Impact of Monthly 120 Mg Denosumab on Bone Metabolism in Bone-metastatic Prostate Cancer Undergoing Androgen Deprivation Therapy
Download
Abstract
Background: While semiannual 60 mg denosumab is a common treatment for osteoporosis, impact of monthly 120 mg denosumab, the common treatment protocol for bone metastases from solid tumors, on bone metabolism is unclear.
Materials and Methods: We reviewed 15 patients with bone-metastatic prostate cancer who initiated monthly 120 mg denosumab in conjunction with androgen deprivation therapy between 2013 and 2014. Bone mineral density (BMD) was measured at lumbar spine and femoral neck using dual energy X-ray absorptiometry (DXA), before treatment and annually thereafter. Bone metabolism markers, including urine N-terminal telopeptide (uNTx) and bone type alkaline phosphatase (BAP), were monitored monthly.
Results: Twelve of 15 (80%) patients had evaluable DXA before treatment, and of them, eight underwent DXA after a year of initiation without discontinuation of denosumab. Percent changes in BMD from baseline were +6.2% at lumbar spine and +7.6% at femoral neck, both of which were significant increases (both P<0.01). Bone metabolism markers were evaluable in 11 (73%) patients: uNTx decreased rapidly, while BAP declined gradually after initiating denosumab. These effects were similar to those seen by the standardized dose for osteoporosis in previous literature. There were no denosumab-related severe adverse events during the follow-up period.
Conclusions: The impact of monthly 120 mg denosumab on bone metabolism was significant, but almost equivalent to that of the standard dose for osteoporosis (60mg semiannually) in bone-metastatic prostate cancer undergoing androgen deprivation therapy. Whereas the higher dose has reportedly reduced skeleton-related events, the effect on bone metabolism seemed plateaued or showed no dose-dependency.
Introduction
In 2012, more than a million men worldwide were estimated to be diagnosed with prostate cancer (PC), resulting in more than 300,000 deaths [1]. Bone metastasis is a common progressive pattern of PC [2,4] and presents with a major cause of mortality in PC patients. While androgen deprivation therapy (ADT) is a common treatment option for metastatic PC, it has several well-recognized adverse effects, including osteoporosis [5,7]. Bone metastases alone or in combination with ADT-related osteoporosis places PC patients at great risk of skeletal morbidity (e.g. pain and fracture), which may significantly impair patients’ quality of life [8,12]. Therefore, bone health management in PC patients with bone metastases and/or undergoing ADT is considered critical to maintain their quality of life and improve prognosis [13, 14].
Denosumab, a receptor activator of NF-kB ligand (RANKL) inhibitor, has recently been reported to reduce skeleton-related events (SREs) and improve survival in patients with bone metastases from solid tumors including PC, when given in a dosage of 120 mg monthly [15,19]. Notably, it has also been reported to improve bone metastasis-free survival in non-metastatic castration-resistant PC [20, 21]. On the other hand, it is also a common treatment for osteoporosis when used in a different dosage: semiannual 60 mg denosumab has been shown to increase bone mineral density (BMD) and affect bone turnover markers in both normal populations [22,27] and PC patients treated with ADT [28,30]. However, no study has investigated the impact of monthly 120 mg denosumab on bone metabolism to date. In this context, the present study evaluated BMD and bone metabolism markers in patients with bone-metastatic PC who underwent monthly 120 mg denosumab along with ADT.
Materials and Methods
We retrospectively reviewed 15 patients with bone-metastatic PC who initiated monthly 120 mg denosumab in conjunction with ADT at our institution between August 2013 and March 2014. BMD was measured at lumbar spine and femoral neck using dual energy X-ray absorptiometry (DXA), before treatment and annually thereafter. Bone metabolism markers, including urine N-terminal telopeptide (uNTx) and bone type alkaline phosphatase (BAP), were monitored monthly. Adverse events were evaluated at every visit of each patient. All research protocols in the present study were approved by the Institutional Review Board of Tokyo Teishin Hospital (approval number: 994).
All statistical analyses were performed using JMP Pro version 12.2.0 (SAS Institute, Cary, NC, USA). Changes in BMD and bone metabolism markers from baseline were analyzed with Wilcoxon signed-rank test. P values were two-sided and were considered as significant at P<0.05. Follow-up information was obtained up to March 2016.
Results
Patients
PC was confirmed histologically and bone metastases were detected with bone scintigraphy ± computed tomography before treatment in all cases. All patients received continuous combined androgen blockade as ADT from the diagnosis of bone-metastatic PC until the last follow-up. No patient had undergone prior local therapy (radical prostatectomy, external beam radiotherapy, brachytherapy, etc.). For the prevention of adverse effects of denosumab, all patients underwent a dental examination before its initiation and were given supplements containing calcium and vitamin D during its administration period.
Baseline characteristics of 15 patients were summarized in Tabl 1. Median interval of time between the diagnosis of bone-metastatic PC and the initiation of denosumab was 6 months (range: 0–106 months). Eight patients utilized alternative ADT and three did docetaxel chemotherapy during the follow-up period.
| Parameter | Value |
| Age, median (range), years | 75 (59–82) |
| Initial prostate specific antigen, median (range), ng/mL | 32 (3.4–1148) |
| Gleason score at biopsy, no. (%) | |
| 7 | 3 (20) |
| 8 | 2 (13) |
| 9 | 7 (47) |
| 10 | 3 (20) |
| Clinical T stage, no. (%) | |
| T1 | 2 (13) |
| T2 | 5 (33) |
| T3 | 5 (33) |
| T4 | 3 (20) |
| Clinical N stage, no. (%) | |
| N0/x | 8 (53) |
| N1 | 7 (47) |
| Metastatic site, no. (%) | |
| Bone | 15 (100) |
| Lung | 2 (13) |
| Other organ | 0 (0) |
| BMD at lumbar spine, median (range), g/cm2 | 1.083 (0.636–1.892) |
| T-score at lumbar spine, median (range) | 0.6 (-1.6–7.1) |
| BMD at femoral neck, median (range), g/cm2 | 0.689 (0.550–0.923) |
| T-score at femoral neck, median (range) | -1.35 (-2.2–0.5) |
| Follow-up time, median (range), months | 21 (1–35) |
a) BMD, bone mineral density
Of 15 patients, 12(80%) had evaluable DXA before initiating denosumab (Fig1). Of these, eight underwent DXA a year later without discontinuation of denosumab, while two died of PC within a year, one discontinued denosumab because of liver failure due to docetaxel, and one did not undergo DXA for another reason. Of eight patients, four underwent DXA two years later without discontinuation of denosumab, whereas three discontinued denosumab within the next year and one did not undergo DXA for another reason.Eleven of 15 (73%) patients had data on uNTx and BAP monitored monthly for more than a year.
Figure 1 :Flow chart representing the study selection process
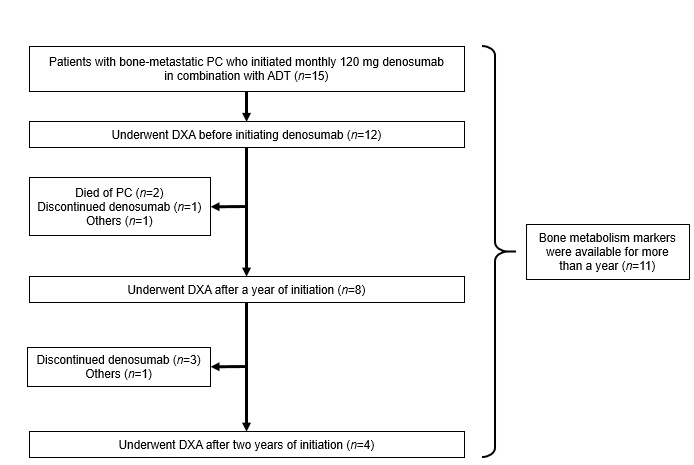
Changes in BMD
In eight patients who underwent DXA a year later, percent changes in BMD from baseline were +6. P=0.0078 and P=0.0078, respectively) (Fig. 2). For reference, median T-scores (ranges)at baseline and a year later in these patients were as follows: -0.5 (-1.6–2.8) and 0 (-1.2–3.4) at lumbar spine; and -1.5 (-2.2–-0.4) and -1.05 (-1.8–0.1) at femoral neck, respectively.2% at lumbar spine and +7.6% at femoral neck, both of which were significant increases (P=0.0078 and P=0.0078, respectively) (Fig. 2). For reference, median T-scores (ranges)at baseline and a year later in these patients were as follows: -0.5 (-1.6–2.8) and 0 (-1.2–3.4) at lumbar spine; and -1.5 (-2.2–-0.4) and -1.05 (-1.8–0.1) at femoral neck, respectively.
Figure 2 :Changes in BMD at lumbar spine (A) and femoral neck (B) during the first year of denosumab administration ( n =8). *, P <0.05.
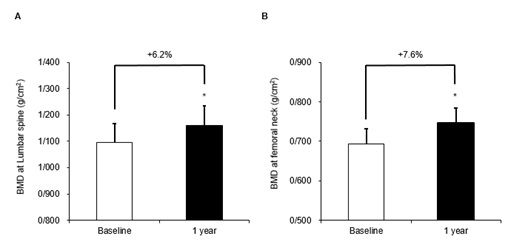
In four patients whose BMDs were followed over 2 years, percent changes in BMD from baseline to a year later and two years later were+6.7% and +8.1% at lumbar spine; and +7.7% and +6.6% at femoral neck, respectively (Fig. S1). While there were increases in BMD at both sites between baseline and a year later, there seemed to be no increases between a year later and two years later, despite a lack of statistical significance due to the small sample size.
Figure S1 :Percent changes in BMD at lumbar spine (A) and femoral neck (B) during two years of denosumab administration ( n =4).
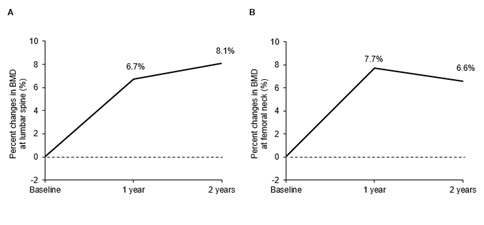
Bone metabolism markers
Fig n=11). uNTx rapidly decreased in the first month after denosumab initiation, and remained at low levels ever after. All values were significantly lower than that of baseline (all P<0.01). On the other hand, BAP gradually declined until four months after initiation of denosumab, and remained at low levels ever after. Again, all values were significantly lower than that of baseline (all P<0.01).3 shows changes in uNTx and BAP during the first 12 months of denosumab administration (n=11). uNTx rapidly decreased in the first month after denosumab initiation, and remained at low levels ever after. All values were significantly lower than that of baseline (all P<0.01). On the other hand, BAP gradually declined until four months after initiation of denosumab, and remained at low levels ever after. Again, all values were significantly lower than that of baseline (all P<0.01).
Figure 3 :Changes in uNTx (A) and BAP (B) during the first year of denosumab administration ( n =11). *, P <0.05.
Adverse events and SREs
In total, four patients discontinued denosumab during the follow-up period for the following reasons: dental treatment, but not for osteonecrosis of the jaw (n=2); depression (n=1); liver failure due to docetaxel (n=1). There were no denosumab-related severe adverse events, such as hypocalcemia, osteonecrosis of the jaw, bone fracture, and renal dysfunction, during the follow-up period.
Although only a patient underwent palliative radiotherapy and used opioid for lumbar pain due to bone metastasis, no other SREs were observed in the remaining patients.
Discussion
To the best of our knowledge, the present study is the first assessment of the impact of monthly 120 mg denosumab on bone metabolism in real-world patients. It demonstrated that BMD evaluated using DXA significantly increased from baseline a year after initiating monthly 120 mg denosumab. It also showed that bone metabolism markers significantly decreased during the first year of initiation, with the decline rate of uNTx faster than that of BAP.
There have been a lot of studies which investigated the effect of semiannual 60 mg denosumab on BMD in both normal populations [22,27] and PC patients treated with ADT [28, 29]. In low BMD postmenopausal women, semiannual 60 mg denosumab has reportedly increased BMD at lumbar spine by 4.6–5.2% and that at femoral neck by 2.1–3.5% after a year of initiation [22, 23]. In the same setting, it has also been reported to increase BMD at lumbar spine by around 6.5% and that at femoral neck by 2.8% after two years of initiation [24, 25]. In low BMD men, the drug has been shown to increase BMD at lumbar spine by 5.8% and that at femoral neck by 2.2% after a year of initiation [27]. On the other hand, semiannual 60 mg denosumab has also been investigated in PC patients treated with ADT: it increased BMD at lumbar spine by 2.5–4% and that at femoral neck by 2–3.7% after a year of initiation; and by 6–8% and by 2–3% after three years of initiation, respectively [28,29]. It should be noted that ADT causes approximately 5% of BMD loss within a year of its initiation [7, 8].
McClung et al conducted a dose-finding study of denosumab for osteoporosis, and demonstrated its optimal dose to be 60mg every six months [23]. They administered denosumab every six months at a dose of 14, 60, 100or 210mg for postmenopausal women with low BMD, and detected the most effective dose to be 60mg, since higher doses were not more effective and a dose of 14mg was less effective. The present study administered 120mg denosumab every month, which meant twelve times higher than a standardized dose for osteoporosis. The dose, which was established based on phase 2 trials seeking the optimal dose for the prevention of SRE (i.e. another endpoint than BMD) in cancer patients with bone metastases [31,33], increased BMD at lumbar spine by 6.2% and that at femoral neck by 7.6% a year later: this seems almost equivalent, or if any, slightly greater effect, compared to the above studies of semiannual 60 mg denosumab for PC patients receiving ADT [28, 29]. These results are completely in line with the dose-finding study by McClung et al, that is, the effect of denosumab on increasing BMD does not exhibit dose-dependency at a more dose than 60 mg every six month.
With regard to bone metabolism markers, we observed that changes in uNTx and BAP exhibited contrasting patterns: uNTx, a bone resorption marker, rapidly decreased, whereas BAP, a bone formation marker, gradually declined. Possible explanations for this are as follows: firstly, tumor cells in bone metastasis activate osteoclasts and promote bone destruction; secondly, ADT also stimulates bone resorption and reduces BMD; thirdly, in response to these, bone formation is promoted; and lastly, high-turnover osteoporosis is induced in bone-metastatic PC patients undergoing ADT. Given that denosumab, an anti-RANKL monoclonal antibody, blocks the action of osteoclasts and inhibits bone resorption, a rapid decrease in a bone resorption marker (uNTx) and a gradual decline in a bone formation marker (BAP) are seemingly reasonable. These explanations are supported by several studies which reported rapid decreases of bone resorption markers including serum C-telopepitide [24,27], [34], tartrate-resistant acid phospatase [26, 28, 34], and uNTx [24, 31-33], and gradual declines of bone formation markers including procollagen type1 N-terminal propeptide [25, 26] and BAP [24].
In the present study, there were no denosumab-related severe adverse events, which might be partly because of prophylactic measures: a dental examination for osteonecrosis of the jaw; and supplements containing calcium and vitamin D for hypocalcemia. Furthermore, in 12 patients with bone-metastatic PC, only a patient experienced SREs during the follow-up. Denosumab might contribute to a decrease in SREs as its originally expected effect, as well as an increase in BMD.
This study was limited by its retrospective design and small sample size. In addition, heterogeneity of concurrent therapies with denosumab (e.g. some patients used alternative ADT and docetaxel chemotherapy) could affect bone metabolism. Further studies with larger populations are needed to confirm these results.
In Conclusion, The impact of monthly 120 mg denosumab on bone metabolism was significant, but almost equivalent to that of the standard dose for osteoporosis (60mg semiannually) after a year of initiation in bone-metastatic PC undergoing ADT. Whereas the higher dose has reportedly reduced SREs, the effect on bone metabolism seemed plateaued or showed no dose-dependency.
Acknowledgements
We received no funding/grant support for this study.
Conflicts of interest statement
The authors declare that they have no competing interests.
References
[1]. Ferlay J SI, Ervik M, et al. GLOBOCAN 2012 v1.0, Available from URL: Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet].accessed on August 29th, 2017. Lyon, France: International Agency for Research on Cancer;http://globocan.iarc.fr,2013.
[2]. Ho CC, Seong PK, Zainuddin ZM, Abdul Manaf MR, Parameswaran M, Razack AH. Retrospective study of predictors of bone metastasis in prostate cancer cases. Asian Pacific journal of cancer prevention : APJCP. 2013;14(5):3289-92.
[3]. Taguchi S, Fukuhara H, Kakutani S, Takeshima Y, Miyazaki H, Suzuki M, et al. Risk factors for clinical metastasis in men undergoing radical prostatectomy and immediate adjuvant androgen deprivation therapy. Asian Pac J Cancer Prev. 2014;15(24):10729-33.
[4]. Chien TM, Lu YM, Geng JH, Huang TY, Ke HL, Huang CN, et al. Predictors of Positive Bone Metastasis in Newly Diagnosed Prostate Cancer Patients. Asian Pacific journal of cancer prevention : APJCP. 2016;17(3):1187-91.
[5]. Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of the "androgen deprivation syndrome" in men receiving androgen deprivation for prostate cancer. Archives of internal medicine. 2006;166(4):465-71.
[6]. Nguyen PL, Alibhai SM, Basaria S, D'Amico AV, Kantoff PW, Keating NL, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. European urology. 2015;67(5):825-36.
[7]. Hamilton EJ, Ghasem-Zadeh A, Gianatti E, Lim-Joon D, Bolton D, Zebaze R, et al. Structural decay of bone microarchitecture in men with prostate cancer treated with androgen deprivation therapy. The Journal of clinical endocrinology and metabolism. 2010;95(12):E456-63.
[8]. Mittan D, Lee S, Miller E, Perez RC, Basler JW, Bruder JM. Bone loss following hypogonadism in men with prostate cancer treated with GnRH analogs. The Journal of clinical endocrinology and metabolism. 2002;87(8):3656-61.
[9]. Greenspan SL, Coates P, Sereika SM, Nelson JB, Trump DL, Resnick NM. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. The Journal of clinical endocrinology and metabolism. 2005;90(12):6410-7.
[10]. Berruti A DL, Terrone C, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167(6):2361–7.
[11]. Morote J, Morin JP, Orsola A, Abascal JM, Salvador C, Trilla E, et al. Prevalence of osteoporosis during long-term androgen deprivation therapy in patients with prostate cancer. Urology. 2007;69(3):500-4.
[12]. Ezat SW SJS, Noraziani K, et al. Skeletal-related events among breast and prostate cancer patients: towards new treatment initiation in Malaysia’s hospital setting. Asian Pac J Cancer Prev. 2013;14(5):3357–62.
[13]. Norgaard M, Jensen AO, Jacobsen JB, Cetin K, Fryzek JP, Sorensen HT. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol. 2010;184(1):162-7.
[14]. DePuy V AK, Castel LD, et al. . Effects of skeletal morbidities on longitudinal patient-reported outcomes and survival in patients with metastatic prostate cancer. Support Care Cancer 2007; .
[15]. Smith MR CR, Klotz L, et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol 2015; .
[16]. Henry DH CL, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29(9):1125–32.
[17]. Stopeck AT LA, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010; 28(35):5132–9.
[18]. Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet (London, England). 2011;377(9768):813-22.
[19]. Henry D V-RS, Hirsh V, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22(3):679–87.
[20]. Smith MR, Saad F, Oudard S, Shore N, Fizazi K, Sieber P, et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time. J Clin Oncol. 2013;31(30):3800-6.
[21]. Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379(9810):39-46.
[22]. Sanchez A, Brun LR, Salerni H, Costanzo PR, Gonzalez D, Bagur A, et al. Effect of Denosumab on Bone Mineral Density and Markers of Bone Turnover among Postmenopausal Women with Osteoporosis. Journal of osteoporosis. 2016;2016:8738959.
[23]. McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, et al. Denosumab in postmenopausal women with low bone mineral density. The New England journal of medicine. 2006;354(8):821-31.
[24]. Lewiecki EM, Miller PD, McClung MR, Cohen SB, Bolognese MA, Liu Y, et al. Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22(12):1832-41.
[25]. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. The New England journal of medicine. 2009;361(8):756-65.
[26]. Bone HG, Bolognese MA, Yuen CK, Kendler DL, Wang H, Liu Y, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. The Journal of clinical endocrinology and metabolism. 2008;93(6):2149-57.
[27]. Langdahl BL, Teglbjaerg CS, Ho PR, Chapurlat R, Czerwinski E, Kendler DL, et al. A 24-month study evaluating the efficacy and safety of denosumab for the treatment of men with low bone mineral density: results from the ADAMO trial. The Journal of clinical endocrinology and metabolism. 2015;100(4):1335-42.
[28]. Smith MR, Saad F, Egerdie B, Szwedowski M, Tammela TL, Ke C, et al. Effects of denosumab on bone mineral density in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2009;182(6):2670-5.
[29]. Smith MR, Egerdie B, Hernandez Toriz N, Feldman R, Tammela TL, Saad F, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. The New England journal of medicine. 2009;361(8):745-55.
[30]. Egerdie RB SF, Smith MR, et al . Responder analysis of the effects of denosumab on bone mineral density in men receiving androgen deprivation therapy for prostate cancer. Prostate Cancer Prostatic Dis2012; .
[31]. Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25(28):4431-7.
[32]. Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, et al. Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(20):6690-6.
[33]. Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27(10):1564-71.
[34]. Smith MR, Saad F, Egerdie B, Sieber P, Tammela T, Leder BZ, et al. Denosumab and changes in bone turnover markers during androgen deprivation therapy for prostate cancer. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(12):2827-33.
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times