Association of Histopathological Parameters and Axillary Lymphnode Metastasis in Primary Breast Carcinoma
Download
Abstract
Introduction: The most common malignancy worldwide among females is breast carcinoma and the second most common malignancy in India, next to cervical cancer. A wide range of potential prognostic features have been studied in breast cancer and are mainly divided into two groups i.e. Histopathological and Molecular. The histological features are cost-effective and provide reliable diagnostic and prognostic information in these tumors. Axillary Lymph node status is one of the most important prognostic factors and greatly affects the morbidity and mortality of the patient.
Materials and Methods: All breast cancer specimens received in the Department of Pathology over a period of five years. The following histopathological parameters were carefully studied like Tumor size, Histological type, Grade, Presence of necrosis, Inflammatory cell infiltrate, Lymphatic invasion, Blood vessel invasion, Perineural invasion, and other Stromal changes were studied in detail, and association of these histopathological parameters with axillary lymph node metastasis were analyzed.
Results: A total of 100 cases were studied, and most of the patients were over the age of 50. The maximum number of cases was in the T2 stage (55%). Infiltrating ductal carcinoma (88%) was the most common type of tumor encountered in the study. The majority of the cases were Grade I tumors. Skin Invasion was seen in 14% and Lymphovascular Invasion was seen in 17% of cases respectively. There was a statistically significant association between the size of the tumor, T stage, Grade of the tumor, necrosis, and inflammatory infiltrate on further analysis.
Conclusion: There was a statistically significant correlation between Tumor size, pathological T stage, Grade of the tumor, Necrosis and inflammatory infiltrate with axillary Lymph node metastasis in the present study. Increased tumor size, T stage, higher grade, presence of necrosis and low inflammatory infiltrate are associated with increased axillary Lymph node metastasis.
Introduction
The most common malignancy worldwide among females is breast carcinoma and in India, the second most common malignancy next to cervical cancer. In India the disease specific mortality of breast cancer is roughly 50% [1]. The incidence of breast cancer in Kolar region is around 6.41% of all malignancies [2]. The worldwide incidence of breast cancer comprises of 10.4% of all malignancies in female population. Breast cancer, a heterogeneous disease has varied morphological appearance, molecular features and behavior of response to therapy. It is becoming increasingly important to assess the prognosis of breast cancer in each patient before treatment [1,2].
A wide range of potential prognostic features have been studied. They are mainly divided into two groups i.e. Histopathological and Molecular. Histopathological prognostic features are relatively simple to assess and provide clinically useful prognostic information. Tumor size, grade, histopathological type and axillary lymph node status are most important histopathological features [3-5] Lymph node staging is an important diagnostic factor and provides prognostic information and showed to be based on histological examination than clinical and radiological examination [4]. Patients with positive axillary lymph node shows higher mortality (four to eight times) than node negative cases. Not only the disease specific mortality increases in node positive patients but also the risk of distant recurrence [6].
This study has been taken up to study association of different histological parameters with axillary Lymph nodes metastasis, which may help in predicting the prognosis and guide the treatment of breast carcinoma.
Materials and Methods
All breast cancer specimens received in the Department of Pathology from R.L.Jalappa Hospital and Research Center attached to Sri Devaraj Urs Medical College, Tamaka, and Kolar over a period of five years. Specimens were cut at 1cm interval and kept in 10% formalin for overnight fixation, and gross examination was done according to standard protocol.Sufficient number of blocks were taken, ensure adequate sampling and routine paraffin embedding was carried out. Standard thin sections (4-6 microns) were taken and stained with routine Haematoxyline & Eosin stain.
Various histopathological parameters were carefully studied in detail. Assessment of Tumor size was done According to American joint committee on cancer; Histological typing was done using WHO. Grading of breast carcinoma was done using modified Bloom Richardson scoring system. Semi quantitive assessment of grading of necrosis was done according to the study done by Richards CH et al [7]. Inflammatory cell infiltrate was studied using a study done by Klintrup et al [8] we also studied lymphatic invasion, Blood vessel invasion, Perineural invasion, Stromal characteristics.
Inclusion Criteria
All operated breast carcinoma specimens with axillary lymph node clearance.
Exclusion Criteria
Carcinoma of breast in male patients and sarcomas of breast. Patients on or receiving radiotherapy or chemotherapy and recurrent tumors were excluded from the study
Results
100 breast carcinoma cases were studies in the present study, out of which 53% were above 50 years. 90% of cases presented with breast Lump, 4% presented with ulceration and nipple discharge each and 2% presented with Pagets diasease of breast. 14% of patients had Tumor size <2 cms, 55% were between 2 to 5cms and 31% were >5 cms. 11% were in T1 stage, 48% were in T2 stage, 22% were in T3 Stage and19% were in T4 Stage. Histopathological typing was done, 88% of patients had Infiltrating Ductal Carcinoma, 2% had Infiltrating Ductal Carcinoma with Lobular Carcinoma component, 2% had Papillary Carcinoma, 1% had Infiltrating Ductal Carcinoma with Medullary Carcinoma component, 3% had Medullary Carcinoma, 1% had Adenoid Cystic Carcinoma, 2% had Infiltrating Ductal Carcinoma with Mucinous Carcinoma and 1% had Lobular Carcinoma. 46% of breast carcinoma cases were Grade 1, 39% were in Grade 2 and 15% were in Grade 3. 28% of cases had Focal, 17% had moderate and 38% had extensive necrosis. In this study, 6% had No Inflammatory Cell Infiltrate, 37% had Mild/ Patchy Increase, 28% had Prominent Inflammatory Reaction and 29% had Florid “Cup Like” Inflammatory Infiltrate. Lymph node stage distribution, 47% of patients were in N0 stage, 30% were in N1 stage, 14% were in N2 stage and 9% were in N3 stage.
Association of pathological parameters with axillary lymph node metastasis:
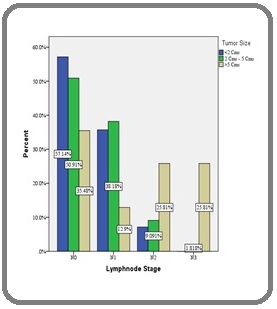
1. Association between Tumor Size and axillary lymph node metastasis: there was significant association between Tumor size and Lymph node stage (χ 2 =25.24, df =9, p = 0.003) (Figure 1).
Figure 1. Association between Tumor Size and Lymph Node Stage in the Study Group.
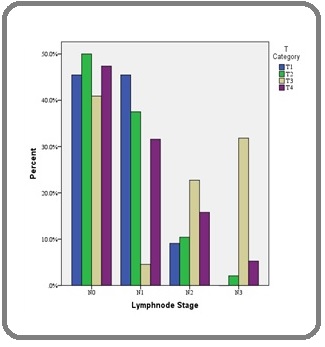
2. Association between Pathological T Stage and axillary Lymph node metastasis: there was significant association between T stage and Lymph node metastasis. (χ 2 =25.24, df =9, p = 0.003* (Figure 2).
Figure 2. Association between Pathological T Stage and Lymph Node Stage.
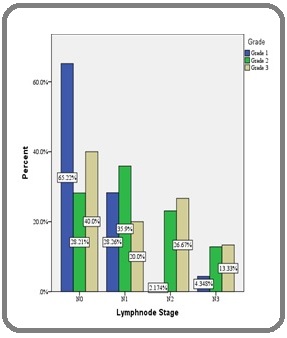
3. Association between Grade and axillary Lymph node metastasis: In the study, there was significant association between Grade and Lymph node metastasis (χ 2 =17.995, df =6, p = 0.006*) (Figure 3).
Figure 3. Association between Tumor Grade and Lymph Node Stage.
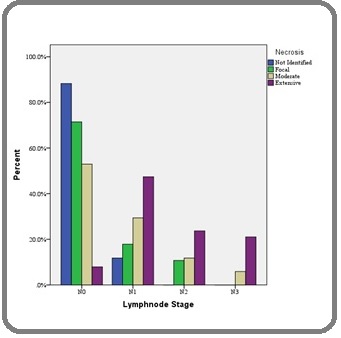
3. Association between Necrosis and axillary Lymph node metastasis: In the study, there was significant association between Necrosis and axillary Lymph node metastasis (χ 2 =44.85, df =9, p <0.001*) (Figure 3).
4. Association between Inflammatory infiltrate and Lymph node Stage: There was significant association between inflammatory infiltrate and axillary Lymph node metastasis (χ 2 =46.299, df =9, p <0.001*) (Figure 4).
Figure 4. Association between Necrosis and Lymph Node Stage in the Study Group.
Discussion
Breast cancer is a leading cause of cancer death among women worldwide. Numerous studies have shown that these malignancies are multifactorial. Life style, genetic and environmental factors play a major role in development of breast carcinomas. In the present study, the age group ranged from 25 years to 92 years with mean age of 51.68 years, which is almost similar to the studies done by EL Saghir et. al. [9] with 49.1% cases less than 50 years and mean age of 49.8 years. Similar observation was made by Najjar et. al. [10] with mean age of 49.4 years which is comparable to the present study. In the present study, tumor size, in majority of the cases were between 2 to 5cms followed by tumor size of more than 5cms. Similar observations were made by Ogawa Y et. al. [11] and Kwon GY et. al. [12] with highest number of cases between tumor size of 2 to 5 cms.
In our study there was significant association between Tumor size and Lymph node stage as shown in the results. As the tumor size increased, positivity of axillary lympnodes also increased. These findings are similar to study done by Marwah, et al, [13] Ahmad, et al, [14] Postaci et al [15] and Sukla et al [16]. The risk of axillary lymph node metastasis increases as tumor size increases which suggests that nodal metastasis is indicative of tumour chronology [17].
In our study, as shown in the results, the maximum numbers of cases were in T2 stage (48%) which was similar to the study done by Wang M et al 70. T2 was followed by T3 stage (22%), T4 stage (19%) and T1 stage (11%) respectively. Pistelli M et al [18] in his study on early stage breast cancers observed that maximum number of cases were in T1 stage (53.6%) followed by T2 stage (30.8%) which was contrary to the observations made in the present study. The reason could be that the stage IV tumors were excluded in the study by Pistelli M et al 19 and the study was done only on early breast cancer patients.
The present study showed that there was strong association between histological grades of tumor with axillary metastasis. Grade II and III tumors had more positive axillary lymph nodes and grade 1 tumors showed low rate of axillary metastasis. Various studies have analyzed the importance of histological grade as a prognostic factor in carcinoma of the breast [13,14, 16,17].
Histopathologically-identified tumor necrosis has been recognized as a potential prognostic marker for a variety of tumors. In the present study, necrosis was seen in 83% of cases in comparable to the study done by Krishnamurthy et. al. [19] whereas Carlomango et. al. [20] observed necrosis in only 20.3% of cases.The observations made in the present study were similar to the study done by Matkowski R et. al. [21]. In our stusy there was a strong association with tumor necrosis and axillary lymph node matastsis. Other Studies have shown that necrosis in the tumor was associated with higher mortality rate, a higher incidence of axillary node metastasis, and a higher mortality rate in patients with axillary node metastasis than were primary malignancy without necrosis.
Several studies outlined the use of the inflammatory infiltrate in breast cancer as a prognostic marker [22, 23]. There was statistically significant correlation between the inflammatory infiltrate and axillary Lymph node metastasis i.e. as the inflammatory infiltrate increases, the chance of axillary Lymph node metastasis decreases. Aaltomaa et al [22] examined 489 breast cancer patients with up to 10 years follow-up. They found lymphocyte infiltrate (LI) positively correlated to axillary lymph node status, tumour diameter and histomorphological variables. Multivariate analysis showed that LI was independently related to axillary lymph node status and was able to predict recurrence free survival as well as breast cancer related survival.
In conclusion, in the present study, there was a statistically significant correlation between Tumor size, pathological T stage, Grade of the tumor, Necrosis and inflammatory infiltrate with axillary Lymph node metastasis. increased tumor size, T stage, higher grade, presence of necrosis and low inflammatory infiltrate are associated with increased axillary Lymph node metastasis. Hence, These histopathological factors can be used as prognostic markers in patients with breast cancer in a resource limited setting.
References
- The Breast. In: Mills SE, Greenson JK, Hornick JL, Longacre TA, Reuter VE editors. Sternberg‟s Diagnostic Surgery Pathology. 6th ed Carter D, Schnitt SJ, Millis RR. Philadelphia: Wolters Kluwer.2015;:317-384.
- Cancer profile in the department of pathology of sri devaraj urs medical college, Kolar: A ten years study Kalyani R, Das S, Singh BindraM.S, Kumar HM.L. Indian Journal of Cancer.2010;47(2). CrossRef
- Tumors of the breast. In: Fletcher CDM. Diagnostic Histopathology of Tumors. 4th ed Ellis IO, Lee AHS, Pinder SE, Rakha AE. Philadelphia: Elsevier.2013;:57-145.
- Predictive and Prognostic Factors in Breast Cancer and their Association with ERPR HER2/neu expression Sinha S, Nath J, Mukherjee A, Chatterjee T. L Carcinog Mutagen.2016;7:1-4.
- Hoda SA, Brogi E, Koerner FC, Rosen PP. Rosen‟s Breast Pathology. 3rd ed. Philadelphia: Wolters Kluwer; 2013.p.235-513. .
- Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update Fisher Bernard, Bauer Madeline, Wickerham D. Lawrence, Redmond Carol K., Fisher Edwin R., Cruz Anatolio B., Foster Roger, Gardner Bernard, Lerner Harvey, Margolese Richard, Poisson Roger, Shibata Henry, Volk Herbert. Cancer.1983;52(9). CrossRef
- The relationships between cellular components of the peritumoural inflammatory response, clinicopathological characteristics and survival in patients with primary operable colorectal cancer Richards C H, Flegg K M, SD Roxburgh C, Going J J, Mohammed Z, Horgan P G, McMillan D C. British Journal of Cancer.2012;106(12). CrossRef
- Inflammation and prognosis in colorectal cancer Klintrup Kai, Mäkinen Johanna M., Kauppila Saila, Väre Päivi O., Melkko Jukka, Tuominen Hannu, Tuppurainen Karoliina, Mäkelä Jyrki, Karttunen Tuomo J., Mäkinen Markus J.. European Journal of Cancer.2005;41(17). CrossRef
- El Saghir NS, Shamseddine AL, Gaera F, Bikhazi K, Rahal B, Salem ZM et.al. Age distribution of breast cancer in Lebanon: increased percentages and age adjusted incidence rates of younger-aged groups at presentation. J Med Liban 2002; 50: 3-9. .
- Age at diagnosis of breast cancer in Arab nations Najjar Hesahm, Easson Alexandra. International Journal of Surgery.2010;8(6). CrossRef
- Erdogan N, Dengizmen A, Akyildizigdem A, Sahan E, Tetikkurt US. Angiogenesis in breast cancers without lymph node metastasis. Turk J Pathol 2010; 26: 136-9. .
- Mast Cell and Macrophage Counts and Microvessel Density in Invasive Breast Carcinoma-Comparison Analysis with Clinicopathological Parameters Kwon Gui Young, Lee Sang Dae, Park Eon Sub. Cancer Research and Treatment.2005;37(2). CrossRef
- Correlation of proliferative index with various clinicopathologic prognostic parameters in primary breast carcinoma: A study from North India Batra Ashima, Marwah Nisha, Marwah Sanjay, Gupta Veena, Shakya Samta, Sen Rajeev. Journal of Cancer Research and Therapeutics.2018;14(3). CrossRef
- Breast carcinoma grading, estimation of tumor size, axillary lymph node status, staging, and nottingham prognostic index scoring on mastectomy specimens Ahmad Zubair, Khurshid Amna, Qureshi Asim, Idress Romana, Asghar Nasira, Kayani Naila. Indian Journal of Pathology and Microbiology.2009;52(4). CrossRef
- Sentinel Lymph Node Biopsy in Breast Cancer: Predictors of Axillary and Non-Sentinel Lymph Node Involvement Postacı Hakan, Zengel Baha, Yararbas Ulkem, Uslu Adam, Eliyatkin Nukhet, Akpinar Goksever, Cengiz Fevzi, Durusoy Raika. Balkan Medical Journal.2013;30(4). CrossRef
- Correlation of axillary lymph nodes involvement and Nottingham prognostic index with various histopathologic prognostic factors in invasive breast carcinoma Shukla A, Jain SC, Swarnkar M. Int Surg J.2019;6:1187-1193.
- Axillary metastasis in breast cancer: When, how, and why? Hartveit Flora. Seminars in Surgical Oncology.1989;5(2). CrossRef
- Prognostic factors in early stage triple negative breast cancer: Lessons and limits from clinical practice Pistelli M, Pagliacci A, Battelli N. Anticancer Res.2013;33:2737-2742.
- Significance of prognostic indicators in infiltrating duct carcinoma breast: Scenario in developing country Krishnamurthy J, Kumar PSandeep. Indian Journal of Cancer.2016;53(1). CrossRef
- Prognostic Significance of Necrosis, Elastosis, Fibrosis and Inflammatory Cell Reaction in Operable Breast Cancer Carlomagno Chiara, Perrone Francesco, Lauria Rossella, De Laurentiis Michelino, Gallo Ciro, Morabito Alessandro, Pettinato Guido, Panico Luigi, Bellelli Teresa, Apicella Anna, Petrella Giuseppe, Bianco Raffaele, De Placido Sabino. Oncology.1995;52(4). CrossRef
- The prognostic role of tumor-infiltrating CD4 and CD8 T lymphocytes in breast cancer Matkowski R, Gisterek I, Halon A, Lacko A, Szewczyk K, Staszek U, et. al . Anticancer Res.2009;29:2445-2452.
- The role of inflammation in progression of breast cancer: Friend or foe? (Review) ALLEN MICHAEL D., JONES LOUISE J.. International Journal of Oncology.2015;47(3). CrossRef
- Lymphocyte infiltrates as a prognostic variable in female breast cancer Aaltomaa S., Lipponen P., Eskelinen M., Kosma V.-M., Marin S., Alhava E., Syrjänen K.. European Journal of Cancer.1992;28(4-5). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2021
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times