Characteristic Imaging Patterns of Muscle Involvement in Polyarteritis Nodosa: Case Report and Review of the Literature
Download
Abstract
Objective: Polyarteritis nodosa (PAN) is a rare disease with complex clinical manifestations that are difficult to diagnose. Imaging diagnoses of previously reported patients have focused on vascular manifestations. Magnetic resonance imaging (MRI) has been used to detect muscle involvement in PAN. Here, we reviewed imaging findings pertaining to muscle involvement in patients with PAN.
Methods: Twelve articles concerning muscle involvement in PAN were published during the period 1980–2020; 21 patients, including our patient, were examined in this study.
Results: Across the published articles, the male to female ratio was 1:1, the mean patient age was 40.76 ± 18.28 years, and there were 17 patients with calf involvement and 3 with thigh involvement. The T1WI and T2WI findings were both isointense in one patient, and the T1WI findings alone were isointense in seven patients. The T1WI findings were slightly hyperintense in five patients, and no T1WI images were available for the remaining seven patients. The T2WI signal was diffusely hyperintense in 10 patients, and “patchy villous hyperintense” in 9 patients. Among the 12 patients with enhanced images, most exhibited diffuse or cotton-like enhancement, while some showed involvement of the fascia and periosteum. The comprehensive imaging analysis of our patient included muscle and blood vessel MRI and computed tomography (CT) examinations. Our patient’s disease involved the calves and thighs, with T1WI isointensity and T2WI patch-like hyperintensity, as well as cotton-like enhancement centered on blood vessels. Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) examinations of the lower limbs showed beadlike changes in the arterial branches, and the lower leg arterial branches were obvious. Head MRA revealed stenosis and occlusion of the right middle cerebral artery. Additionally, the superior mesenteric artery was locally dilated around a tumor, with the greatest width being approximately 9 mm. Cerebral perfusion analysis indicated cerebral blood flow (CBF) was lower in the right cerebellar hemisphere.
Conclusions: PAN should be considered in the presence of patchiness or diffuse muscle signal changes on MRI of the lower leg (or thigh), followed by vessel-centered cotton-like enhancement accompanied by fascial or periosteal enhancement. Our findings suggest that systemic examination of small and medium arteries in patients with PAN can aid early prevention and treatment.
Introduction
Polyarteritis nodosa (PAN) is uncommon in patients with systemic vasculitis, and has a low incidence of 1.6–2.4 per 1 million people worldwide [1-2]. In its early stages, PAN encompasses the majority of vasculitis manifestations. In recent years, in-depth research on vasculitis has revealed specific subtypes. Research concerning the pathogenic effect of antineutrophil cytoplasmic antibodies (ANCA) in vasculitis has led to a distinction being drawn between ANCA-positive patients with vasculitis and patients with PAN. Furthermore, eosinophilic granulomatous polyangiitis is currently classified as ANCA-associated vasculitis, formerly known as Churg–Strauss syndrome. PAN with rheumatoid arthritis is now regarded as “rheumatoid arthritis vasculitis” [3-5].
PAN is a segmentalized, necrotizing vasculitis that primarily involves small and medium arteries, as well as arterioles. It was defined at the Chapel Hill Conference in 2012. This rare disease may present with multiple organ involvement and complicated clinical manifestations, and is difficult to diagnose early [3]. The present report summarizes the typical magnetic resonance imaging (MRI) manifestations of patients with PAN, as described in the English-language medical literature. The shin exhibits patchy-like T1WI isointensity and T2WI hyperintensity, with diffuse or vascular foci. After enhancement, the shin exhibits cotton-like enhancement with vascular foci and focal accumulation in the periosteum and fascia. In our patient, plain and contrast-enhanced muscle MRI showed typical imaging manifestations, but plain and contrast-enhanced computed tomography (CT) revealed no abnormalities. The clinical manifestations of other extramuscular organs were normal, but computed tomography angiography (CTA) and magnetic resonance angiography (MRA) findings suggested stenosis of cranial vessels or aneurysmal dilation of the mesentery, and vascular lesions precluded clinical manifestations.
Case Report
Clinical manifestations and treatment process
The patient was a 55-year-old man who had a 1-year history of bilateral nodal erythema with swelling, but no itching desquamation, fever, or discomfort. He presented to the First Affiliated Hospital of Guangzhou University of TCM and the Fifth Affiliated Hospital of Sun Yat-sen University. He was first diagnosed with nodular erythema, and treated with hydroxychloroquine, prednisone, and Chinese medicine. These treatments did obviously relieve his symptoms, and he gradually developed nodal erythema on the wrist, left elbow, and thighs. Approximately 4 months later, he developed pain in both knee joints, accompanied by pain during exertion of the bilateral thigh muscles. He also experienced muscle fatigue, swelling and pain in the left ring finger, and limited fist clenching ability in both hands. However, there was no morning stiffness or skin tightness. Three months later, he developed nonspecific fever, primarily at night, with a maximum temperature of 39.0℃. This temperature returned to normal in the mornings. Based on the absence of other symptoms (e.g., oral and genital ulcers, sore throat, cough and expectoration, and abdominal pain), the patient was diagnosed with PAN. He was treated with prednisone acetate (30 mg/day) and methotrexate (12.5 mg/week). These treatments reduced the intermittent fever.
2.2 Laboratory examination
The initial laboratory findings were as follows: monocytes, 14.34%; absolute monocyte count, 1.19 × 109/L; PLT, 391.00 × 109/L; PT, 13.7 s; INR, 1.22 ↑; fibrinogen, 6.78 g/L; activated partial thromboplastin time, 33.8 ↑s; ESR, 44 mm/h; complement C3, 1.72 g/L; and high-sensitivity C-reactive protein, 107.73 mg/L. The patient had negative findings for cytoplasmic ANCA, formaldehyde-resistant perinuclear ANCA (pANCA), formaldehyde-sensitive pANCA, glomerular basement membrane antibody, proteinase 3 target antigen, myeloperoxidase-resistant Sm antibodies, anti-SSB antibody, anti-SSA antibody, Ro-52 antibody, Scl-70 antibody, Jo-1 antibody, centromere-resistant double-stranded DNA antibody, and nucleosome-resistant antibody. The extremity electromyography (EMG) findings were as follows: abnormal bilateral median nerve F wave, bilateral ulnar nerve F wave, and bilateral tibial nerve H reflex; these findings suggested proximal nerve root involvement.
Follow-up laboratory findings were as follows: lymphocytes, 68.5%; monocytes, 10.8%; absolute monocyte count, 0.87 × 109/L; Hb, 128.00 g/L; HCT, 39.7%; MCH, 26.7 pg; RDW, 15.5%; MPV, 8.90 fl; PDW, 9.50 fl; ratio of large platelets, 16.3%; aspartate aminotransferase, 13.2 U/L; and 2019-nCoV nucleic acid, negative. The patient again had negative findings for cytoplasmic ANCA, formaldehyde-resistant pANCA, formaldehyde-sensitive pANCA, glomerular basement membrane antibody, proteinase 3 target antigen, myeloperoxidase-resistant Sm antibodies, anti-SSB antibody, anti-SSA antibody, Ro-52 antibody, Scl-70 antibody, Jo-1 antibody, centromere-resistant double-stranded DNA antibody, and nucleosome-resistant antibody.
Imaging findings
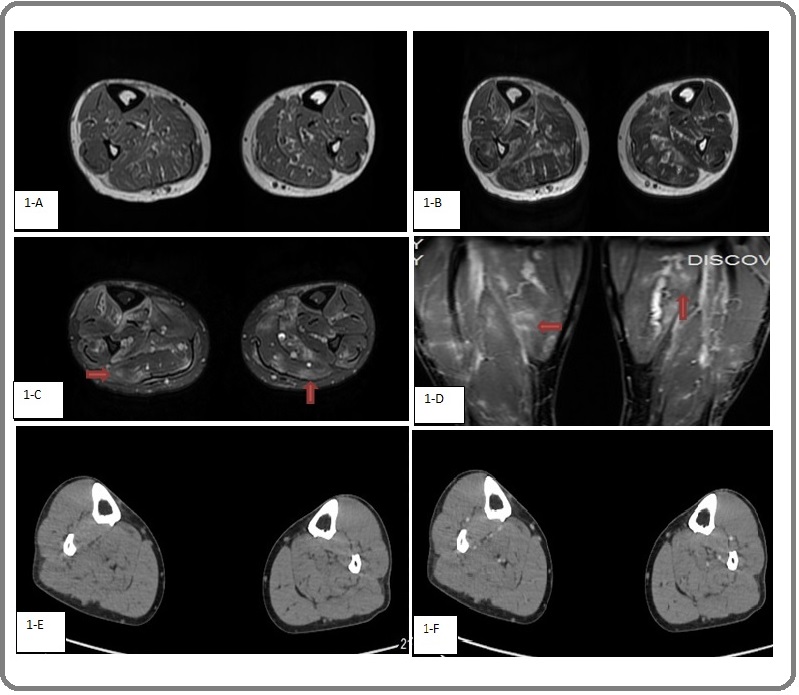
MRI of both lower limbs revealed swelling of both lower legs, as well as large abnormal shadows scattered in the calf muscle groups on both sides. T1WI showed mainly uneven hypointensity, and a few slightly hyperintense “internal shadows”. Fat-saturated T2WI and T2WI showed large shadows with blurred edges. Occasional hypointense signals on T1WI and hyperintense signals on T2WI were evident in the middle tibiofibular region on both sides, along with cotton-like enhancement centered on blood vessels (Figure 1A–D). CT of both lower extremities revealed no abnormalities (Figure 1E, F).
Figure 1. 1-A, T1WI image; 1-B, T2WI image; 1-C, fat-saturated T2WI image; 1-D, fat-saturated T1WI image; 1-E, Plain CT scan; 1-F, enhanced CT scan. On MRI, large abnormal shadows were scattered in bilateral calf muscle groups, with a few slightly hypointense signals on T1WI, hyperintense shadows on T2WI and fat-saturated T2WI, and blurred edges and cotton-like enhancement.
Bilateral lower limb MRA revealed normal findings in the abdominal aorta, bilateral common iliac arteries, bilateral external iliac arteries, bilateral internal iliac arteries, bilateral femoral arteries, bilateral popliteal arteries, bilateral anterior tibial, and posterior tibial arteries and their branches, although they had slightly rough walls. The vessels of the lower limb arteries showed beadlike changes, and the branches of the bilateral calf artery were obvious (Figure 2A).
Figure 2. 2-A, MRA of Lower Extremity Vessels; 2-B, CTA of Lower Extremity Vessels. MRA and CTA showed beadlike changes in the branches of lower extremity arteries, as well as obvious branches of lower leg arteries.
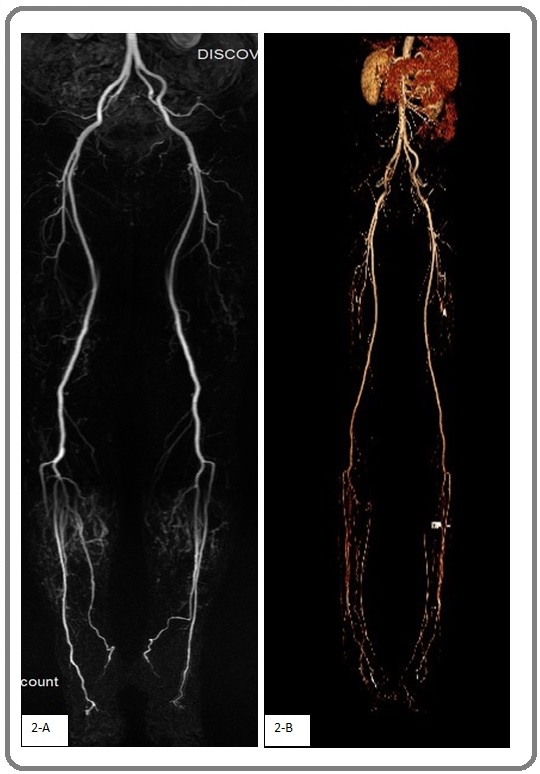
CTA of the bilateral lower limbs revealed normal findings in the abdominal aorta, renal artery, celiac axis and its branches, superior mesenteric artery, bilateral common iliac arteries, bilateral intenal iliac arteries, bilateral external iliac arteries, shallow bilateral femoral artery, deep artery, popliteal artery, peripheral artery, and posterior tibial artery and its branches. The vessels exhibited rough walls, with a double leg artery and its branches, the local change a beaded samples, superior mesenteric artery local expansion in tumor samples, at its widest point is about 9 mm, bilateral popliteal artery, double pretibial, posterior tibial artery and its branches development shallow light, double leg visible early show great saphenous vein, small saphenous vein, abnormal muscle did not see reinforcement (Figure 2B).
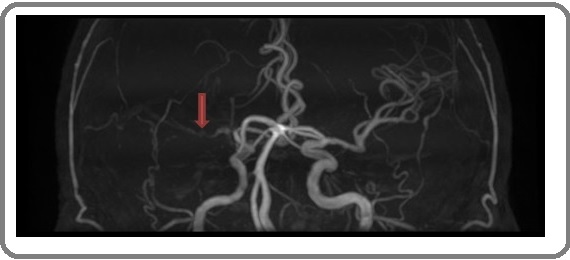
Cranial MRA revealed right middle cerebral artery stenosis and occlusion, but the other intracranial vessels were normal (Figure 3).
Figure 3. Right Middle Cerebral Artery Stenosis and Occlusion (arrow). The remaining intracranial vessels were normal.
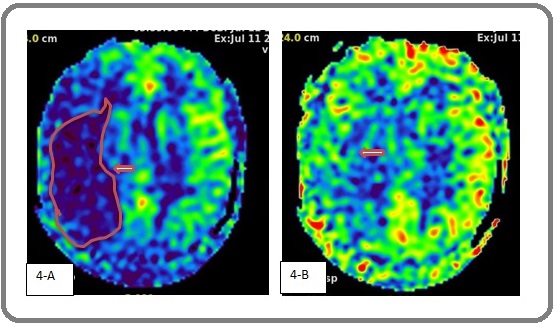
Brain perfusion imaging and head MRI showed that cerebral blood flow (CBF) was lower in the right cerebellar hemisphere than in the left cerebellar hemisphere at DLP1.5, although both sides were equivalent at DLP2.5. These findings were indicative of ischemic changes in the right cerebellar hemisphere (Figure 4).
Figure 4. 4- A, Cerebral Perfusion of CBF at DLP1.5; 4-B, Cerebral Perfusion of CBF at DLP2.5. Magnetic resonance perfusion imaging indicated that CBF of the right cerebellar hemisphere decreased at DLP1.5, compared with that of the left cerebellar hemisphere. CBF was similar on both sides at DLP2.5 (arrows).
EMG of both lower extremities revealed an abnormal tibial nerve H reflex on both sides, which implied proximal nerve root involvement.
Diagnosis and treatment process
See Table 1.
| May 2018 | March 2019 | March 2020 | March 2020 (follow-up) | July 2020 | |
| Diagnosis and treatment | No venous abnormalities were observed in either lower limb. | Erythema nodosum on both legs was suspected by the First Affiliated Hospital of Guangzhou University of TCM and the Fifth Affiliated Hospital of Sun Yat-sen University, but the appropriate treatment was unclear. | Subcutaneous nodule biopsy findings were consistent with ANCA-negative erythema nodosum and fever. Multidisciplinary consultation led to a diagnosis of PAN. Arthritis pain was relieved by prednisone acetate and methotrexate treatment, and his body temperature returned to normal | Mesenteric arteriovenous CTA showed a slightly wider localized superior mesenteric artery. Ultrasound showed no abnormalities in the bilateral renal arteries, carotid arteries, or vertebral arteries. | Prednisone acetate and methotrexate treatments yielded joint pain relief and normal temperature. Injection of recombinant human tumor necrosis factor receptor Ⅱ antibody led to substantial lower limb pain and fatigue reduced, but no fever. |
Pathological examination
Skin biopsy of the left leg revealed findings consistent with erythema nodosum.
Discussion
PAN is a relatively rare rheumatic immune disease with various clinical manifestations and no specific serological markers. Therefore, it is difficult to diagnose and clinicians have insufficient knowledge of this disease. The pathogenesis of PAN remains unclear. Some clinicians have suggested that it is heredity, where deletion of the autosomal recessive CecRL gene can lead to PAN [6]. Furthermore, patients with familial Mediterranean fever may present with PAN [7]. Viral infection, such as hepatitis B virus, hepatitis C virus, and human immunodeficiency virus, is an important potential etiology [8].
Patients with PAN may have nonspecific symptoms such as fever, weight loss, joint pain, and muscle pain. The corresponding organs or tissues may exhibit ischemia or bleeding due to narrowing or blockage of inflamed arteries, or microaneurysm rupture. PAN combined with cardiovascular lesions has the following imaging characteristics: extensive artery involvement, most commonly in the aorta and secondary branches; and diverse arterial lesions, primarily in the gut and lower extremities, including the renal artery and its branches. Coronary artery involvement is also common. The reported incidence of cardiac involvement of PAN ranges from 4% to 65% [9]. Vascular lesions of PAN disease show segmental changes, mainly in vascular branches, along with immune complex deposition and granuloma formation. Extensive inflammatory cell infiltration (mainly lymphocytes) is present in the lesions. Fibrosis-like necrosis is a common manifestation of active lesions, often accompanied by neutrophil infiltration [10-13].
The MRI manifestations of 21 patients (including our patient, as shown in Table 2) described in 12 English-language articles [17-28] were retrospectively analyzed: there were 17 patients (80.9%) with calf involvement, 3 (14.3%) with thigh involvement, and 1 (4.8%) with both thigh and calf involvement.
| Reference | Gender | Age | Position | T2-Weighted STIR Images | T1-Weighted Images | Contrast Enhancement | Fascial Lesion | Periosteal Lesion |
| Nakamura et al. [17] | F | 38 | leg | Diffuse increase | Not available | Not available | Not available | Not available |
| Yang et al. [18] | M | 26 | leg | Diffuse increase | Not available | Present | Not available | Not available |
| Hofman et al. [19] | F | 36 | leg | Diffuse increase | Not available | Not available | Not available | Not available |
| Révelon et al. [20] | M | 43 | leg | Patchy increase | Not available | Cotton-wool appearance | Present | Not available |
| Ahmed et al [21]. | F | 36 | leg | Diffuse increase | Not available | Not available | Present | Not available |
| Gallien et al. [22] | F | 41 | leg | Patchy increase | Not available | Present | Not available | Present on bone scintigraphy |
| Gallien et al. [22] | F | 45 | leg | Diffuse increase | Within normal limit | Not available | Not available | Not available |
| Mac Donald and Blake [23] | M | 20 | leg | Patchy increase | Not available | Not available | Present | Present on bone scintigraphy |
| Esteva-Lorenzo et al. [24] | M | 56 | leg | Diffuse increase | Decrease | Not available | Absent | Present |
| Reading et al. [25] | M | 38 | thigh | Diffuse increase | Not available | Not available | Present | Absent |
| Kang, Y [26] | F | 42 | leg | Diffuse increase | Slight hyperintensity | Cotton-wool appearance | Absent | Absent |
| M | 7 | leg | Diffuse increase | Within normal limit | Cotton-wool appearance | Absent | Present | |
| M | 64 | leg | Diffuse increase | Within normal limit | Diffuse enhancement | Present | Absent | |
| F | 20 | leg | Within normal limit | Within normal limit | Absent | Present | Absent | |
| M | 7 | leg | Patchy hyperintensity | Within normal limit | Cotton-wool appearance | Preset | Present | |
| M | 30 | leg | Patchy hyperintensity | Slight hyperintensity | Cotton-wool appearance | Absent | Absent | |
| F | 56 | leg | Patchy hyperintensity | Within normal limit | Cotton-wool appearance | Absent | Present | |
| F | 49 | leg | Patchy hyperintensity | Within normal limit | Cotton-wool appearance | Absent | Present | |
| Masahiro Aoshima [27] | F | 78 | leg | Patchy high signal Adipoid signal | Adipoid signal | Not available | Not available | Not available |
| Hiroshi Takei [28] | M | 69 | thigh | Discretely granular | Slightly high signal | Cotton-wool appearance | Absent | Absent |
| This case | M | 55 | leg thigh | Patchy hyperintensity | Within normal limit | Cotton-wool appearance | Absent | Absent |
The patients included 11 males and 10 females (mean age, 40.76 ± 18.28 years; range: 7–78 years). The T1WI and T2WI findings were both isointense in one patient, while the T1WI findings alone were isointense in seven patients. The T1WI findings were slightly hyperintense in five patients, and no T1WI images were available for the remaining seven patients. The T2WI signal was diffusely hyperintense in 10 patients, and “patchy villous hyperintense” in 9 patients. Among the 12 patients with enhanced images, most exhibited diffuse or cotton-like enhancement, and some patients had involvement of the fascia and periosteum. Our patient showed early disease manifestations in the calves, and late manifestations in the thighs. MRI revealed that the thigh and calf muscles were involved, with T1WI isointensity and T2WI patch- like hyperintensity, as well as cotton-like enhancement centered on blood vessels. Enhanced muscle scans did not show abnormalities (Figure 1). Focal accumulation was indicative of vascular stenosis. The bilateral leg artery and its branches showed localized beadlike changes with many lesions. The wall of the profunda femoris artery was rough, with slightly localized stenosis and few lesions. It also showed aneurysmal dilation of the superior mesentery, as well as middle cerebral artery stenosis and occlusion, and ischemic compensatory changes in the right cerebellar hemisphere. However, no clinical symptoms were evident (Figures 2-4).
Currently, diagnosis of PAN is made using the American College of Rheumatology 1990 classification [14]. However, due to improvements in our understanding of vasculitis, this standard now clearly has limitations, especially because the ANCA antibody test does not reliably detect microscopic polyangiitis. PAN was defined at the Chapel Hill Conference in 2012 and determined to have no association with ANCA [3]. Some researchers have proposed combining the American College of Rheumatology, Laham Standard, and Chapel Hill Conference standards, such that granulomatous polyangiitis is ruled out first. If eosinophilic granulomatous polyangiitis, granulomatous polyangiitis, and microscopic polyangiitis can be excluded, then PAN may be the appropriate diagnosis [15].
Glucocorticoids are the currently preferred treatment, combined with immunosuppressants as necessary. Surgical treatment may be necessary in patients with serious complications, such as gastrointestinal perforation, visceral rupture, ischemia, or bleeding. In a retrospective database analysis conducted in 2011, the French Vasculitis Research Group found that factors associated with 5-year mortality in patients with PAN included age > 65 years, renal insufficiency (serum creatinine ≥ 150 µmol/L), symptomatic cardiac insufficiency and, especially, severe gastrointestinal involvement (e.g., perforation, hemorrhage, and/or pancreatitis) [16].
In Conclusion, in cases with patchiness or diffuse muscle signal changes in the lower leg (or thigh) on MRI, cotton-like or diffuse enhancement centered on blood vessels may be accompanied by fascial or periosteal enhancement. PAN should be considered in the differential diagnosis, and vascular CTA or MRA examinations should be added to facilitate the diagnosis. In our patient, only the skin and lower leg showed clinical symptoms, but the profunda femoris artery, celiac trunk, and cranial vessels were abnormal. Therefore, patients with PAN should undergo examinations of small and medium arteries throughout the body, to ensure early detection of diseased vessels. This may prevent or reduce damage to target organs. If focal accumulation is suspected, MRI and MRA examinations are recommended.
References
- Effect of classification on the incidence of polyarteritis nodosa and microscopic polyangiitis Watts Richard A., Jolliffe Victoria A., Carruthers David M., Lockwood Martin, Scott David G. I.. Arthritis & Rheumatism.1996;39(7). CrossRef
- Polyarteritis nodosa when applying the Chapel Hill nomenclature--a descriptive study on ten patients Selga D., Mohammad A., Sturfelt G., Segelmark M.. Rheumatology.2006;45(10). CrossRef
- 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides Jennette J. C., Falk R. J., Bacon P. A., Basu N., Cid M. C., Ferrario F., Flores-Suarez L. F., Gross W. L., Guillevin L., Hagen E. C., Hoffman G. S., Jayne D. R., Kallenberg C. G. M., Lamprecht P., Langford C. A., Luqmani R. A., Mahr A. D., Matteson E. L., Merkel P. A., Ozen S., Pusey C. D., Rasmussen N., Rees A. J., Scott D. G. I., Specks U., Stone J. H., Takahashi K., Watts R. A.. Arthritis & Rheumatism.2012;65(1). CrossRef
- PolyaIteritis and relate disorders . Kelley’s textbook of rheumatology Lin, H.Y , W.F. Fagan , P.E. Jabin . sauders company.2009;:1539-1547.
- PolyaIteritis and relate disorders.Kelley’s textbook of rheumatology[M].8th ed.WB John S,sergent . sauders company.2009;:1539-1547.
- Mutant Adenosine Deaminase 2 in a Polyarteritis Nodosa Vasculopathy Navon Elkan Paulina, Pierce Sarah B., Segel Reeval, Walsh Tom, Barash Judith, Padeh Shai, Zlotogorski Abraham, Berkun Yackov, Press Joseph J., Mukamel Masha, Voth Isabel, Hashkes Philip J., Harel Liora, Hoffer Vered, Ling Eduard, Yalcinkaya Fatos, Kasapcopur Ozgur, Lee Ming K., Klevit Rachel E., Renbaum Paul, Weinberg-Shukron Ariella, Sener Elif F., Schormair Barbara, Zeligson Sharon, Marek-Yagel Dina, Strom Tim M., Shohat Mordechai, Singer Amihood, Rubinow Alan, Pras Elon, Winkelmann Juliane, Tekin Mustafa, Anikster Yair, King Mary-Claire, Levy-Lahad Ephrat. New England Journal of Medicine.2014;370(10). CrossRef
- Familial Mediterranean fever caused by homozygous E148Q mutation complicated by Budd-Chiari syndrome and polyarteritis nodosa Standing A. S. I., Eleftheriou D., Lachmann H. J., Brogan P. A.. Rheumatology.2010;50(3). CrossRef
- Clinical features and outcomes in 348 patients with polyarteritis nodosa: A systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French vasculitis study group database Pagnoux Christian, Seror Raphaèle, Henegar Corneliu, Mahr Alfred, Cohen Pascal, Le Guern Véronique, Bienvenu Boris, Mouthon Luc, Guillevin Loïc. Arthritis & Rheumatism.2010;62(2). CrossRef
- Clinical features, prognosis, and response to treatment in polyarteritis Cohen, R.D , D.L. Conn, , D.M. Ilstrup . Mayo Clin Proc.1980;55(3):146-155.
- Clinical Features of Polyarteritis Nodosa in Korea Bae Young Deok, Choi Hyo Jin, Lee Jung Chan, Park Jeong Jin, Lee Yun Jong, Lee Eun Bong, Song Yeong Wook. Journal of Korean Medical Science.2006;21(4). CrossRef
- Dynamic pattern of endothelial cell adhesion molecule expression in muscle and perineural vessels from patients with classic polyarteritis nodosa Coll-Vinent Blanca, Cebri�n Mireia, Cid Maria C., Font Carme, Esparza Jordi, Juan Manel, Yag�e Jordi, Urbano-M�rquez �lvaro, Grau Josep M.. Arthritis & Rheumatism.1998;41(3). CrossRef
- Immunohistochemical characterization of inflammatory cells and immunologic activation markers in muscle and nerve biopsy specimens from patients with systemic polyarteritis nodosa Cid Maria-Cinta, Grau Josep Maria, Casademont Jordi, Campo Elías, Coll-Vinent Blanca, López-Soto Alfons, Ingelmo Miguel, Urbano-Márquez Alvaro. Arthritis & Rheumatism.1994;37(7). CrossRef
- Systemic and isolated vasculitis. A rational approach to classification and pathologic diagnosis Lie J.T. Pathol Annu.1989;24 Pt 1:25-114.
- The American college of rheumatology 1990 criteria for the classification of polyarteritis nodosa Lightfoot Robert W., Michel Beat A., Bloch Daniel A., Hunder Gene G., Zvaifler Nathan J., McShane Dennis J., Arend William P., Calabrese Leonard H., Leavitt Randi Y., Lie J. T., Masi Alfonse T., Mills John A., Stevens Mary Betty, Wallace Stanley L.. Arthritis & Rheumatism.2010;33(8). CrossRef
- Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies Watts R., Lane S., Hanslik T., Hauser T., Hellmich B., Koldingsnes W., Mahr A., Segelmark M., Cohen-Tervaert J. W, Scott D.. Annals of the Rheumatic Diseases.2006;66(2). CrossRef
- The Five-Factor Score Revisited Guillevin Loïc, Pagnoux Christian, Seror Raphaele, Mahr Alfred, Mouthon Luc, Toumelin Philippe Le. Medicine.2011;90(1). CrossRef
- Polyarteritis nodosa limited to calf muscles: a case report and review of the literature Nakamura Tadashi, Tomoda Kunihiko, Yamamura Yuji, Tsukano Michishi, Honda Itsuo, Iyama Ken-ichi. Clinical Rheumatology.2003;22(2). CrossRef
- Muscular Polyarteritis Nodosa Yang Seung Nam, Cho Nam Soon, Choi Hyun Soo, Choi Seong Jae, Yoon Eul-Sik, Kim Dong Hwee. JCR: Journal of Clinical Rheumatology.2012;18(5). CrossRef
- Demonstration of calf abnormalities by magnetic resonance imaging in polyarteritis nodosa Hofman D. M., Lems W. F., Witkamp T. D., V.D.Putte S. C. J., Bijlsma J. W. J.. Clinical Rheumatology.1992;11(3). CrossRef
- Acute swelling of the limbs: magnetic resonance pictorial review of fascial and muscle signal changes Révelon Géraldine, Rahmouni Alain, Jazaerli Nedal, Godeau Bertrand, Chosidow Olivier, Authier Jérôme, Mathieu Didier, Roujeau Jean-Claude, Vasile Norbert. European Journal of Radiology.1999;30(1). CrossRef
- A case of polyarteritis nodosa limited to the right calf muscles, fascia, and skin: a case report Ahmed Saad, Kitchen Joanne, Hamilton Samuel, Brett Francesca, Kane David. Journal of Medical Case Reports.2011;5(1). CrossRef
- Magnetic resonance imaging of skeletal muscle involvement in limb restricted vasculitis Gallien S. Annals of the Rheumatic Diseases.2002;61(12). CrossRef
- Periostitis and Localized Myositis in Polyarteritis Nodosa MacDonald William B. G., Blake Martin P.. Clinical Nuclear Medicine.2004;29(11). CrossRef
- Case report 866 Esteva-Lorenzo F. J., Ferreiro J. L., Tardaguila F., de la Fuente A., Falasca G., Reginato A. J.. Skeletal Radiology.1994;23(7). CrossRef
- A sartorial challenge Reading PJ, Hudgson P, Johnson MA, Jenkins A. The Lancet.1999;354(9183). CrossRef
- Muscle Involvement in Polyarteritis Nodosa: Report of Eight Cases With Characteristic Contrast Enhancement Pattern on MRI Kang Yusuhn, Hong Sung Hwan, Yoo Hye Jin, Choi Ja-Young, Park Jin Kyun, Park Jina, Kang Heung Sik. American Journal of Roentgenology.2016;206(2). CrossRef
- Ectopic Adipose Tissue with Vasculitis in the Calf Muscle Explaining Systemic Symptoms in Leg-limited Cutaneous Polyarteritis Nodosa Aoshima M, Fukuchi K, Tatsuno K, Ito T, Tokura Y. Acta Dermato Venereologica.2016;96(1). CrossRef
- Intriguing Findings of the Muscle on Magnetic Resonance Imaging in Polyarteritis Nodosa Takei Hiroshi, Hanaoka Hironari, Kaneko Yuko, Yamaoka Kunihiro, Sasaki Aya, Takeuchi Tsutomu. Internal Medicine.2016;55(21). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2021
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- Xml downloaded - 0 times