, Lymphovascular invasion (LVI), perineural invasion (PNI), number of involved lymph nodes, total number of lymph nodes in the specimen and lymph node ratio (ratio of involved lymph nodes to the total number of lymph nodes in the post-operative specimen). The information was entered into pre-designed Performa followed by analysis of epidemiological characteristics, survival and their correlations. Disease-free survival (DFS) was defined from the start of primary therapy to the date of disease recurrence, or last follow-up. Overall survival (OS) was defined as the time from the date of the start of primary therapy to date of death or the last follow-up.
Statistical analysis
Statistical evaluation was done using SPSS version25. Baseline demographic and tumor characteristics of TNBC were analyzed. Univariate analysis of prognostic factors was done using the Log Rank test. Co-relation between tumor size and lymph node involvement, upfront surgery and recurrence rates, lymph node status and type of recurrence, and relapses were analyzed. Chi-square test was done to assess the statistical significance of these correlations. Survival estimation was done using the Kaplan Meier method. Multivariate analyses were performed using the Cox regression model. A ‘p’ value of <0.05 was considered statistically significant.
Results
A total of 457 nonmetastatic breast cancer patients were registered at our institute from January 2014 to August 2018, of which 137 were triple-negative breast cancer (TNBC). This accounted for 29.9% of nonmetastatic breast cancer during this period. 137 patients were eligible for this study as non-metastatic TNBC. The median age at diagnosis was 45 years (20-75). Clinical features including pain, lump, lump with pain, nipple discharge, and lump with ulcers were 19%, 48.9%, 15.3%, 1.5%, and 15.3% respectively. The tumor was right-sided in 49.6% and left-sided in 50.4% at presentation. The median duration of symptoms was 30 weeks (8 – 54). The age group distribution show 20-30 years, 31-40 years, 41-50 years,51-60 years, 61-70 years, and > 70 years were 13.9%, 27%, 30.7%, 21.2%, 6.6%, and 0.7% respectively. The age group distribution of the patient concern to the stage given in Figure 1.
Figure 1: Distribution of Age Group of Patients According to Stage at Diagnosis.
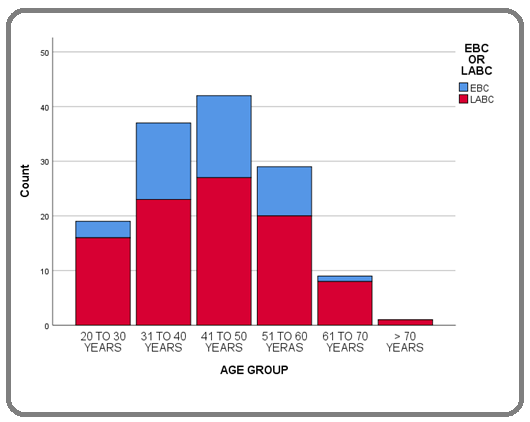
13.9% of patients were ≤ 35 years of age and 86.1% were > 35 years. The majority of the patients were post-menopausal with 53.3% and 46.7% were premenopausal. The nulliparity and history of breastfeeding were 12.4%, and 87.6% respectively. 10.9% of patients were unmarried and 89.15% of patients were married. The median age at first childbirth was 29 years. Most of the patients were considered for upfront surgery, 75.2% were considered for upfront surgery while 24.8% of patients received neoadjuvant chemotherapy (NACT) followed by surgical intervention. A modified radical mastectomy (MRM) was done in 88.3% of cases and 11.7% underwent breast conservative surgery (BCS). Adjuvant chemotherapy was considered in 98.5% of cases and 1.5% of patients defaulted for adjuvant chemotherapy. Chemotherapy regimen mostly consisted of anthracycline (A) and Cyclophosphamide (C) of 4 cycles followed by taxanes (T) of 4 cycles (4 x AC→4 X T) constituting 56.9% and followed by 6 cycles taxane, anthracycline and Cyclophosphamide (TAC) constituting 41.6% of cases. In the postoperative histopathological report review, it was observed that subtypes of invasive ductal breast carcinoma were not otherwise specified (NOS), cribriform, medullary and invasive lobular carcinoma as 87.6%, 7.3%, 3.6% and 1.5% respectively. The grading of the tumors were grade I, grade II, and grade III as 1.5%, 32.1% and 66.4% respectively of the tumors. Pathologically tumor size T1, T2, T3 and T4 were 3.6%, 37.2%, 34.3% and 24.8% respectively. The pathological nodal status of the tumors was N0, N1, N2 and, N3 as 38.7%, 24.1%, 27%, and 10.2% respectively. The lymph node positivity was statistically significantly associated with large tumor size (p=0.040), but not statistically significantly associated with LVI (p=0.074), PNI (p=0.139) and higher tumor grade (p=0.765). The median total number of lymph nodes removed during surgical intervention was nine (9) and the median number of positive lymph nodes was two (2). Lymph node ratio (LNR) was calculated as the ratio of the number of positive lymph nodes to the total number of lymph nodes removed during surgical intervention. The median LNR was 0.25 (0.00 – 1.00). LNR was not available in 8.02% (11) of patients as there were no pathologically identifiable nodal tissues were found in the postoperative specimen. Patients were classified as low risk, intermediate-risk, and high risk based on LNR.
Patients with LNR 0.00-0.20, 0.21-0.65, and > 0.65 were defined as low risk, intermediate-risk, and high risk respectively. LNR with low risk, intermediate risk, high risk, and not available were in 42.3%, 28.5%, 21.2%, and 8.02% respectively. Overall 61.3% of patients presented with node-positive disease while 38.7% presented with node-negative disease. Early breast cancer was seen in 30.7% and locally advanced breast cancer was observed in 69.3% of cases. Postoperative staging shows that stage IA, IIA, IIB, IIIA, IIIB, and IIIC were 2.2%, 14.6%, 27%,24.1%, 21.9% and 10.2% respectively. Lymphovascular invasion (LVI), perineural invasion (PNI), and margin positive were observed in 88.3%, 67.2%, and 1.5% respectively. Adjuvant radiation was indicated in 97.8% of cases, but taken by 92% of patients and adjuvant radiation was defaulted by 5.8% of cases. Twenty- nine percent of patients show recurrences at a median follow up of 38 months. Brain was the most common site of recurrence with 37.2%, liver 23.2%, lung 13.9%, ipsilateral 11.6%, contralateral metastasis 9.3% and bone 4.6% respectively. Comparative analysis of different clinicopathological parameters presented in Table 1.
| EBC | LABC | |||||
| Count | N % | Count | N % | p-value | ||
| Age < 35 | Yes | 7 | 5.1 | 30 | 21.9 | 0.070 |
| No | 35 | 25.5 | 65 | 47.4 | ||
| Side | Right | 23 | 16.8 | 45 | 32.8 | 0.425 |
| Left | 19 | 13.9 | 50 | 36.5 | ||
| Presenting complains | Pain | 13 | 9.5 | 13 | 9.5 | 0.004 |
| Lump | 23 | 16.8 | 44 | 32.1 | ||
| Lump with pain | 5 | 3.6 | 16 | 11.7 | ||
| Nipple discharge | 1 | 0.7 | 1 | 0.7 | ||
| Lump with ulcer | 0 | 0.0 | 21 | 15.3 | ||
| Age Group | 20 to 30 years | 3 | 2.2 | 16 | 11.7 | 0.365 |
| 31 to 40 years | 14 | 10.2 | 23 | 16.8 | ||
| 41 to 50 years | 15 | 10.9 | 27 | 19.7 | ||
| 51 to 60 years | 9 | 6.6 | 20 | 14.6 | ||
| 61 to 70 years | 1 | 0.7 | 8 | 5.8 | ||
| > 70 years | 0 | 0.0 | 1 | 0.7 | ||
| Married | Yes | 40 | 29.2 | 82 | 59.9 | 0.123 |
| No | 2 | 1.5 | 13 | 9.5 | ||
| Pregnancies | Never | 2 | 1.5 | 15 | 10.9 | 0.071 |
| One or more | 40 | 29.2 | 80 | 58.4 | ||
| Breast feeding | Yes | 40 | 29.2 | 80 | 58.4 | 0.071 |
| No | 2 | 1.5 | 15 | 10.9 | ||
| NACT | Yes | 4 | 2.9 | 30 | 21.9 | 0.006 |
| No | 38 | 27.7 | 65 | 47.4 | ||
| Surgery upfront | Yes | 38 | 27.7 | 65 | 47.4 | 0.006 |
| No | 4 | 2.9 | 30 | 21.9 | ||
| Type of surgery | BCS | 16 | 11.7 | 0 | 0.0 | < 0.001 |
| MRM | 26 | 19.0 | 95 | 69.3 | ||
| Nodal status | Positive | 19 | 13.9 | 65 | 47.4 | 0.010 |
| Negative | 23 | 16.8 | 30 | 21.9 | ||
| HPE subtype | Cribriform | 2 | 1.5 | 8 | 5.8 | 0.081 |
| ILC | 1 | 0.7 | 1 | 0.7 | ||
| Medullary | 4 | 2.9 | 1 | 0.7 | ||
| NOS | 35 | 25.5 | 85 | 62.0 | ||
| BR grade | Grade I | 0 | 0.0 | 2 | 1.5 | 0.268 |
| Grade II | 17 | 12.4 | 27 | 19.7 | ||
| Grade III | 25 | 18.2 | 66 | 48.2 | ||
| Pathological T | pT1 | 3 | 2.2 | 2 | 1.5 | < 0.001 |
| pT2 | 39 | 28.5 | 12 | 8.8 | ||
| pT3 | 0 | 0.0 | 47 | 34.3 | ||
| pT4 | 0 | 0.0 | 34 | 24.8 | ||
| Pathological N | pN0 | 23 | 16.8 | 30 | 21.9 | < 0.001 |
| pN1 | 19 | 13.9 | 14 | 10.2 | ||
| pN2 | 0 | 0.0 | 37 | 27.0 | ||
| pN3 | 0 | 0.0 | 14 | 10.2 | ||
| Postoperative stage | Stage IA | 3 | 2.2 | 0 | 0.0 | < 0.001 |
| Stage IIA | 20 | 14.6 | 0 | 0.0 | ||
| Stage IIB | 19 | 13.9 | 18 | 13.1 | ||
| Stage IIIA | 0 | 0.0 | 33 | 24.1 | ||
| Stage IIIB | 0 | 0.0 | 30 | 21.9 | ||
| Stage IIIC | 0 | 0.0 | 14 | 10.2 | ||
| LNR group | Not available | 2 | 1.5 | 9 | 6.6 | <.0.001 |
| Low risk | 29 | 21.2 | 29 | 21.2 | ||
| Intermediate risk | 10 | 7.3 | 29 | 21.2 | ||
| High risk | 1 | 0.7 | 28 | 20.4 | ||
| Margin | Negative | 42 | 30.7 | 93 | 67.9 | 0.344 |
| Positive | 0 | 0.0 | 2 | 1.5 | ||
| LVI | Negative | 10 | 7.3 | 6 | 4.4 | 0.003 |
| Positive | 32 | 23.4 | 89 | 65.0 | ||
| PNI | Negative | 21 | 15.3 | 24 | 17.5 | 0.004 |
| Positive | 21 | 15.3 | 71 | 51.8 | ||
| Adjuvant Chemotherapy | Yes | 42 | 30.7 | 93 | 67.9 | 0.344 |
| Not indicated | 0 | 0.0 | 0 | 0.0 | ||
| Not taken | 0 | 0.0 | 2 | 1.5 | ||
| Chemotherapy regimen | AC T | 24 | 17.5 | 54 | 39.4 | 0.635 |
| TAC | 18 | 13.1 | 39 | 28.5 | ||
| Not taken | 0 | 0.0 | 2 | 1.5 | ||
| Chest wall RT/ whole breast RT | Yes | 37 | 27.0 | 89 | 65.0 | 0.03 |
| Not indicated | 3 | 2.2 | 0 | 0.0 | ||
| Not taken | 2 | 1.5 | 6 | 4.4 | ||
| Axilla RT | Yes | 37 | 27.0 | 89 | 65.0 | 0.03 |
| Not indicated | 3 | 2.2 | 0 | 0.0 | ||
| Not taken | 2 | 1.5 | 6 | 4.4 | ||
| SCF RT | Yes | 34 | 24.8 | 89 | 65.0 | < 0.001 |
| Not indicated | 6 | 4.4 | 0 | 0.0 | ||
| Not taken | 2 | 1.5 | 6 | 4.4% | ||
| Site of recurrence | No recurrence | 39 | 28.5 | 55 | 40.1 | 0.007 |
| Ipsilateral recurrence | 1 | 0.7 | 4 | 2.9 | ||
| Lung metastasis | 0 | 0.0 | 6 | 4.4 | ||
| Liver metastasis | 0 | 0.0 | 10 | 7.3 | ||
| Bone metastasis | 0 | 0.0 | 2 | 1.5 | ||
| Brain metastasis | 2 | 1.5 | 14 | 10.2 | ||
| Contralateral metastasis | 0 | 0.0 | 4 | 2.9 | ||
| Recurrence | Yes | 3 | 2.2 | 40 | 29.2 | < 0.001 |
| No | 39 | 28.5 | 55 | 40.1 |
*EBC- early breast cancer; LABC-locally advanced breast cancer; NACT-neoadjuvant chemotherapy; HPE-histopathological examination; BR grade-Bloom-Richardson grade; T- pathological tumor size; N- pathological nodal status; LNR-lymph node ratio; LVI-Lymphovascular invasion; PNI-Perineural invasion; RT-radiation; SCF-supraclavicular; NOS- not otherwise specified
Recurrence was statistically significantly correlating with age at presentation (p=0.019; nominal by interval; Eta=0.309), age ≤ 35 (p= < 0.001), pathological N status (p= < 0.001), pathological stage (p= < 0.001), nodal status at diagnosis (p= < 0.001), PNI (p= < 0.001), number of positive lymph nodes (p= < 0.001), and LNR (p=< 0.001). Recurrence was not correlating statistically significantly with pathological T status (p=0.084), LVI (p=0.083), grade (p=0.58), and total number of lymph nodes removed (p=0.32).
The recurrence according to stage and age group represented as in Figure 2.
Figure 2: The Recurrence According to Stage and Age Group.
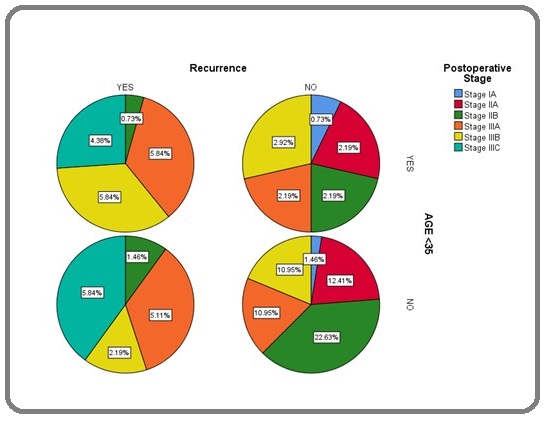
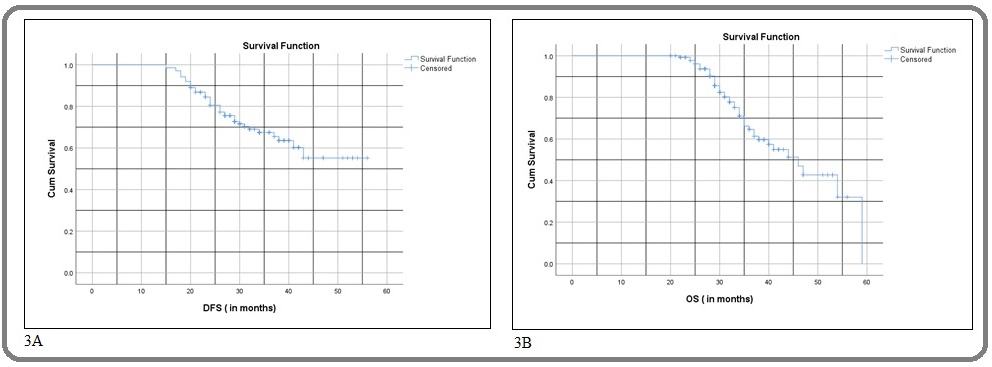
The median DFS was not reached but mean DFS was 43.6 months (95% CI; 40.58 – 46.72). The median OS was 46 months (95% CI; 39.1 – 52.8). Three-year DFS and OS were 65.5% and 66.2 % respectively. The Kaplan-Meier estimate of survival for DFS (Figure 3A)(Figure 3) and OS is represented in (Figure 3B).
Figure 3:A. The Cumulative Disease-free Survival Kaplan-Meier Curve;B. The cumulative overall survival Kaplan-Meier Curve.
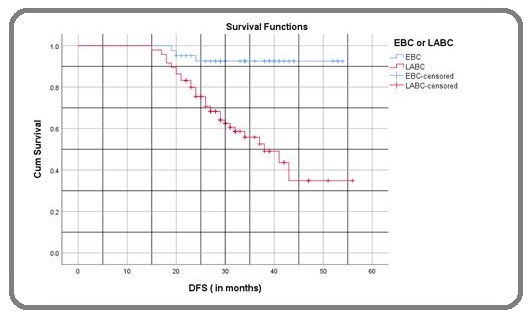
The 3-year DFS for patients with EBC and LABC was 92.5% and 55.8% respectively (p= < 0.001), the Kaplan-Meier survival curve is represented in Figure 4.
Figure 4: Disease-free Survival (DFS) in Months Comparing to Stage (EBC Vs LABC); Log rank p= < 0.001.
In univariate analysis age ≤ 35, stage, nodal status, pathological T status, and pathological N status, have a significant impact on DFS and OS given in the Table 2.
| Variable | Disease-free Survival (DFS) | Overall Survival (OS) | |||
| % | p-value | % | p-value | ||
| Age <35 | Yes | 32.5 | 36.3 | ||
| No | 47.8 | < 0.001 | 49.8 | < 0.001 | |
| Postmenopausal | Yes | 48.2 | 48.3 | ||
| No | 36.9 | 0.003 | 40.38 | 0.003 | |
| Stage | EBC | 51.5 | 51.5 | ||
| LABC | 38.7 | < 0.001 | 40.7 | < 0.001 | |
| Nodal status | Positive | 33.5 | 38.4 | ||
| Negative | 55.3 | < 0.001 | 55.3 | < 0.001 | |
| LVI | Positive | 42.1 | 43.1 | ||
| Negative | 49.5 | 0.041 | 50.2 | 0.020 | |
| PNI | Positive | 39.6 | 40.3 | ||
| Negative | 48.9 | < 0.001 | 55.5 | < 0.01 | |
| Pathological T | T1 | 48.6 | 49.8 | ||
| T2 | 46.4 | 45.4 | |||
| T3 | 41.4 | 0.023 | 44.5 | 0.002 | |
| T4 | 32.2 | 33.9 | |||
| Pathological N | N0 | 55.5 | 55.5 | ||
| N1 | 41.0 | 40.8 | |||
| N2 | 31.9 | < 0.001 | 37.8 | < 0.001 | |
| N3 | 22.2 | 32.5 |
Discussion
TNBC is known for its heterogeneity and early recurrence. One of the important things to consider in TNBC is that the ineffectiveness of the therapies targeted against ER, PR, and Her2neu receptors. Patients expressing these receptors having different therapeutic strategies due to the available number of anti-targeted agents. Therefore, the non-TNBCs have a good prognosis in comparison to TNBC. When TNBCs diagnosed earlier and treated adequately, the survival rates are comparable to non-TNBCs [13]. In this study TNBC accounted for 29.9% of non-metastatic breast cancer. Studies by Indian authors have reported a wide range of TNBCs from 11.8% to 31.9% [14][15]. Sarin et al. reported an incidence of 20%, similarly Chintalapani et al. reported an incidence of 19.3% of TNBC [16][17]. Murtaza et al. reported TNBC incidence in their study as 43.5% [18]. In our study the median age at presentation was 45 years which was similar to other studies as Lakshmaiah et al. and Suresh et al. the median age in their studies were 45 years and 49 years respectively [19][20]. Previous reports have also suggested a younger age at diagnosis in TNBCs (Hudis and Gianni, 2011; Sen et al., 2012). The median age at presentation in the Western population in a study was 53 years [21]. In this study the most commonly involved age group was 41-50 years with 30.7% followed by 31-40 years with 27%. A study by Chowdhary et al. of 185 TNBC patients, almost reported the similar findings [22]. In this study the tumor was right-sided in 49.6% and left-sided in 50.4% at presentation. Doval et al. in their study of 148 patients found 53.45% right-sided and left-sided in 46.6% [23]. In our study the majority of the patient were postmenopausal 53.3% which was similar to that of a study by Chintalapani et al. in their study 56.6% of patients were postmenopausal while Lakshmaiah et al. reported 40.47% of postmenopausal among the analysis of 84 patients [19]. A study by Doval et al. shows postmenopausal patients with 69.9%, which is higher than our study [23]. These studies suggest that the hormonal status of the patient in the postmenopausal state may have a role in the tumor growth or angiogenesis (Demicheli et al., 2004). In this study most of the patients (75.2%) underwent upfront surgical intervention and the rest of 24.8% were considered for NACT followed by surgical intervention. In this study MRM was the main surgical intervention followed by BCS similar reports were also found in other Indian studies [17][19][20][21][22][23][24]. The type of surgical procedure depends on the extent of the presenting disease, patient’s preference, and access to tertiary health care center. In this study majority of the patient were pathological stage III (56.2%) and grade III (66.4%). Indian literature regarding TNBC also reported similar findings as most of the TNBC presented with stage III [25][19-23]. In this study the pathological T2 (37.2%) was the most common finding followed by T3 (34.3%), similar findings were reported by Lakshmaiah et al. in their study with pathological T2 (35.7%), Hakim A, et al. in their study also reported pathological T2 (31.4%) [26], while Doval et al. reported pathological T2 (62.1%) in their study which was not consistent with our study [23]. In our study majority (61.3%) of patients presented with node-positive almost similar findings were reported by Lakshmaiah et al. in their study with 63% node-positive. Other Indian studies reported axillary node positivity in their studies from 50% to 74% [27][28][18] while Doval et al. reported 36.8% node-positive in their study [23], which was not consistent with our study. In our study 30.7% of patients were EBC and 69.3% were LABC. A study by Suhani et al. reported 56.1% of patients of TNBC presented as LABC [29]. Most of the Indian studies reported the presentation of LABC from 35% to 60% [18, 26-23-19]. The recurrence rate in this study was 29.2% at the median follow-up of 38 months. In this study the brain was the most common site of recurrence followed by liver and lung in TNBCs. Rathi et al. in their study reported lungs as the most common site of recurrence [28]. The mean DFS and OS were 43.6 and 46 months respectively. Three-year DFS and OS were 65.5% and 66.2% respectively. Rathi et al. reported 74.2% of 3-year DFS while Suresh et al. and Sarin et al. reported 3-years DFS more than 80% [28, 23-16]. The data from Chinese studies reported DFS and OS 77.8% and 79.9% [30]. A study from the USA reported a 3-year RFS of 63% and an OS of 71% [31]. These data reveals wide variability of survival outcomes around the regions of the world. This may be due to stage at presentation and survival analysis without stage IV disease etc. In our study the lower DFS may be due to a higher percentage of patients with LABC compared to other Indian studies on TNBC. The survival was better in EBC compared to LABC. The survival analysis revealed that better DFS and OS are significantly associated with EBC. The patients with EBC were managed with surgical intervention followed by adjuvant systemic chemotherapy and radiotherapy (when indicated) to reduce the risk of recurrence. Those patients presented with LABC, the majority of them were managed with NACT followed by surgery and adjuvant chemotherapy and radiotherapy. Axillary lymph node involvement results in poor DFS and OS which is statistically significant. This node involvement is well known prognostic factor in breast cancer that can predict the recurrence. The result of this study is per other studies (Tian et al. 2008; Ovcaricek et al., 2011). The pathological feature LVI did not influence DFS or OS but PNI was associated with poor DFS and OS with statistical significance. TNBC responds well to anthracycline and taxane-based systemic chemotherapy, which provide good response to treatment, though it may result in early recurrence [32][33].
In conclusion, triple-negative breast cancers constitute a significant proportion of breast cancer which is ER- negative, PR-negative, and Her2neu negative. They are high-grade tumors mostly presented in locally advanced stages and most of the patients are young. Locally advanced TNBCs are clinically more aggressive than early breast cancers. TNBCs are clinically aggressive with high risk of metastasis to visceral organs compared to non-TNBCs. However TNBCs respond well to systemic chemotherapy, thus better and less toxic management options to be considered along with there is also a need for newer targeted therapy. The survival of TNBCs in the Indian scenario is less in comparison to the Western population, probably due to racial factors, socioeconomic factors and health care access facility. The present study has a limitation of selection bias which may be due to retrospective nature.
Financial support and sponsorship
Nil
Acknowledgments
We acknowledge the help extended by the Department of General Surgery, Institute of Post Graduate Medical Education and Research, Kolkata, India.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- World Health Organization. Global Health Observatory. Geneva: World Health Organization; 2018. Available from http://gco.iarc.fr/ .
- Globocan India 2018. Population fact sheets p. 1-2. Available from http://www.gco.iarc.fr/today/data/factsheets/ populations/356-india-fact-sheets.pdf .
- Global cancer statistics Jemal Ahmedin, Bray Freddie, Center Melissa M., Ferlay Jacques, Ward Elizabeth, Forman David. CA: A Cancer Journal for Clinicians.2011;61(2). CrossRef
- Triple-Negative Breast Cancer Foulkes William D., Smith Ian E., Reis-Filho Jorge S.. New England Journal of Medicine.2010;363(20). CrossRef
- Triple Negative Breast Cancer Cetin Idil, Topcul Mehmet. Asian Pacific Journal of Cancer Prevention.2014;15(6). CrossRef
- Female Genital System and Breast. In: Kumar V, Abbas AK, Aster JC Sattar HA. Robbins Basic Pathology. 9th Eds. Philadelphia, Elsevier 2013.;:Pp. 681-714.
- Clinicopathologic features of triple negative breast cancers: an experience from Pakistan Hashmi Atif, Edhi Muhammad, Naqvi Hanna, Faridi Naveen, Khurshid Amna, Khan Mehmood. Diagnostic Pathology.2014;9(1). CrossRef
- Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications Sorlie T., Perou C. M., Tibshirani R., Aas T., Geisler S., Johnsen H., Hastie T., Eisen M. B., van de Rijn M., Jeffrey S. S., Thorsen T., Quist H., Matese J. C., Brown P. O., Botstein D., Lonning P. E., Borresen-Dale A.-L.. Proceedings of the National Academy of Sciences.2001;98(19). CrossRef
- Repeated observation of breast tumor subtypes in independent gene expression data sets Sørlie Therese, Tibshirani Robert, Parker Joel, Hastie Trevor, Marron J. S., Nobel Andrew, Deng Shibing, Johnsen Hilde, Pesich Robert, Geisler Stephanie, Demeter Janos, Perou Charles M., Lønning Per E., Brown Patrick O., Børresen-Dale Anne-Lise, Botstein David. Proceedings of the National Academy of Sciences.2003;100(14). CrossRef
- Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network Lin Nancy U., Vanderplas Ann, Hughes Melissa E., Theriault Richard L., Edge Stephen B., Wong Yu-Ning, Blayney Douglas W., Niland Joyce C., Winer Eric P., Weeks Jane C.. Cancer.2012;118(22). CrossRef
- WHO classification of tumors of the breast, 4 edn. Geneva: World Health Organization 2012 Lakhani SR, Ellis IQ, Schnitt SJ, Tan PH, van DE, Vijver MJ, eds . .
- pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up ELSTON C.W., ELLIS I.O.. Histopathology.1991;19(5). CrossRef
- Triple-negative breast cancer: the impact of guideline-adherent adjuvant treatment on survival—a retrospective multi-centre cohort study Schwentner L., Wolters R., Koretz K., Wischnewsky M. B., Kreienberg R., Rottscholl R., Wöckel A.. Breast Cancer Research and Treatment.2011;132(3). CrossRef
- Five year retrospective survival analysis of triple negative breast cancer in North-West India Kalwar A, Sharma N, Kapoor A, Kumar N, Satyanarayan , Sharma B. Indian Journal of Cancer.2013;50(4). CrossRef
- Triple Negative Breast Cancer in People of North East India: Critical Insights Gained at a Regional Cancer Centre Sharma Mousumi, Sharma Jagannath Dev, Sarma Anupam, Ahmed1 Shiraj, Kataki Amal Chandra, Saxena Rahul, Sharma Dilutpal. Asian Pacific Journal of Cancer Prevention.2014;15(11). CrossRef
- Epidemiological and survival analysis of triple-negative breast cancer cases in a retrospective multicenter study male breast cancer: Epidemiological data from the North of Peru Sarin R, Khandrika L, Hanitha R, Avula A, Batra M, Kaul S, et al . Indian J Cancer.2016;53:353-359.
- Triple-negative breast cancer: Pattern of recurrence and survival outcomes Konatam MeherLakshmi, Chintalapani ShynyReddy, Bala Stalin, Gundeti Sadashivudu, Kuruva SivaPrasad, Hui Monalisa. Indian Journal of Medical and Paediatric Oncology.2019;40(1). CrossRef
- Triple negative breast cancer: an Indian perspective Akhtar Murtuza, Dasgupta Subhrajit, Rangwala Murtuza. Breast Cancer: Targets and Therapy.2015. CrossRef
- A study of triple negative breast cancer at a tertiary cancer care center in southern India Das U, Suresh TM, Lokanatha D, Babu GK, Jacob LA, Babu S, Lakshmaiah KC. Annals of Medical and Health Sciences Research.2014;4(6). CrossRef
- Epidemiological and clinical profile of triple negative breast cancer at a cancer hospital in North India Suresh P, Batra Ullas, Doval DC. Indian Journal of Medical and Paediatric Oncology.2013;34(2). CrossRef
- Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence Dent R., Trudeau M., Pritchard K. I., Hanna W. M., Kahn H. K., Sawka C. A., Lickley L. A., Rawlinson E., Sun P., Narod S. A.. Clinical Cancer Research.2007;13(15). CrossRef
- Chowdhary, Sarthak Mishra; An analysis of incidence and prevalence and prognostic outcomes for women with triple-negative breast cancer in an Indian setting G. S . International Journal of scientific research.2019;8(12):2277-8179. CrossRef
- Eight Year Survival Analysis of Patients with Triple Negative Breast Cancer in India Dinesh Chandra Doval , P Suresh , Rupal Sinha , Saud Azam , Vineet Talwar , Kapil Kumar , Anurag Mehta , Ullas Batra . Asian Pacific Journal of Cancer Prevention.2016;17(6):2995-2999.
- “Triple-negative breast cancer- experience at a tertiary care center, South India” Satyanarayan V, Ashok Akula . International Journal of Current Research.;8(11):42382-42383.
- A study of triple-negative breast cancer at a cancer institute in India. Ram Prabu M. P., Raina V., Shukla N. K., Mohanti B. K., Deo S. V. S.. Journal of Clinical Oncology.2011;29(15_suppl). CrossRef
- Epidemiology of Breast Cancer in a Single Institute in North India with High Incidence of Triple- Negative Breast Cancers Hakim A A, et al . Int J Ped & Neo Heal.2019;3(1):27-31.
- Clinicopathological Features of Triple Negative Breast Carcinoma Reddy Gowry Maram. JOURNAL OF CLINICAL AND DIAGNOSTIC RESEARCH.2017. CrossRef
- Incidence and Clinical Profile of Triple-Negative Breast Cancer (TNBC) Deepak Kumar Rathi , S Chaudhary , M Sharma , et al. . IOSR Journal of Dental and Medical Sciences (IOSR-JDMS).2018;17(1):pp.04-06. CrossRef
- Triple-negative breast cancers: Are they always different from nontriple-negative breast cancers? An experience from a tertiary center in India Parshad Rajinder, Suhani , Kazi Mufaddal, Seenu V, Mathur Sandeep, Dattagupta Siddharth, Haresh KP. Indian Journal of Cancer.2017;54(4). CrossRef
- Clinicopathological and Prognostic Characteristics of Triple-Negative Breast Cancer (TNBC) in Chinese Patients: A Retrospective Study Li Chun-Yan, Zhang Sheng, Zhang Xiao-Bei, Wang Pei, Hou Guo-Fang, Zhang Jin. Asian Pacific Journal of Cancer Prevention.2013;14(6). CrossRef
- Triple Receptor–Negative Breast Cancer: The Effect of Race on Response to Primary Systemic Treatment and Survival Outcomes Dawood Shaheenah, Broglio Kristine, Kau Shu-Wan, Green Marjorie C., Giordano Sharon H., Meric-Bernstam Funda, Buchholz Thomas A., Albarracin Constance, Yang Wei T., Hennessy Bryan T.J., Hortobagyi Gabriel N., Gonzalez-Angulo Ana Maria. Journal of Clinical Oncology.2009;27(2). CrossRef
- Response to Neoadjuvant Therapy and Long-Term Survival in Patients With Triple-Negative Breast Cancer Liedtke Cornelia, Mazouni Chafika, Hess Kenneth R., André Fabrice, Tordai Attila, Mejia Jaime A., Symmans W. Fraser, Gonzalez-Angulo Ana M., Hennessy Bryan, Green Marjorie, Cristofanilli Massimo, Hortobagyi Gabriel N., Pusztai Lajos. Journal of Clinical Oncology.2008;26(8). CrossRef
- Prognostic impact of clinicopathologic parameters in stage II/III breast cancer treated with neoadjuvant docetaxel and doxorubicin chemotherapy: paradoxical features of the triple negative breast cancer Keam Bhumsuk, Im Seock-Ah, Kim Hee-Jun, Oh Do-Youn, Kim Jee Hyun, Lee Se-Hoon, Chie Eui Kyu, Han Wonshik, Kim Dong-Wan, Moon Woo Kyung, Kim Tae-You, Park In Ae, Noh Dong-Young, Heo Dae Seog, Ha Sung Whan, Bang Yung-Jue. BMC Cancer.2007;7(1). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2020
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times