Epidemiological and Pathologic Characteristics of Cancer Morbidity in Elderly Nigerians: A Call for Action
Download
Abstract
Objective: Geriatric cancer is a major public health problem with increasing incidence due to population growth and ageing.This study hopes to analyze the epidemiology and pathology of geriatric cancer in a Nigerian tertiary healthcare center.
Method: The study is a ten-year (2010-2019) descriptive retrospective study of histopathologically confirmed geriatric cancer cases in the University of Benin Teaching Hospital, Nigeria. The age, gender, anatomic site and histological diagnosis were used for the study. Analysis was with Microsoft Excel spreadsheet and results summarized in tables and figures.
Result: Geriatric cancer accounted for 33.7% of all cancers, affecting 871 males and 593 females with mean age and age range of 70.4 and 60-101 years respectively. Cases in 2010, 2011, 2012, 2013, 2014, 2015, 2016, 2017, 2018 and 2019 were 7.9%, 10.5%, 10.7%, 11.3%, 7.0%, 8.5%, 8.55%, 10.5%, 11.8% and 12.4% respectively. Exactly 26.0%, 25.4%, 19.1%, 15.0%, 9.0%, 3.2%, 1.3%, 0.8%, and 0.2% of cancer cases were encountered among the 60-64, 65-69, 70-74, 80-84, 85-89, 90-94, 95-99 and 100-104 years age groups respectively. Prostate, cervical, breast, colorectal and gastric cancer accounted for 41.3%, 12.3%, 12.3%, 5.8% and 3.1% of the cases respectively.
Conclusion: Geriatric cancer incidence is on the increase with male predominance and a peak at 60-64 years. About 75% of geriatric cancer affected the prostate, uterine cervix, breast, stomach and colorectum. Geriatric cancer care is still a low priority in Nigeria. A stronger health and social system is desirable for the worsening geriatric cancer burden in this region.
Introduction
Cancer is a major public health and socioeconomic challenge worldwide, affecting all age groups [1]. It is generally seen as a disease of old age because it is usually driven by age-related accumulation of mutations and carcinogenesis, decreased capacity to repair genetic damage and greater tissue sensitivity to these carcinogenic agents [2]. In developed countries, over 60% of cancer and about 70% of cancer related mortality are encountered among the 65+ years age group [3]. Geriatric cancer has been associated with more aggressive cancer biology, increased risk of treatment toxicity, co-morbidities, decreased physiological reserve, reduced access to health care, and ultimately higher cancer death rate relative to cancer in the younger counterparts [4]. As the world continues to experience a demographic shift, due to improvement in living standard and advances in medical care, the number of elderly patients with cancer will continue to rise, further increasing the global burden of cancer [5].
In Nigeria, there are only few and sub-optimal population-based cancer registries and the quality of cancer data from these regions are usually poor [6]. These challenges invariably obscure the true picture of geriatric cancer morbidity and mortality. With a relatively low life expectancy and with only about 3.0% of the population within the age range of 65 years and above,[7] the choice of the 60+ years age group as the definition of the Nigerian elderly is justified [8].
The pattern and trend of cancer morbidity in Nigerian elders is scarcely addressed in existing literature.
In this paper, we analyzed data from the histopathology department of one of the apex tertiary care centers in Nigeria with the hope of describing the epidemiological and histopathological characteristics of geriatric cancer in this region. It is hoped that findings from this study will close the knowledge gap and guide health policy makers, legislators, public health educators and clinical researchers in taking the necessary action in preventing cancer and improving the healthcare of elderly patients with cancer.
Materials and Methods
University of Benin Teaching Hospital (UBTH) is the apex reference tertiary care center in Edo State of the South-southern part of Nigeria with a capacity of over 600 bed spaces. It is also one of the few hospitals with histopathology and immunohistochemistry services in this region.
The index study is a hospital-based descriptive retrospective study carried out in the Department of Pathology, UBTH, Nigeria, from 1st January 2010 to 31st December 2019.
The materials for this study included duplicate copies of the histopathology reports, histopathology slides, and tissue blocks of all confirmed cancer cases of patient aged 60 years and above during the study period.
The data retrieved included the age and sex of patients, site of lesion and corresponding histological diagnosis. The sites of the cancer were reclassified using ICD10 classification of cancer [9]. Where necessary, fresh tissue sections were made and re-examined under the microscope. Special histochemical and immunohistochemical stains were utilized when necessary, to confirm challenging cases.
Data analysis was done using Excel spreadsheet 2007 and the statistical summary presented in Tables and Figures.
Results
In ten years, we had 4344 histologically confirmed cancer cases, out of which 1464 cases (33.7%) were patients of 60+ years. The average incidence is 146.4 cancer cases per annum. Of these patients, 871 (59.5%) were males while 593 (40.5%) were females, which gave a male to female ratio of 1.5:1. The age of the patients ranged from 60-101 years with a mean age of 70.4 years. The elderly male and female mean ages were 71.3 and 69.0 years respectively.
The yearly distribution of cancer among the older patients is shown in Table 1 with 7.9%, 10.5%, 10.7%, 11.3%, 7.0%, 8.5%, 8.55%, 10.5%, 11.8% and 12.4% of cases recorded in 2010, 2011, 2012, 2013, 2014, 2015, 2016, 2017, 2018 and 2019 respectively. This is depicted in Figure 1.
| SITE/ORGAN | Male | Female | All Cases | ||||||
| No | Percentage (%) | Mean age (yrs) | No | Percentage (%) | Mean age (yrs) | No | Percentage (%) | Mean age (yrs) | |
| Prostate | 604 | -69.4 | 72.3 | 604 | -41.3 | 72.3 | |||
| Cervix | 180 | -30.4 | 69.2 | 180 | -12.3 | 69.2 | |||
| Breast | 17 | -2 | 71.8 | 163 | -27.5 | 67.3 | 180 | -12.3 | 67.7 |
| Colorectum | 42 | -4.8 | 68.6 | 43 | -7.3 | 69.9 | 85 | -5.8 | 69.2 |
| Stomach | 24 | -2.8 | 72.5 | 22 | -3.7 | 72.1 | 46 | -3.1 | 72.4 |
| Nose, sinuses | 23 | -2.6 | 69.1 | 13 | -2.2 | 67.2 | 36 | 2.50) | 68.9 |
| Bladder | 20 | -2.3 | 69.7 | 13 | -2.2 | 72.3 | 33 | -2.3 | 70.7 |
| Skin non melanoma | 17 | -2 | 13 | -2.2 | 73.2 | 30 | ((2.10) | 70.7 | |
| Uterine corpus | 26 | -4.4 | 66.8 | 26 | -1.8 | 66.8 | |||
| Larynx | 24 | -2.8 | 67.8 | 1 | 60 | 25 | -1.7 | 67.4 | |
| Lung | 11 | -1.3 | 67.5 | 11 | -1.9 | 68.9 | 22 | -1.5 | 68.2 |
| NHL | 9 | -1 | 69.1 | 18 | -3 | 66.3 | 27 | -1.8 | 67.5 |
| Esophagus | 10 | -1.2 | 66.8 | 8 | -1.4 | 70.3 | 18 | -1.2 | 68.3 |
| Kaposi | 15 | -1.7 | 67.5 | 3 | -0.5 | 65.7 | 18 | -1.2 | 67.2 |
| Ovary | 16 | -2.7 | 67.9 | 16 | -1.1 | 67.9 | |||
| Soft tissue | 11 | -1.3 | 69.7 | 4 | -0.7 | 70.5 | 15 | -1 | 69.9 |
| Vulva | 12 | -2 | 69.3 | 12 | -0.8 | 69.3 | |||
| Skin melanoma | 5 | -0.6 | 65.6 | 5 | -0.8 | 77.4 | 10 | -0.7 | 71.5 |
| Liver | 3 | -0.3 | 65.7 | 6 | -1 | 66 | 9 | -0.6 | 65.9 |
| Kidney | 2 | -0.2 | 65 | 6 | -1 | 74.3 | 8 | -0.6 | 72 |
| Thyroid | 3 | -0.3 | 65.7 | 5 | -0.8 | 72 | 8 | -0.6 | 72.2 |
| Hypopharynx | 5 | -0.6 | 66.8 | 1 | -0.2 | 66 | 6 | -0.4 | 66.7 |
| Salivary gland | 1 | -0.1 | 69 | 3 | -0.5 | 70.3 | 4 | -0.3 | 70 |
| Small intestine | 4 | -0.7 | 74.5 | 4 | -0.3 | 74.5 | |||
| Brain & Nervous system | 1 | -0.1 | 62 | 2 | -0.3 | 65.5 | 3 | -0.2 | 64.3 |
| Eye | 2 | -0.2 | 60 | 2 | -0.3 | 87 | 4 | -0.3 | 78,0 |
| Pancreas | 1 | -0.1 | 60 | 2 | -0.3 | 60.5 | 3 | -0.2 | 60.3 |
| Tonsil | 2 | -0.2 | 75.5 | 1 | -0.2 | 80 | 3 | -0.2 | 77 |
| Vagina | 0 | 3 | -0.5 | 69 | 3 | -0.2 | 69 | ||
| Oropharynx | 2 | -0.2 | 68.5 | 1 | -0.2 | 82 | 3 | -0.2 | 73 |
| HL | 3 | -0.3 | 66.3 | 3 | -0.2 | 66.3 | |||
| Others | 14 | -1.6 | 6 | -1 | 20 | -1.4 | - | ||
| Total | 871 | -100 | 593 | -100 | 1464 | -100 | 70.4 |
*NS (Nervous system); NHL (Non-hodgkins lymphoma); HL (Hodgkins lymphoma);Yrs (years)
Figure 1: Yearly Trend of Geriatric Cancer in Nigeria.
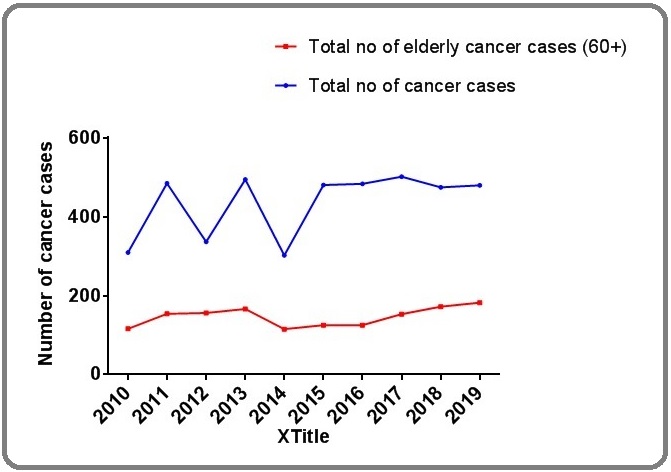
The age distribution of cancer among older adults is shown in Figure 2.
Figure 2: Age-wise Distribution of Geriatric Cancer in Nigeria.
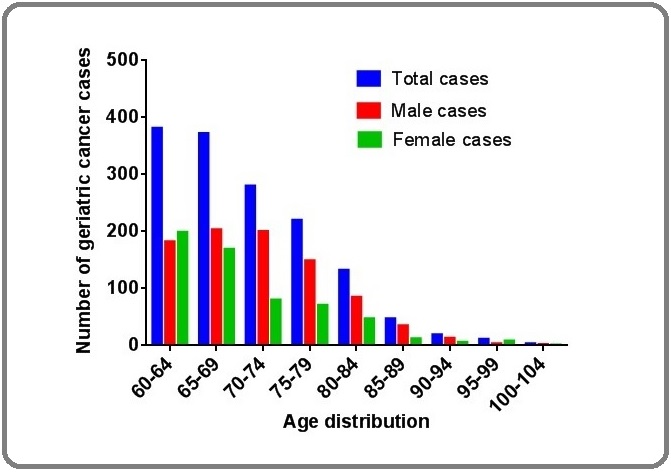
Among the males, 20.9%, 23.3%, 23.0%, 17.1%, 9.8%, 4.0%, 1.5%, 0.3% and 0.2% of the cancer cases were encountered in the 60-64, 65-69, 70-74, 80-84, 85-89, 90-94, 95-99 and 100-104 years age groups respectively. The relative proportions of the cancer cases in older females were as follows: 60-64 years (33.6%), 65-69 years (28.5%), 70-74 years (13.5%), 75-79 years (12.0%), 80-84 years (7.9%), 85-89 years (2.0%), 90-94 years (1.0%), 95-99 years (1.4%) and 100-104 years (0.2%). Among both genders, 26.0%, 25.4%, 19.1%, 15.0%, 9.0%, 3.2%, 1.3%, 0.8%, and 0.2% of cancer cases were encountered in the 60-64, 65-69, 70-74, 80-84, 85-89, 90-94, 95-99 and 100-104 years age groups respectively. Details of the organ distribution of geriatric cancer by gender are shown in Table 1. The most common cancer among males in descending order were prostate (69.4%), colorectal (4.8%), laryngeal (2.8%), stomach (2.8%), nose and paranasal sinuses (2.6%) and bladder cancer (2.3%). Leading cancer in older women were cervical (30.4%), breast (27.5%), colorectal (7.3%), corpus uteri (4.4%) and stomach (3.7%) cancers. Among all geriatric cancer, prostate (41.3%), cervical (12.7%), breast (12.3%), colorectal (5.8%) and stomach cancer (3.1%) are the leading cancer.
Details of regional distribution of cancer among the different age groups is shown in Table 2.
| Site/Organ | Age Group in Years | NO | ||||||||
| 60-64 | 65-69 | 70-74 | 75-79 | 80-84 | 85-89 | 90-94 | 95-99 | 100-104 | ||
| Prostate | 95 (15.7%) | 133 (22.0%) | 144 (23.8%) | 121 (20.0%) | 73 (12.1%) | 26 (4.3%) | 10 (1.7%) | 2 (0.3%) | 604 (41.3%) | |
| Cervix | 54 (30%) | 54 (30.0%) | 25 (13.9%) | 25 (13.9%) | 13 (7.2%) | 3 (1.6%) | 3 (1.7%) | 3 (1.7%) | 180 (12.3%) | |
| Breast | 67 (37.2%) | 58 (32.2%) | 25 (13.9%) | 18 (10.0%) | 5 (2.8%) | 3 (1.7%) | 3 (1.7%) | 1 (0.6%) | 180 (12.3%) | |
| Colorectum | 29 (34.1%) | 17 (20%) | 20 (23.5%) | 8 (9.4%) | 8 (9.4%) | 3 (3.5%) | 85 (5.8%) | |||
| Stomach | 10 (21.7%) | 11 (23.9%) | 5 (10.9%) | 10 (21.7%) | 7 (15.2%) | 2 (6.5%) | 1 | 46 (3.1%) | ||
| Nose, sinuses | 13 (36.1%) | 13 (36.1%) | 4 (11.1%) | 2 (5.6%) | 1 (2.8%) | 2 (5.6%) | 1 (2.8%) | 36 (2.5%) | ||
| Bladder | 12 (33.3%) | 4 (12.1%) | 6 (18.2%) | 7 (21.2%) | 3 (9.1%) | 2 (6.1%) | 33 (2.3%) | |||
| NMSC | 8 (26.7%) | 7 (23.3%) | 6 (20.0%) | 3 (10.0%) | 3 (10.0%) | 2 (6.7%) | 1 (3.3%) | 30 (2.1%) | ||
| Uterine corpus | 14 (53.9%) | 8 (30.8%) | 2 (7.7%) | 1 (3.9%) | 1 (3.9%) | 26 (1.8%) | ||||
| Larynx | 11 (44.0%) | 3 (12.0%) | 9 (36.0%) | 1 (4.0%) | 1 (4.0%) | 25 (1.7%) | ||||
| Lung | 4 (18.2%) | 8 (36.4%) | 9 (40.9%) | 1 (4.6%)) | 22 (1.5%) | |||||
| NHL | 12 (44.4%) | 7 (27.3%) | 3 (11.1%) | 4 (14.8%) | 1 (4.6%) | 27 (1.8%) | ||||
| Esophagus | 5 (27.8%) | 6 (33.3%) | 1 (5.6%) | 5 (27.8%) | 1 (5.6%) | 18 (1.2%) | ||||
| Kaposi | 6 (33.3%) | 7 (38.9%) | 2 (11.1%) | 3 (16.7%) | 18 (1.2%) | |||||
| Ovary | 6 (37.5%) | 5 (31.3%) | 2 (12.5%) | 1 (6.3%) | 2 (12.5%) | 16 (1.1%) | ||||
| Soft tissue | 3 (20%) | 6 (40.0%) | 3 (20.0%) | 1 (6.7%) | 1 (6.7%) | 15 (1.0%) | ||||
| Vulva | 4 (33.3%) | 4 (33.3%) | 2 (16.7%) | 1 (8.3%) | 1 (8.3%) | 12 (0.8%) | ||||
| Skin melanoma | 2 (20.0%) | 2 (20.0%) | 2 (20.0%) | 3 (30.0%) | 1 (10.0%) | 10 (0.7%) | ||||
| Liver | 4 (44.4%) | 3 (33.3%) | 1 (11.1%) | 1 (11.1%) | 9 (0.6%) | |||||
| Kidney | 2 (25.0%) | 1 (12.5%) | 1 (12.5%) | 1 (12.5%) | 3 (37.5%) | 8 (0.6%) | ||||
| Thyroid | 2 (25.0%) | 3 (37.5%) | 1 (12.5%) | 2 (25.0%) | 8 (0.6%) | |||||
| Hypopharynx | 2 (33.3%) | 3(50.0%) | 1 (16.7%) | 6 (0.4%) | ||||||
| Salivary gland | 1 (25.0%) | 1 (25.0%) | 1 (25.0%) | 1 (25.0%) | 4 (0.3%) | |||||
| Small intestine | 1 (25.0%) | 1 (25.0%) | 1 (25.0%) | 1 (25.0%) | 4 (0.3%) | |||||
| Eye and orbit | -50.00% | 1 (25.0%) | 1 (25.0%) | 3 (0.2%) | ||||||
| Oropharynx | 2 (66.7%) | 1 (33.3%) | 4 (0.3%) | |||||||
| Brain& NS | 2 (66.7%) | 1 (33.3%) | 3 (0.2%) | |||||||
| Pancreas | 3 (100%) | 3 (0.2%) | ||||||||
| Tonsil | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) | 3 (0.2%) | ||||||
| Vagina | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) | 3 (0.2%) | ||||||
| HL | 2 (66.7%) | 1 (33.3%) | 3 (0.2%) | |||||||
| Others | 4 (20.0%) | 3 (15.0%) | 5 (25.0%) | 2 (10.0%) | 2 (10.0%) | 2 (10.0%) | 1 (5.0%) | 1 (5.0%) | 20 (1.4%) | |
| Total no | 381 | 372 | 280 | 220 | 132 | 47 | 19 | 11 | 3 | 1464 |
| % of all cancer | 26.00% | 25.40% | 19.10% | 15.00% | 9.00% | 3.20% | 1.30% | 0.80% | 0.20% | 100% |
*NMSC, Non-melanoma skin cancer; NS, Nervous system; HL, Hodgkins lymphoma
Table 3 and 4 compared the distribution of the top five geriatric cancer across the globe with the present study.
| Country | Descending Order of Cancer Incidence | ||||
| 1 | 2 | 3 | 4 | 5 | |
| USA22 | Prostate | Lung/Bronchus | Colon | Bladder | Skin melanoma |
| Denmark23 | Prostate | Lung/Bronchus | Colon | Stomach | Bladder |
| Turkey24 | Lung/Bronchus | Prostate | Bladder | Colon | Stomach |
| China25 | Lung/Bronchus | Stomach | Colon | Esophagus | Liver |
| India21 | Lung/Bronchus | Prostate | Esophagus | Larynx | Liver |
| Kenya26 | Prostate | Esophagus | Stomach | Colon | Nasopharynx |
| South Africa26 | Esophagus | Prostate | Lung/Bronchus | Lips/Oral | Larynx |
| Uganda26 | Prostate | Esophagus | Nasopharynx | Kaposi | Stomach |
| Zimbabwe26 | Prostate | Stomach | Esophagus | TBL | Nasopharynx |
| Index study | Prostate | Colon | Stomach | larynx | Nasopharynx |
| Country | Order of Cancer Inidence | ||||
| 1 | 2 | 3 | 4 | 5 | |
| USA22 | Breast | Lung/Bronchus | Colon | Corpus uteri | NHL |
| Denmark23 | Breast | Colon | Lung/Bronchus | Stomach | Corpus uteri |
| Turkey24 | Breast | Colon | Stomach | Lung/Bronchus | Corpus uteri |
| China25 | Lung | Colon | Breast | Esophagus | Liver |
| India21 | Breast | Cervix | Ovary | Esophagus | Lung |
| Kenya26 | Breast | Cervix | Esophagus | Stomach | Ovary |
| South Africa26 | Cervix | Esophagus | Breast | Corpus uteri | Liver |
| Uganda26 | Cervix | Breast | Esophagus | Liver | Ovary |
| Zimbabwe26 | Cervix | Breast | Stomach | Esophagus | Liver |
| Index study | Cervix | Breast | Colon | Corpus uteri | Stomach |
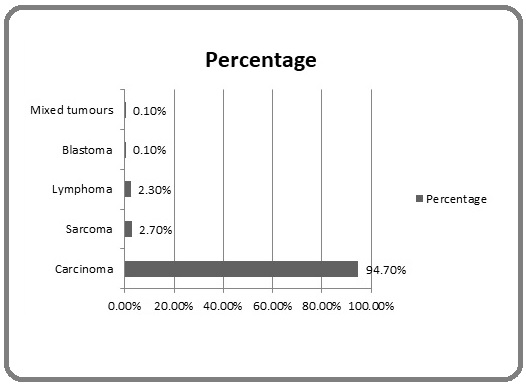
Figure 3 show the distribution of the cancer into the major histological groups with carcinomas, sarcomas, lymphomas, mixed tumours and blastomas accounting for 94.7%, 2.7%, 2.3%. 0.1% and 0.1% of the cases respectively.
Figure 3: Distribution of Geriatric Cancers into Histologic Groups.
Discussion
To the best of our knowledge, this is the first epidemiological and pathologic assessment of the burden of geriatric cancer in this part of the globe. The strength of the analysis lies in the long duration of the study, the reliable diagnostic technique utilized, and the large population served by the hospital. The findings from this study will further strengthen already existing record from Nigerian cancer registries.
In this study, geriatric cancer accounted for 34.5% of all histopathologically confirmed cancer cases in the study center which concurred with earlier reports from other parts of Nigeria, namely Port Harcourt (37.1%) [10] and Akwa Ibom (37.4%) [11]. In a report from both Ibadan and Abuja cancer registries, 38% of the cancer cases involved patients from the age of 55 years [12]. In Ghana [13] and Togo [14]. 36% and 15.2-17.4% of cancer patients respectively were of 60+ years old. These proportions from the African setting were however lower than 60% reported among Chinese,[15] United States of America (US) [16] and European elderly [17]. Nigeria currently has a relatively younger population structure with so many barriers limiting the access of older adults to healthcare [7]. With improvements in the socioeconomic structure and the quality of medical care, and current population growth, the proportion of older adults in the country is bound to increase, resulting in a corresponding increase in geriatric cancer burden in the future. This development could exert substantial pressure on our health system unless necessary changes are implemented.
In this study, we observed a gradual increase in the number of geriatric cancer cases up to the year 2013. A sharp decline was noted in 2014, and afterwards, a continuous rise till date. It is important to note that the study center serves as a major referral center to both Delta and Edo States of Nigeria. The most likely explanation for the abrupt drop in the number of cases is the competing interest from Delta State University Teaching Hospital, which has increased its capacity and therefore changed the destination of many referred cancer cases, especially among those domiciled in Delta State of Nigeria. In general, it can be concluded that the number of cancer cases has continued to increase among the Nigerian elderly patients. Our rate of increase is however comparatively lower than might be seen in developed countries like Europe and Asia where older adults of 65+ years constitute about 22-39% of the population, where population ageing is much higher [18]. In this study, males had a higher cancer burden than females with a male to female ratio of 1.5:1, which compared favorably with 2.1:1 reported in Akwa-Ibom but at variance with 1.9:2 reported in Port Harcout, Nigeria. [10-11]. In a worldwide study across 184 countries, an excess of cancer in elderly males was a general observation with a male to female ratio of 1.1-19:1 [18]. This male excess is even greater than its face value since globally, there are consistently, substantially more elderly females than males in every region [19]. Some explanation to the sex discrepancy may be provided by the differences in biology, gender role and carcinogen exposure. Men play more of productive roles while the women played more of reproductive roles at the early stage of their life cycles. The gender role further influences men’s exposure to occupational carcinogens, exposure to sunlight, smoking, alcohol abuse, unhealthy diets and higher levels of stress. Most of these cancer associated with these risk factors manifest much later in life [20].
There is a marked variation in the topographic distribution of cancer in this report. In the entire study, the leading cancer were prostate, colorectal, stomach, laryngeal and nasopharyngeal cancer for males and cervical, breast, colorectal, uterine corpus and stomach cancer for females (in decreasing order). Earlier studies on geriatric cancer epidemiology show striking differences in distribution of contributors of cancer in various regions, across the globe, as highlighted in Tables 3 and 4 [21-26]. In this study, the observation of prostate cancer as the leading cancer among elderly males is in concordance with earlier reports that prostate cancer is the most common cancer in most countries [1]. In an analysis of cancer among older men by Pilleron et al., prostate cancer was also the most common globally, except in some Asian countries [18]. Although the risk factors for prostate cancer have been poorly characterized, early detection has been associated with marked reduction in mortality, especially when backed up with effective treatment [1]. Conversely, lung cancer, which was shown to be one of the leading cancers in most developed countries among the elderly in other regions, is uncommon in our region, possibly due to differences in tobacco consumption and genetic factors [1-18]. Among older women in this study, cancer of the uterine cervix is the most common cancer, which is at variance with reports from non-African countries [1-18]. Africa has been the major contributor of the global burden of cervical cancer because national cervical cancer screening programmes and vaccination against the oncogenic strain of human papilloma virus have remained an abysmal failure. On the contrary, the adoption of these activities in most developed countries is the major explanation for the consistent decline in cervical cancer in these regions in recent decades [1-18]. Across the globe, breast cancer is the most common cancer among elderly women, except in China and Africa where it is preceded by lung and cervical cancer respectively [18]. The almost consistently high rate of breast cancer in the elderly females in most countries could be a reflection of decreasing parity and the obesity epidemic. A unique pattern in our investigation is that sex-specific cancer (male genital tract cancer among elderly males and breast and female genital tract cancer among elderly females) accounted for over 70% of cancer in the elderly.
Colorectal cancer accounted for 5.8% of the geriatric cancer burden in this study and is the second most common among elderly males and third most common among elderly females. Among elderly females, it is also the second most common in Turkey, China and Denmark and the third most common in the US [22-25]. Among elderly males, it is the third most common in the US and Denmark and the fourth most common in Turkey and Kenya [22-24,26]. The close epidemiological pattern may be explained by the change to a westernized low fiber diet, which is a major risk factor for colorectal cancer. Reduction in colorectal cancer incidence and mortality in developed countries is a general observation and is attributed to cancer screening programmes using routine fecal occult blood and colonoscopic examination, which allows for detection and removal of premalignant lesions. [1-18]. These preventive measures are yet to be widely implemented in Nigeria.
With respect to age distribution, the peak occurred at the beginning of the study and decreased continually till the end. Individually, apart from prostate cancer, most cancer peaked right from the 60-64 years age group in this study. Earlier studies had shown that most cancers peaked much earlier than the age of 60 years [27]. The implication for policy makers and legislators is that planning of cancer prevention through public enlightenment on behavioral changes and risk factor modification, cancer screening and vaccination, should target much younger age groups as carcinogenesis is a multi-step evolving process that should be terminated early.
Carcinoma is the most common geriatric malignancy in this study, accounting for 94.7% of the cancer. This is expected because the major organs accounting for cancer in the elderly are lined by epithelial cells. Sarcoma is the second most common groups of cancer, accounting for 2.7% of the cancer burden. Interestingly, the most common sarcoma in this region is Kaposi sarcoma, which also has an overwhelming male predominance. Although increasing rate of Kaposi sarcoma were attributed to HIV infection in an earlier study,[28] the relationship in this study was not confirmed.
Non Hodgkin’s lymphoma was relatively more common than Hodgkin’s lymphoma in this study. Literature on lymphoma among Nigerian elderly is however scanty.
In this part of the globe, where environmental hygiene is poor and infection is still a major health challenge, a high incidence of infection related cancer is not unexpected. This may explain why cervical cancer (Human papilloma virus), stomach cancer (Helicobacter pylori), liver cancer (hepatitis B and C virus), nasopharyngeal cancer (Epstein-Barr virus), and Kaposi sarcoma (Human immunodeficiency virus) are common in this region. Improving hygiene and vaccination where possible, will go a long way in reversing this trend.
Despite the current cancer burden in the elderly, there are many impediments to the fight against geriatric cancer. These include poverty and low socioeconomic status of the elderly, absence of social security for senior citizens, negative cultural belief systems, high treatment costs, dwindling family support arising from westernized lifestyle and the collapsing extended family system, out- of-pocket payment of hospital bills, belief in traditional medicine and faith therapy, the presence of few standard oncology treatment centers with qualified personnel and necessary infrastructure for optimal care of cancer patients, the current brain drain arising from mass exodus of qualified personnel in search of greener pastures and above all, the lack of political will to develop geriatric oncology in this most populous country of Africa [29-30]. There is no better time for a radical solution than now, if the geriatric cancer crisis has to be prevented from growing out of control. Both individuals and the government have roles to play. Public health education campaigns against cancer-promoting life styles (such as smoking, alcohol consumption and unhealthy diet, obesity and physical inactivity), and the elimination of stigma, misinformation and misconception are invaluable. There is need for a national framework on preventive measures against cancer through vaccination and treatment against hepatitis B and C viruses, human immunodeficiency virus, Helicobacter pylori and Human papilloma virus as well as screening and for cervical, breast, colorectal and prostate cancer. Treatment of cancer is financially intensive; therefore universal health insurance should be adopted to bridge this gap. The current healthcare budget has to be increased to improve existing health infrastructure and promote training and research in geriatric oncology. It is time for high profile cancer institution across the globe to mentor and collaborate with Nigerian institutions, especially the National Postgraduate Medical College and the West African College of Physicians to promote training of certified geriatric health care personnels. This is the time to approach donor agencies, international philanthropic organizations and explore public-private partnerships to assist in strengthening our weak health system. While the government should be encouraged to ensure that the Nigerian environment becomes more attractive to young doctors, there is need for its citizens to be more patriotic. This will go a long way to prevent the current brain drain. At local level, there is need for interdisciplinary co-ordination of care and greater personal commitment among health workers towards this goal. The time for the paradigm shift is now [29].
Limitation
Our study is a hospital based pathologic analysis. It therefore excludes cancer cases diagnosed using other techniques such as imaging. It also excludes cases diagnosed and managed in other healthcare centers in this region. These factors may lower the epidemiological significance of this report.
In conclusion, the Nigerian elderly have a considerable cancer burden, with over 70% of them arising from the prostate, uterine cervix, breast, colorectum and stomach.
Geriatric cancer incidence is on the increase with a peak at 60-64 years. It is more common in males and seems to be dominated by sex-specific cancers, and infection related cancer. Geriatric cancer care is still a low priority in Nigeria. A stronger health and social system is desirable for the worsening geriatric cancer burden in this region.
Acknowledgements
The authors are highly indebted to the staff and management of the Department Pathology of the University of Benin Teaching Hospital, Benin City, Nigeria.
Funding statement
Funding was by both authors. There was no external support.
References
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray Freddie, Ferlay Jacques, Soerjomataram Isabelle, Siegel Rebecca L., Torre Lindsey A., Jemal Ahmedin. CA: A Cancer Journal for Clinicians.2018;68(6). CrossRef
- The Biology of Aging and Cancer: A Brief Overview of Shared and Divergent Molecular Hallmarks Aunan Jan R., Cho William C, Søreide Kjetil. Aging and Disease.2017;8(5). CrossRef
- Cancer in the Elderly CINAR DERYA. Northern Clinics of Istanbul.2015. CrossRef
- Comorbidity in older adults with cancer Williams Grant R., Mackenzie Amy, Magnuson Allison, Olin Rebecca, Chapman Andrew, Mohile Supriya, Allore Heather, Somerfield Mark R., Targia Valerie, Extermann Martine, Cohen Harvey Jay, Hurria Arti, Holmes Holly. Journal of Geriatric Oncology.2016;7(4). CrossRef
- Demographic transition – Cancer trends in geriatric population of North India Kansal Surbhi, Rao Seema. Journal of Geriatric Oncology.2019;10(2). CrossRef
- Developing National Cancer Registration in Developing Countries – Case Study of the Nigerian National System of Cancer Registries Jedy-Agba Elima E., Oga Emmanuel A., Odutola Michael, Abdullahi Yusuf M., Popoola Abiodun, Achara Peter, Afolayan Enoch, Banjo Adekunbiola Aina Fehintola, Ekanem Ima-Obong, Erinomo Olagoke, Ezeome Emmanuel, Igbinoba Festus, Obiorah Christopher, Ogunbiyi Olufemi, Omonisi Abidemi, Osime Clement, Ukah Cornelius, Osinubi Patience, Hassan Ramatu, Blattner William, Dakum Patrick, Adebamowo Clement A.. Frontiers in Public Health.2015;3. CrossRef
- Care of the elderly in Nigeria: Implications for policy Tanyi Perpetua Lum, André Pelser, Mbah Peter, Tong Kar-wai. Cogent Social Sciences.2018;4(1). CrossRef
- WHO. Proposed working definition of an older person in Africa for the MDS Project Available at: https://www.who.int/healthinfo/survey/ageingdefnolder/en/..
- Classification and coding. In: International Agency for Research on Cancer/WHO. Cancer incidence in five continents Volume XI Jacques Ferlay , Klaus Kraywinkel , Brian Rous , Ariana Znaor . Available at https://ci5.iarc.fr/CI5-XI/Default.aspx..
- Cancer incidence in the Niger Delta region of Nigeria. A population based review of Port Harcourt Cancer registry Obiora CC, Osagbemiro BB, Akani NA. TNHJ.2018;19(2):85-94.
- The pattern and distribution of cancers in Akwa Ibom State, Nigeria Nwafor CC, Nwafor NN. Nigerian Journal of Clinical Practice.2018;21(5). CrossRef
- Cancer incidence in Nigeria: A report from population-based cancer registries Jedy-Agba Elima, Curado Maria Paula, Ogunbiyi Olufemi, Oga Emmanuel, Fabowale Toyin, Igbinoba Festus, Osubor Gloria, Otu Theresa, Kumai Henry, Koechlin Alice, Osinubi Patience, Dakum Patrick, Blattner William, Adebamowo Clement A.. Cancer Epidemiology.2012;36(5). CrossRef
- Cancer incidence in Ghana, 2012: evidence from a population-based cancer registry Laryea Dennis O, Awuah Baffour, Amoako Yaw A, Osei-Bonsu E, Dogbe Joslin, Larsen-Reindorf Rita, Ansong Daniel, Yeboah-Awudzi Kwasi, Oppong Joseph K, Konney Thomas O, Boadu Kwame O, Nguah Samuel B, Titiloye Nicholas A, Frimpong Nicholas O, Awittor Fred K, Martin Iman K. BMC Cancer.2014;14(1). CrossRef
- Cancers in the Elderly Seen in Anatomical Pathology Laboratory in Lomé, Togo Darré Tchin, Walla Atchi, Kpatcha Tchilabalo Matchonna, Aboubakari Abdoul-Samadou, Maneh Nidain, Koulinga Mikotakatola, Amégbor Koffi, Koura Gado Napo. Open Journal of Pathology.2016;06(01). CrossRef
- Epidemiological Characteristics of Cancer in Elderly Chinese Zou Xiao Nong, Wan Xia, Dai Zhen, Yang Gong Huan. ISRN Oncology.2012;2012. CrossRef
- Cancer burden in the aged Yancik Rosemary. Cancer.1997;80(7). CrossRef
- Cancers in the Elderly in Brazzaville Nkoua M’Bon J.B, Nsondé Malanda J, Moukassa D. Carcinol Clin Afrique.2011;10:43-46.
- Global cancer incidence in older adults, 2012 and 2035: A population-based study Pilleron Sophie, Sarfati Diana, Janssen-Heijnen Maryska, Vignat Jérôme, Ferlay Jacques, Bray Freddie, Soerjomataram Isabelle. International Journal of Cancer.2018;144(1). CrossRef
- The ratio of older women to men: Historical perspectives and cross-national comparisons Guralnik J. M., Balfour J. L., Volpato S.. Aging Clinical and Experimental Research.2000;12(2). CrossRef
- Sex Disparities in Cancer Incidence by Period and Age Cook Michael B., Dawsey Sanford M., Freedman Neal D., Inskip Peter D., Wichner Sara M., Quraishi Sabah M., Devesa Susan S., McGlynn Katherine A.. Cancer Epidemiology Biomarkers & Prevention.2009;18(4). CrossRef
- Geriatric Cancers in India: An Epidemiological and Demographic Overview Yeole BB, Kurkure AP, Koyande SS. Asian Pacific J Cancer Prev.2008;9:271-274.
- Cancer Risk Among Older Adults: Time for Cancer Prevention to Go Silver White Mary C, Holman Dawn M, Goodman Richard A, Richardson Lisa C. The Gerontologist.2019;59(Supplement_1). CrossRef
- Common Cancers in the Elderly Hansen Johnni. Drugs & Aging.1998;13(6). CrossRef
- Cancer in the Elderly CINAR DERYA. Northern Clinics of Istanbul.2015. CrossRef
- High Cancer Burden in Elderly Chinese, 2005–2011 Li Shugang, Zhang Xuefei, Yan Yizhong, Wang Kui, Rui Dongsheng, Pang Lijuan, Li Feng. International Journal of Environmental Research and Public Health.2015;12(10). CrossRef
- Cancer incidence in older adults in selected regions of sub‐Saharan Africa, 2008–2012 Pilleron Sophie, Soerjomataram Isabelle, Charvat Hadrien, Chokunonga Eric, Somdyala Nontuthuzelo I. M., Wabinga Henry, Korir Anne, Bray Freddie, Jemal Ahmedin, Maxwell Parkin D.. International Journal of Cancer.2019;144(8). CrossRef
- Cancer Incidence in Nigeria: A Tertiary Hospital Experience Uchendu Obiora Jude. Asian Pacific Journal of Cancer Care.2020;5(1). CrossRef
- Kaposi's sarcoma in Nigeria Onunu Abel N., Okoduwa Cynthia, Eze Emeka U., Adeyekun Ademola A., Kubeyinje Emmanuel P., Schwartz Robert A.. International Journal of Dermatology.2007;46(3). CrossRef
- The State of Oncology in Africa 2015; International Prevention Research Institute, Lyon Boyle P, Ngoma T, Sullivan R, Ndlovu N, Autier P, Stefan C, et al . ;:1-560.
- The health workforce crisis: the brain drain scourge Ike SO. Niger J Med.2007;16(3):204-211.
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2021
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- Xml downloaded - 0 times