Acute Promyelocytic Leukemia: Simplifying Treatment in a Resource Poor Setting
Download
Abstract
Introduction: Acute promyelocytic leukemia (APL) is a distinct leukemia which can be treated with differentiating agents alone.Treatment without chemotherapy decreases the cost of treatment and the need for supportive care. Here we present analysis of APL patients treated with arsenic trioxide (ATO)-all trans retinoic acid (ATRA) without chemotherapy in our hospital.
Patients and Methods: Forty three patients with newly diagnosed APL were treated with arsenic trioxide(ATO ) and All trans retinoic acid (ATRA) during induction treatment. For consolidation ATRA 45 mg /m2 for two weeks every four weeks was administered for twenty eight weeks. ATO was administered for four cycles, with a cycle length of eight weeks. The drug was administered at 0.15 mg/kg/d for five days per week for four weeks during each cycle.Patients were followed up with once in three month hemogram and once in six month reverse-transcriptase polymerase chain reaction (RT-PCR) for two years and yearly thereafter.
Results: The morphologic complete remission (CR) rate was 86.04%.The most common cause of remission failure was early death due to bleeding. None of the low risk patients died during induction therapy. The most important prognostic factor for early mortality was a high white blood cell (WBC) count at presentation. The median overall survival (OS) has not been reached.The two year OS was 83.4% and the three year OS was 74.8%.The estimated five year survival was 74.8%. At a median follow up of 42.6 months the estimated five year survival in the low-intermediate risk group was 93.3% and 59.1% in the high risk group
Conclusion: ATO –ATRA can be considered as a treatment option for frontline treatment of all risk APL patients in resource poor settings.The results can be better with better supportive care to prevent early mortality and by salvaging patients who relapse.
Introduction
Acute promyelocytic leukemia (APL) is a highly curable form of leukemia with distinct clinical and biological features. It is characterised by a balanced reciprocal translocation between chromosome 15 and 17 resulting in differentiation arrest at the stage of promyelocyte [1]. The translocation generates a fusion transcript joining the PML (promyelocyte) and RAR-A (retinoic acid receptor-A) genes [2]. Arsenic trioxide (ATO) is a highly effective antileukaemic agent in APL. All trans retinoic acid (ATRA) and ATO degrade PML-RARA synergistically, resulting in differentiation and eventual eradication of the disease [3].
APL represents the first example of a malignant disease that is highly curable with molecularly targeted therapy against its specific genetic abnormality [4,5]. Guidelines suggest the use of ATO and ATRA combination for low-intermediate risk APL. However the combination is an option for treatment of high risk APL in resource poor countries to keep the costs low and to avoid the adverse effects of chemotherapy. Here we present our data of APL patients treated with ATO and ATRA without chemotherapy.
Materials and Methods
Patients
Between September 2013 and March 2021,486 patients were diagnosed with Acute Myeloid leukemia at our hospital. Amongst these patients we identified 64 (13.1%) patients with APL. The initial diagnosis was based on peripheral smear and bone marrow examination. The diagnosis was confirmed with FISH for t (15:17) translocation and PML-RARA by RT-PCR. Treatment was begun immediately when a diagnosis of APL was suspected, and treatment was continued until the confirmation or exclusion of APL diagnosis by molecular methods. Written informed consent was obtained before treatment initiation from every patient.
Treatment
Induction comprised of ATRA 45 mg/m2/d in two divided doses daily plus ATO 0.15 mg/kg/d as a two hour infusion till remission was achieved. Blood product support was administered to achieve platelet count greater than 50000/mm3 and prothrombin time, activated partial thromboplastin time to as normal as possible and fibrinogen greater than 150 mg/dl. For prophylaxis of differentiation syndrome dexamethasone 10 mg once daily was started when white blood cell (WBC) count crossed 10000/mm3 and continued till the WBC count started to fall. For patients with manifest differentiation syndrome, dexamethasone dose was increased to 10 mg twice daily for 3-5 days and then tapered. Twice weekly electrocardiographic surveillance was combined with 3 times per week electrolyte assessment to minimize the risk of arrhythmias due to arsenic-associated Q-Tc (corrected QT interval) prolongation. ATO was stopped for 2-3 days for patients with Q-Tc greater than 500ms. Potassium, magnesium and calcium levels were maintained in the high normal range all throughout the treatment.
Bone marrow aspiration was done at WBC count recovery to confirm remission.Molecular testing was not done at the end of induction.
For consolidation ATRA 45 mg /m2 for 2 weeks every 4 weeks was administered for 28 weeks. ATO was administered for four cycles, with a cycle length of 8 weeks. The drug was administered at 0.15 mg/kg/d for 5 days per week for 4 weeks during each cycle. Electrolyte and ECG monitoring was not done during consolidation. At the end of four consolidation cycles with ATO, bone marrow examination was done to document continued remission. Molecular analysis with quantitative RT PCR was done on bone marrow sample at the same time to document molecular remission.
Follow up
Patients were followed with complete blood count and peripheral smear once in 3 months for 2 years ,6 monthly thereafter for 5 years and once yearly thereafter. Quantitative RT PCR was done once in 6 months for 2 years on peripheral blood and yearly thereafter for 5 years. RT PCR was not monitored after 5 years of follow up unless indicated.
Statistical Analysis
Overall survival (OS) curves were performed using the Kaplan-Meier method. The OS was measured from diagnosis until last follow-up or death. Patients were risk stratified according to the WBC count into high risk (WBC count >10000/mm3) or low-intermediate risk APL (WBC count <10000/mm3).
Results
The characteristics of all patients are described in the Table 1.
| Charactersitic | Total PTS (64) | Treated PTS (43) |
| Age | ||
| Range | 9-65 Years | 10 – 65 |
| Median | 34 | 33 |
| Sex | ||
| M:F | 27:37:00 | 17:26 |
| Treatment | ||
| YES/NO | 43/21 | |
| Hemoglobin | ||
| Range | 2.9-14.2 gm/dl | 2.9-14.2 gm/dl |
| Median | 7.1 gm/dl | 6.6 gm/dl |
| TLC | ||
| Range | 1200 – 318500 | 1200 – 78800 |
| Median | 17450 | 11400 |
| Platelets | ||
| Range | 4000-400000 | 4000-400000 |
| Median | 15000 | 15000 |
| Risk | ||
| Low/High | 22/42 | 20/23 |
| Induction Days | ||
| Range | 2 – 51 | |
| Median | 31 | |
| Follow Up | ||
| Range | 0.1 – 89.4 months | |
| Median | 42.6 months | |
| Status | ||
| Alive/Expired | 33/10 |
Of the 64 patients, 21 patients did not consent for treatment. The major reason for the lack of consent was financial constraint .One another reason for lack of consent was the relative poor prognosis in high risk group as majority of them had high risk APL.
Of the 43 patients who agreed for treatment 20 were low risk and 23 were high risk. 6 patients died within the first 12 days of treatment. The major cause of early mortality was bleeding .4 patients died of intrapulmonary bleeding and 2 died of intracranial bleeding. All were high risk patients. None of the low –intermediate risk patients died due to bleeding. Only 2 patients developed infections requiring antibiotic treatment and both of them were related to intravenous access site infections.
The median time to complete induction treatment was 33 days (range 28 to 45 days). The highest WBC count reached due to differentiation was 1,78,000/mm3. The lowest WBC count reached due to differentiation was 5800/mm3. Treatment was withheld for 2 days in a single patient due to symptomatic differentiation syndrome. He responded to increased dose of steroids. No patient was given cytoreductive therapy. Treatment with ATO was withheld for 2 days in a single patient due to QT interval prolongation beyond 500ms. However ATRA was continued in the patient. Q-Tc normalised within 2 days with stoppage of ATO and electrolyte correction. All patients who completed induction achieved complete remission.
Four patients died due to relapse during follow up. Three of them were high risk patients and 1 was low risk patient. None of the patients consented for salvage treatment. All the relapses occurred within 36 months. There were no relapses in either groups after 36 months. One patient developed squamous cell carcinoma of external ear at 87 months of follow up. He underwent surgery for the same and has been planned for adjuvant chemoradiation.
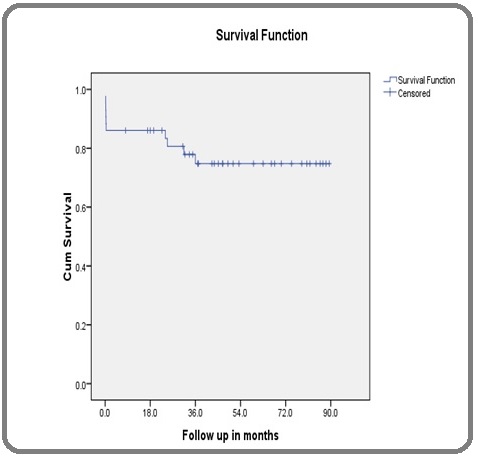
The median OS has not been reached. The 2 year OS was 83.4% and the 3 year OS was 74.8%. The estimated 5 year survival was 74.8% (Figure 1).
Figure 1. Overall survival.
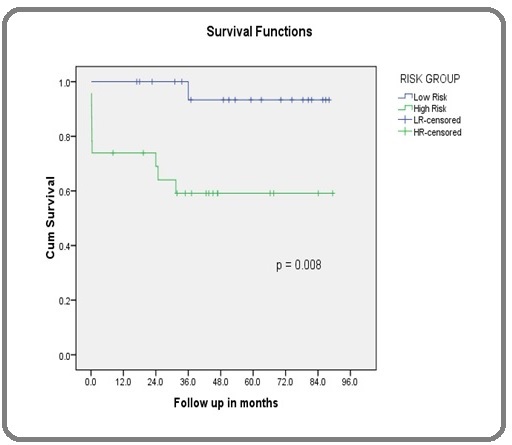
There was a statistically significant mean OS difference between the low-intermediate and high risk groups (84.6 months vs 56.8 months p = 0.008). At a median follow up of 42.6 months the estimated 5 year survival in the low- intermediate risk group was 93.3% and 59.1% in the high risk group (Figure 2).
Figure 2. Overall Survival Comparison between Low and High Risk APL.
We further tried to subgroup high risk patients into 2 groups – patients with WBC count <25000 and those with WBC count >25000 to see if there is a survival difference. Though there was an estimated survival difference of 27 months (68 months vs 41 months), it was not statistically significant (p = 0.15).
Discussion
Chemotherapy has a lot of disadvantages viz myelosuppression, resulting infections and long-term complications like cardiotoxicity and secondary myeloid neoplasms [6]. Additionally, the health resource utilization and treatment costs are also significantly higher with chemotherapy approaches in APL [7]. Hence avoiding chemotherapy has a lot of advantages in treatment of APL. In addition ATO –ATRA combination can be an outpatient treatment later in induction after the coagulopathy improves and in consolidation treatment. ATO –ATRA treatment is lot cheaper compared to chemotherapy approaches.
In the APL 0406 trial in non-high risk APL at a median follow up of 40.6 months , the event-free survival, incidence of relapse, and overall survival at 50 months for patients in the ATRA-ATO versus ATRA- chemotherapy arms were 97.3% v 80%, 1.9% v 13.9%, and 99.2% v 92.6%, respectively (P < .001, P = .0013, and P = .0073, respectively) [8]. The updated results from study at a median follow up of 66.4 months, state that the 6-year event-free survival was significantly better with ATO + ATRA as compared to ATRA + chemotherapy (96.6% versus 77.4%, p 0.0002). The cumulative incidence of relapse was significantly lower with ATO + ATRA as compared to ATRA + chemotherapy (1.7% versus 15.5%, p 0.02) [9]. In our study the estimated OS at 5 years was 74.8%. The estimated OS for the low –intermediate risk was 93.3% and for high risk it was 59.1%.Our results in low –intermediate risk APL are comparable to the above study whereas the results in high risk are poor. Our relapse rate 5% (1 out of 20 patients) is lesser than the above study for non-high risk APL. Our relapse rate in high risk APL is 17.6% (3 out of 17 high risk patients who completed treatment which is comparable to the above study.
Another trial, conducted by the National Cancer Research Institute (NCRI) cooperative group, compared ATRA plus chemotherapy with ATRA plus ATO in patients with APL regardless of WBC count [10]. The results confirmed significantly higher event-free survival and lower cumulative incidence of relapse rates in patients receiving ATO plus ATRA whereas overall survival was not statistically different in the 2 arms [11]. The lack of difference in overall survival rates between the two arms can be explained by immediate initiation of salvage treatment with ATO at molecular relapse in the chemotherapy arm.There was significantly less requirement for supportive care in the non-chemotherapy arm.A similar observation has been noted in our study with no infection related mortality and very few infections.
The results of a single institution study [12] suggested that ATRA plus ATO results in sustained responses in patients with WBC counts ≤10 × 109/L. The trial included all risk patients. High risk patients received idarubicin or a single dose of Gemtuzumab ozogamicin in addition to ATO –ATRA. The induction mortality was 4%,5 year OS was 89% for low risk and 86% for high risk. In the AML 4 study all risk patients were treated with idarubicin, ATO and ATRA followed by maintenance ATRA, methotrexate and 6 mercaptopurine. The 5 year OS was 96% in the low –intermediate risk group and 87% for the high risk group [13].
Our results are comparable to the above 2 studies in the low risk group. The reason for poor survival in high risk group can be high early mortality and no salvage treatment at relapse in our patients.
In a SWOG high risk APL study, ATO –ATRA was given with GO in induction therapy. The consolidation consisted of ATO-ATRA, daunorubicin and GO. This was followed by maintenance ATRA, methotrexate and 6 mercaptopurine. The induction mortality was 11% and 3 year OS was 86% [14]. Our results are poor due to high early mortality (26% in high risk, 6/23 patients) as majority of them presented with bleeding.
In Conclusion, ATO –ATRA can be considered as a treatment option for frontline treatment of all risk APL patients in resource poor settings. The results can be better with better supportive care to prevent early mortality and by salvaging patients who relapse.
References
- 15/17 TRANSLOCATION, A CONSISTENT CHROMOSOMAL CHANGE IN ACUTE PROMYELOCYTIC LEUKAEMIA Rowley JanetD., Golomb HarveyM., Dougherty Charlotte. The Lancet.1977;309(8010). CrossRef
- The acute promyelocytic leukemia-specific PML-RARα fusion protein inhibits differentiation and promotes survival of myeloid precursor cells Grignani Francesco, Ferrucci Pier Francesco, Testa Ugo, Talamo Giampaolo, Fagioli Marta, Alcalay Myriam, Mencarelli Amedea, Grignani Fausto, Peschle Cesare, Nicoletti Ildo, Pelicci Pier Giuseppe. Cell.1993;74(3). CrossRef
- Eradication of acute promyelocytic leukemia-initiating cells through PML-RARA degradation Nasr Rihab, Guillemin Marie-Claude, Ferhi Omar, Soilihi Hassan, Peres Laurent, Berthier Caroline, Rousselot Philippe, Robledo-Sarmiento Macarena, Lallemand-Breitenbach Valérie, Gourmel Bernard, Vitoux Dominique, Pandolfi Pier Paolo, Rochette-Egly Cécile, Zhu Jun, de Thé Hugues. Nature Medicine.2008;14(12). CrossRef
- Acute promyelocytic leukemia: from highly fatal to highly curable Wang Zhen-Yi, Chen Zhu. Blood.2008;111(5). CrossRef
- Alltrans-retinoic acid as a differentiating agent in thetreatment of acute promyelocytic leukemia Degos L, Dombret H, Chomienne C, et al . Blood.1995;85(10):2643-2653.
- Time to abandon traditional chemotherapy for acute promyelocytic leukaemia? Ravandi Farhad, Kantarjian Hagop. The Lancet Oncology.2015;16(13). CrossRef
- Resource utilization and cost effectiveness of treating acute promyelocytic leukaemia using generic arsenic trioxide Bankar Aniket, Korula Anu, Kulkarni Uday P., Devasia Anup J., NA Fouzia, Lionel Sharon, Abraham Aby, Balasubramanian Poonkuzhali, Janet Nancy Beryl, Nair Sukesh C., S Sezlian, Jeyaseelan Visali, N Jeyaseelan, Prasad Jasmine, George Biju, Mathews Vikram. British Journal of Haematology.2019;189(2). CrossRef
- Improved Outcomes With Retinoic Acid and Arsenic Trioxide Compared With Retinoic Acid and Chemotherapy in Non–High-Risk Acute Promyelocytic Leukemia: Final Results of the Randomized Italian-German APL0406 Trial Platzbecker Uwe, Avvisati Giuseppe, Cicconi Laura, Thiede Christian, Paoloni Francesca, Vignetti Marco, Ferrara Felicetto, Divona Mariadomenica, Albano Francesco, Efficace Fabio, Fazi Paola, Sborgia Marco, Di Bona Eros, Breccia Massimo, Borlenghi Erika, Cairoli Roberto, Rambaldi Alessandro, Melillo Lorella, La Nasa Giorgio, Fiedler Walter, Brossart Peter, Hertenstein Bernd, Salih Helmut R., Wattad Mohammed, Lübbert Michael, Brandts Christian H., Hänel Mathias, Röllig Christoph, Schmitz Norbert, Link Hartmut, Frairia Chiara, Pogliani Enrico Maria, Fozza Claudio, D’Arco Alfonso Maria, Di Renzo Nicola, Cortelezzi Agostino, Fabbiano Francesco, Döhner Konstanze, Ganser Arnold, Döhner Hartmut, Amadori Sergio, Mandelli Franco, Ehninger Gerhard, Schlenk Richard F., Lo-Coco Francesco. Journal of Clinical Oncology.2017;35(6). CrossRef
- Long-term results of all-trans retinoic acid and arsenic trioxide in non-high-risk acute promyelocytic leukemia: update of the APL0406 Italian-German randomized trial Cicconi Laura, Platzbecker Uwe, Avvisati Giuseppe, Paoloni Francesca, Thiede Christian, Vignetti Marco, Fazi Paola, Ferrara Felicetto, Divona Mariadomenica, Albano Francesco, Efficace Fabio, Sborgia Marco, Di Bona Eros, Breccia Massimo, Borlenghi Erika, Cairoli Roberto, Rambaldi Alessandro, Melillo Lorella, La Nasa Giorgio, Fiedler Walter, Brossart Peter, Hertenstein Bernd, Salih Helmut R., Annibali Ombretta, Wattad Mohammed, Lubbert Michael, Brandts Christian H., Hanel Mathias, Rollig Christoph, Schmitz Norbert, Link Hartmut, Frairia Chiara, Fozza Claudio, Maria D’Arco Alfonso, Di Renzo Nicola, Cortelezzi Agostino, Fabbiano Francesco, Dohner Konstanze, Ganser Arnold, Dohner Hartmut, Amadori Sergio, Mandelli Franco, Voso Maria Teresa, Ehninger Gerhard, Schlenk Richard F., Lo-Coco Francesco. Leukemia.2019;34(3). CrossRef
- Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial Burnett Alan K, Russell Nigel H, Hills Robert K, Bowen David, Kell Jonathan, Knapper Steve, Morgan Yvonne G, Lok Jennie, Grech Angela, Jones Gail, Khwaja Asim, Friis Lone, McMullin Mary Frances, Hunter Ann, Clark Richard E, Grimwade David. The Lancet Oncology.2015;16(13). CrossRef
- Attenuated arsenic trioxide plus ATRA therapy for newly diagnosed and relapsed APL: long-term follow-up of the AML17 trial Russell Nigel, Burnett Alan, Hills Robert, Betteridge Sophie, Dennis Mike, Jovanovic Jelena, Dillon Richard, Grimwade David. Blood.2018;132(13). CrossRef
- Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab Abaza Yasmin, Kantarjian Hagop, Garcia-Manero Guillermo, Estey Elihu, Borthakur Gautam, Jabbour Elias, Faderl Stefan, O’Brien Susan, Wierda William, Pierce Sherry, Brandt Mark, McCue Deborah, Luthra Rajyalakshmi, Patel Keyur, Kornblau Steven, Kadia Tapan, Daver Naval, DiNardo Courtney, Jain Nitin, Verstovsek Srdan, Ferrajoli Alessandra, Andreeff Michael, Konopleva Marina, Estrov Zeev, Foudray Maria, McCue David, Cortes Jorge, Ravandi Farhad. Blood.2017;129(10). CrossRef
- Use of arsenic trioxide in remission induction and consolidation therapy for acute promyelocytic leukaemia in the Australasian Leukaemia and Lymphoma Group (ALLG) APML4 study: a non-randomised phase 2 trial Iland Harry J, Collins Marnie, Bradstock Ken, Supple Shane G, Catalano Alberto, Hertzberg Mark, Browett Peter, Grigg Andrew, Firkin Frank, Campbell Lynda J, Hugman Amanda, Reynolds John, Di Iulio Juliana, Tiley Campbell, Taylor Kerry, Filshie Robin, Seldon Michael, Taper John, Szer Jeff, Moore John, Bashford John, Seymour John F. The Lancet Haematology.2015;2(9). CrossRef
- A phase 2 study of ATRA, arsenic trioxide, and gemtuzumab ozogamicin in patients with high-risk APL (SWOG 0535) Lancet Jeffrey E., Moseley Anna B., Coutre Steven E., DeAngelo Daniel J., Othus Megan, Tallman Martin S., Litzow Mark R., Komrokji Rami S., Erba Harry P., Appelbaum Frederick R.. Blood Advances.2020;4(8). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2021
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times