Ovarian Germ Cell Tumor with Myasthenia Gravis: Co-incidence or a Possible link?
Download
Abstract
Teratomas are a common form of germ cell tumor. Teratomas are commonly found in the gonadal organs, such as the ovaries and testes. Treatment of choice for ovarian teratomas is complete surgical excision, which exhibits a good prognosis in benign teratomas; however, chemotherapy treatment is needed for malignant components. Neurological paraneoplastic presentation of gynecological tumors is rare; however, ovarian tumors account for 10% of this presentation. In literature, paraneoplastic limbic encephalitis, anti-N-methyl-D-aspartate receptor encephalitis, and paraneoplastic cerebellar degeneration have been reported in ovarian teratomas and tumors; however, myasthenia gravis has been reported only twice. In both of those cases, manifestation of myasthenia gravis was preceding the diagnosis of ovarian cancer. We describe the first case of a 21-year-old female who presented with new-onset myasthenia gravis after finishing chemotherapy for ovarian teratoma. Another unusual aspect of our case is the rare co-occurrence of gliomatosis peritonei with mature teratoma.
Introduction
Ovarian teratomas (OT) include mature cystic teratomas (dermoid cysts), immature teratomas, and monodermal teratomas (e.g struma ovarii, carcinoid tumors, neural tumors). Ovarian teratoma has documented autoimmune associations e.g paraneoplastic limbic encephalitis and cerebellar degeneration; however, association with myasthenia gravis has been reported only once before [1]. Myasthenia gravis (MG) is mainly caused by autoantibodies against the acetylcholine receptor (AChR) [2]. In approximately 15% of patients of thymoma, MG is considered a paraneoplastic phenomenon [3,4]. However, extrathymic malignancies have been also reported to coincide with myasthenia gravis. The potential mechanism for the association of MG with ovarian teratoma in our patient was indistinct, but teratoma may have contained myoid cells with antigenicity for the anti- acetylcholine receptor (anti-AchR), as has been observed in case of the thymus [5].
Case Presentation
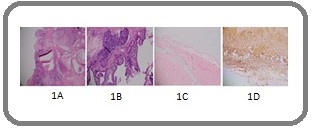
A 21-year-old female presented in medical oncology clinic with a four month history of abdominal swelling, generalized weakness, and reduced appetite. She underwent left sided surgical oophorectomy, the histopathology of which showed stage IIC mixed germ cell tumor (Figure 1A, B).
Figure 1. (A-D) Histopathological Images. 1A,B, Mixed germ cell ovarian tumor showing mature (70%) and immature (30%) teratomatous components. 1C,D, Peritoneal wall biopsy, showing mature glial tissue only; gliomatosis peritonei (GFAP showing diffuse staining in 1D).
Laboratory workup indicated cancer antigen (Ca) 125: 90 U/mL, beta-human chorionic gonadotropin (β-HCG): <2 mIU/mL, alpha-fetoprotein (AFP): 50 ng/mL and serum lactate dehydrogenase (LDH): 252 U/L.
Magnetic resonance imaging (MRI) pelvis revealed a complex left adnexal mass with peritoneal carcinomatosis (Figure 2A, B).
Figure 2. (A-D) MRI Pelvis Images. 2A,B, Complex left adnexal mass (5 x 3.2 cm) and omentoperitoneal disease. 2C,D, Interval reduction in left adnexal mass lesion.
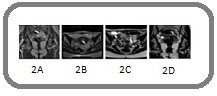
As there was omentoperitoneal disease, it was decided to offer her BEP (bleomycin, etoposide, and cisplatin) chemotherapy and then assess for surgery at a later stage. MRI pelvis after 4 cycles of chemotherapy showed partial treatment response by virtue of interval reduction in size of the left adnexal mass (Figure 2C, D). Tumor markers also got normalized after chemotherapy.
Meanwhile, she developed bleomycin-induced pulmonary toxicity. For her residual pelvic disease, she was planned to be offered staging laparoscopy and excision of the left-sided mass.
Meanwhile, she presented in the emergency department one day with a one-week history of left eyelid drooping, inability to comb hair, difficulty in speaking and swallowing along with a feeling of getting tired with passage of the day. Her weakness was fatiguable with early morning improvement. Physical examination revealed ptosis of the left eyelid, bilateral equal and reactive pupils, and expressive aphasia along with intact facial nerve territory, gag reflex, and cough reflex. Tone, power, reflexes, and sensations were normal in all the four limbs. Keeping in mind the differentials of myasthenia gravis (MG), 3rd nerve palsy, and Wernicke’s aphasia, investigations were ordered. Serum creatine kinase, thyroid profile, fasting plasma glucose, and MRI brain turned out to be normal. However, acetylcholine receptor antibodies level was raised i.e 27 (normal 0.4). CT thorax was also negative for thymic hyperplasia or thymoma (no evidence of cystic component or calcification). Her weakness improved when given pyridostigmine. So, the neuro physician recommended an increased dose of pyridostigmine and prednisolone. Considering her new-onset myasthenia gravis issue, her pelvic surgery was planned to be deferred until regaining of complete power. After an escalating dose of pyridostigmine to 60 mg five times a day along with prednisolone 45 mg/day, her fatiguability got settled and her single breath count became 32. So, after optimizing her bleomycin-induced lung injury and myasthenia gravis, she underwent staging laparoscopy and peritoneal deposit biopsy. Per-operative findings were widespread metastatic disease involving all quadrants of abdomen suggesting irresectability. Histopathology showed mature glial tissue only (no other component) with the possibility of gliomatosis peritonei (Figure 1 C, D). As the disease was irresectable and tumor markers were normal, she was put on surveillance. Thereafter, her 3 monthly staging CT chest abdomen pelvis showed stable residual disease and her tumor markers continued to be within normal limits. Since then, she has been doing quite well and is on active follow up with medical oncology and neurology teams.
Discussion
Mature cystic teratomas of the ovary are often discovered as incidental findings on physical examination, during radiological investigations, or during abdominal surgery performed for other reasons. Mature ovarian teratomas are asymptomatic in many patients. When symptoms are present, they include abdominal mass or swelling, abdominal pain, or pelvic pain, which is caused by torsion of the ovary or irritation of its ligaments.
Myasthenia gravis (MG) is characterized by neuromuscular transmission failure due to autoantibodies to the acetylcholine receptor (AchR). The paraneoplastic myasthenic syndrome is associated with thymoma in 15% of MG patients. Extra-thymic malignancy with myasthenia gravis is a reported entity.The relationship between Lambert-Eaton myasthenic syndrome and malignant disorders is well known [6]; however, the association of extra-thymic malignancies with myasthenia gravis is an interesting entity. To our knowledge, there is only one reported case of retroperitoneal teratoma causing ocular myasthenia, which was treated successfully by a complete resection of the tumor; and one case of papillary serous adenocarcinoma of ovary associated with myasthenia gravis [7,8]. However, in those two case, myasthenic symptoms were manifested preceding the diagnosis of retroperitoneal teratoma and ovarian cancer; unlike our case, in which features of MG developed after chemotherapy was completed for teratoma. Another peculiar aspect in our case was a rare combination of gliomatosis peritonei (GP) accompanying mature teratoma. GP is a mature neural glial tissue implanted onto the peritoneal surface. It is mostly seen in immature teratomas and rarely in mature teratomas [9,10].
In our case, the patient had been diagnosed with a mixed germ cell tumor (mature cystic teratoma 70% and immature teratoma) after she underwent surgical oophorectomy. The immature component of the germ cell tumor was treated with chemotherapy and the mature component was planned to be treated by surgical resection. However, due to chemotherapy-induced lung injury, surgery of the patient got delayed. Afterward, the mature component of ovarian teratoma being irresectable was left as the residual disease. The potential mechanism for the association of residual teratoma in our case with new-onset MG is unclear. However, mature teratoma in our patient may have contained myoid cells with antigenicity for AchR. The residual disease (mature teratoma) could have lead to continued stimulation of the anti-AchR antibody.
It was unclear whether there was an endocrine substance secreted by the residual teratoma that lead to weakness and easy fatiguability.
In conclusion, It is very difficult to ascertain whether the manifestation of myasthenia gravis on the background of ovarian teratoma is just a co-incidence, or there is a possible link. However, myasthenia gravis should be considered as one of the differentials in a germ cell tumor patient presenting with new-onset weakness or easy fatiguability.
References
- Paraneoplastic neurological syndromes associated with ovarian tumors Zaborowski Mikolaj Piotr, Spaczynski Marek, Nowak-Markwitz Ewa, Michalak Slawomir. Journal of Cancer Research and Clinical Oncology.2014;141(1). CrossRef
- Clinical Practice Guideline Series Update Blissitt Patricia A.. Journal of Neuroscience Nursing.2013;45(5). CrossRef
- Paraneoplastic Neurological Syndromes Toothaker Thomas B., Rubin Michael. The Neurologist.2009;15(1). CrossRef
- Update on Paraneoplastic Neurologic Disorders Rosenfeld Myrna R., Dalmau Josep. The Oncologist.2010;15(6). CrossRef
- Clinical and scientific aspects of acetylcholine receptor myasthenia gravis Keijzers Marlies, Nogales-Gadea Gisela, de Baets Marc. Current Opinion in Neurology.2014;27(5). CrossRef
- THE LAMBERT-EATON MYASTHENIC SYNDROME O'NEILL J. H., MURRAY N. M. F., NEWSOM-DAVIS J.. Brain.1988;111(3). CrossRef
- Retroperitoneal teratoma causing ptosis: A case report XU LIUYU, ZHAO QINGLI, ZHANG XUEBEI, LI QING, HUANG SHENGLIANG. Oncology Letters.2015;10(4). CrossRef
- Myasthenia as a paraneoplastic manifestation of ovarian Cancer Simonsen Marcelo, Miyabe Marcos Minamoto, Ouki Helio Toshio, Galvão Antônio Cezar Ribeiro, Leite Denise, Murayama Bárbara Alencar Rolim, Laginha Fabio Martins, Samora Michelle. Gynecologic Oncology Reports.2018;25. CrossRef
- Immature (malignant) teratoma of the ovary.A clinical and pathologic study of 58 cases Norris Henry J., Zirkin Howard J., Benson William L.. Cancer.1976;37(5). CrossRef
- Ovarian teratoma with gliomatosis peritonei Chuang Jiin-Haur, Chen Leung. Journal of Pediatric Surgery.1992;27(5). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2021
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times