Demographic Pattern, Tumor Size and Stage of Breast Cancer in Africa: A Meta-analysis
Download
Abstract
Purpose: Understanding the epidemiology of breast cancer (BC) in Africa, as well as regional variation is essential for planning future intervention. Our objective was to describe summary estimates of socio-demographic and clinical characteristics of BC in Africa, thus providing researchers and policymakers baseline data for planning diagnostic and treatment programs to improve BC outcomes in the future.
Method: We screened African publications on BC between 2010 and 2019 in PubMed, AJOL, Google, ScienceDirect, and ResearchGate to estimate the distribution of socio-demographic and clinical tumor characteristics. The meta-analysis used the random effect model.
Result: Eighty articles were eligible, including 33,199 total patients. Overall, 58% of patients were <50 years old. In East Africa, 38% (95% CI 31-45) were diagnosed before 40 years. Conversely, in Southern Africa, 37% were diagnosed after 60 years, with Caucasian-like age distribution. The overall prevalence of male BC was high (3%), with East Africa having the highest prevalence (5% (95% CI 5.0-6.0)). Only 2% (95% CI 1-2) of patients were diagnosed with carcinoma-in-situ. Invasive tumors were 7% stage I, 26% stage II, 50% stage III, and 17% stage IV. Seventy per-cent (95% CI 60-80) had clinical nodal involvement. The smallest tumors were in North Africa. The largest and most advanced tumors were in West Africa. Trend analysis showed decreasing age, an increasing population of unmarried BC patients, a relatively high proportion of uneducated BC patients, and a stable proportion of late-stage disease in the last decade.
Conclusion: Regional variation in the presentation of BC throughout Africa necessitates region/country-specific targets for improving BC control.
Introduction
According to 2018 global cancer statistics [1], breast cancer (BC) is one of the two most common adult cancers, accounting for nearly 25% of cancers in women worldwide. Africa has disproportionately high age-standardized mortality due to BC [2]. The World Health Organization (WHO) and other experts in the field [3] recommend early diagnosis combined with timely and effective treatment as cost-effective measures for improving BC outcomes in Africa. Understanding the epidemiology of BC in Africa, as well as regional variation, is essential for planning future interventions.
Prior researches have aggregated data to better understand BC in Africa, but there is a notable gap in the existing literature. Previous meta-analyses described BC incidence, stage at presentation [4], and biological characteristics in sub-Saharan Africa (SSA). However, none of the existing reviews adequately summarize patient demographics, clinical pattern, or regional variation of BC. These factors are of significant prognostic value and are critical determinants of resource allocation that can allow for tailored screening, early detection, diagnostic and treatment programs to be adapted for specific local or regional contexts.
This meta-analysis aims to describe summary estimates of patient demographics, tumor size, and BC stage in Africa. A secondary aim is to compare the clinical pattern between African regions and countries. Our ultimate goal is to provide researchers and policymakers a baseline and region-specific targets for planning future interventions.
Method
This research aligned with the Preferred Reported Items for Systematic Reviews and Meta-analysis (PRISMA) recommendations [5]. The needs assessment and preliminary literature review [in PubMed, African Journal Online (AJOL), Cochrane library, and Prospero reference ID CRD42020153269] confirmed no similar meta-analysis was ongoing or previously conducted. The full literature search was performed in PubMed.gov between November 10, 2019, and December 31, 2019, using an iterative process with the search term “breast cancer AND country name” for each African country and only as “breast cancer” in AJOL. Hand-search was done on Google, Google Scholar, ScienceDirect, PubMed central, ResearchGate, and Academia. Snow-balling search was in the reference list of original articles and already published review articles. We sent an exclusive request email to authors for full articles not available online or to clarify data.
Article screening and data extraction
Full-text screening used predetermined Population, Intervention, Control, Outcome, Time, Study design (PICOTS) criteria (Table 1).
| Participants/Population | We included freely available publications of studies conducted in Africa and reporting on the total female breast cancer patients or both sexes or a representative sample. We excluded articles reporting on breast cancer patients’ subpopulations, such as early presentation alone, young women, older women, or treatment subgroups. |
| Intervention | Not applicable |
| Control | Not applicable |
| Outcomes | The outcomes were: patient demographics (including age, sex, marital status, educational status, and menopausal status), and locoregional characteristic (including the primary tumor size, lymph node status, combined tumor staging, proportion of invasive and in-situ tumors, and tumor laterality). |
| The sex distribution was extracted in studies where the proportion of both sexes were reported. Age distribution was extracted in the range <40, 40-49, 50-59, and ≥60 years and in the binary distribution ≤30 years/ >30 years, and <50 years /≥50 years. Marital status was extracted into three categories: married, unmarried (separated, divorced, or widowed), and single (never married). Education was extracted into three categories: none/primary, secondary, and tertiary. Tumor laterality was extracted from articles that reported both unilateral and bilateral disease. | |
| The proportion of invasive disease and carcinoma in-situ were extracted using articles that reported the two. The primary tumor size was based on the American Joint Committee on Cancer (AJCC) classification for the articles reporting in T1-4 fashion, and staging was based on articles where all four stages could be distinctly identified. | |
| Nodal status was extracted as the presence or absence of nodal metastasis using the clinical or pathologic description according to the American Joint Committee on Cancer (AJCC) clinical staging criteria version 6 or 7. | |
| Time | Articles published between January 2010 and December 2019. Articles including data earlier than January 2000 were excluded. |
| Study design | Study design was not a strict exclusion criterion because demographic characteristics are expected to be fundamental elements in the reporting of any study. Language was also not an exclusion criterion. We included any original article with a sample size of at least 30 subjects providing at least one data point or observation according to the outcomes list above. We excluded review articles. Original articles involving more than one country were included if the observation (s) could be extracted separately for each country. |
Author AO performed the article title and abstract screening while AO and AI performed the full article review independently. The same authors also conducted data extraction. The authors discussed to resolve any disagreement.
Quality assessment
Five quality assessment variables were designed using domains in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist [6]. The quality assessment classified the report into one of 5 levels, A-E, depending on the quality score, with A being the highest score of five and E being a score of one or zero. The quality score was not used in meta-analytical weighing.
Statistical analysis
The primary outcome was the summary estimate of each outcome variable defined in our PICOTS criteria. The meta-analytical procedure was conducted in MetaXL (www.epigear.com) add-in for Microsoft Excel. A random-effect model was implemented to obtain summary estimates using the double arcsine transformation to avoid overweighting studies with values close to 0 or 100%. I-squared [I2] values above 75% indicated high heterogeneity. Subgroup analysis was conducted based on the United Nations regional classification of African nations as Central Africa (CA), East Africa (EA), Northern Africa (NA), West Africa (WA), and Southern Africa (SNA). By-country analysis was also conducted to compare variables and explain the potential source of heterogeneity. We analyzed summary estimates of all variables where there were at least two observations for the continent, the region, or the country. The variables were analyzed as proportions of the total in each publication (n/N).
Tumor characteristics according to the clinical or pathologic AJCC, were analyzed separately. Results were presented in percentages with 95% confidence intervals (95% CI). The parent forest plots for all analyses are available in the supplementary file. Funding: No funding source.
Results
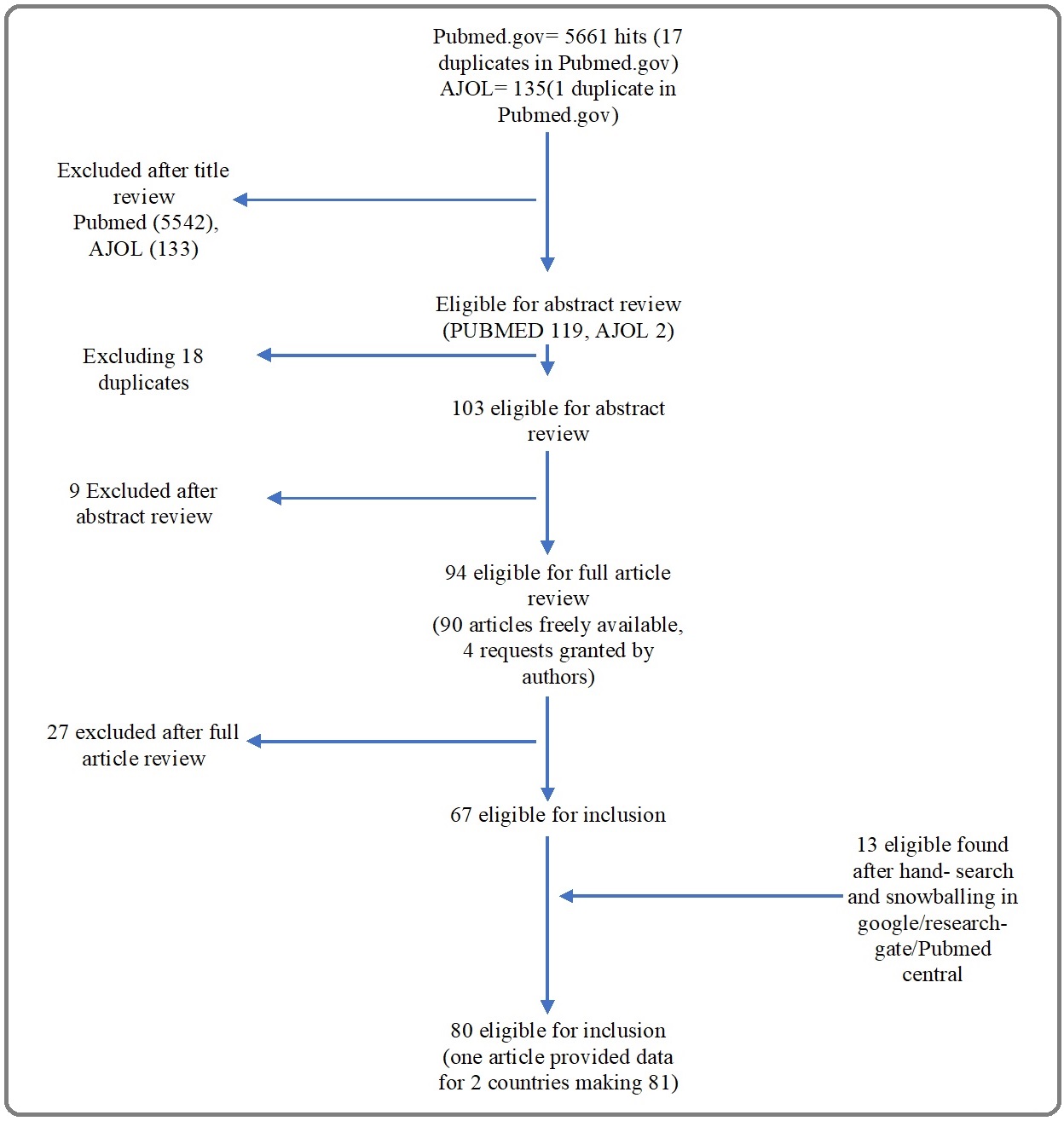
Full electronic search returned 5661 articles; 80 articles were eligible after the article selection process (Figure 1, Table 2).
Figure 1. Article Screening Flow Chart.
| Author | Year | Country | United Nations Region | Race | Period | Location of hospital | Design | N | Study level age statistic. range/mean/ median |
| Adejumo, et al. [22] | 2019 | Nigeria | WA | NS | 2015-2018 | FMC Keffi | pros | 199 | NS/NS/NS |
| Adebamiji, et al. [23] | 2016 | Nigeria | WA | NS | 2003-2007 | University of Ilorin Teaching Hospital Oke Oyi | retro | 203 | 21-99/49.2/NS |
| Agbo, et al. [24] | 2014 | Nigeria | WA | NS | 2007-2011 | Usman Dan Fodio Teaching Hospital Sokoto | retro | 816 | NS/48.2/NS |
| Agodirin, et al. [25] | 2018 | Nigeria | WA | NS | 2016-2018 | Multicenter | survey | 100 | 26-80/50.5/NS |
| Akanbi, et al. [26] | 2015 | Nigeria | WA | NS | 2012-2014 | NS | cross- | 120 | NS |
| Akinkuolie, et al. [27] | 2016 | Nigeria | WA | NS | 2007-2013 | Wesley Guild Hospital Ilesha | cross | 46 | 25-81/NS/NS |
| Anyanwu, et al. [28] | 2011 | Nigeria | WA | NS | 2004-2008 | Nnamdi Azikwe University Teaching Hospital Nnewi | pros | 275 | 18-80/45.2/NS |
| Ayoade, et al. [29] | 2015 | Nigeria | WA | NS | 2011-2014 | Olabisi Onabanjo University Teaching Hospital Sagamu | survey | 113 | NS/47.8/NS |
| Balekouzou, et al. [12] | 2016 | Central African Republic | CA | NS | 2003-2015 | Tungji Med College & Bangui University | retro | 174 | 16-90/ 45.83/ NS |
| Balekouzou, et al. [11] | 2018 | Central African Republic | CA | NS | 2003-2015 | National Lab in Bangui and General and Gynecologic Service | retro | 174 | 16-90/NS/45.5 |
| Bambara, et al. [30] | 2017 | Burkina faso | WA | NS | 2015-2016 | Yaldago Ouedraogo Teaching Hospital | cross | 80 | 28-80/48.2/NS |
| Bennis, et al. [31] | 2012 | Morocco | NA | NS | 2007-2010 | Hazzan University Hospital Fez | retro | 366 | 18-82/45/NS |
| Boder, et al. [32] | 2010 | Libya | NA | NS | 2002-2006 | African Oncology Institute | database | 234 | NS/46/NS |
| Brinton, et al. [33] | 2017 | Ghana | WA | NS | NS | Korle Bu Teaching Hospital & Komfo Anoyle Kumasi & Peace and Love Kumasi | case | 1184 | 18-74/NS/NS |
| Burson, et al. [21] | 2010 | Tanzania | EA | NS | 2007-2009 | Ocean Road Cancer Institute dar es Salam | retro | 488 | NS/43.4/NS |
| Cubasch, et al. [34] | 2018 | South Africa | SNA | blk (542) nblk (55) | 2009-2011 | CHBAH breast Clinic | database | 602 | |
| Dagne, et al. [35] | 2019 | Ethiopia | EA | NS | 2011-2012 | Tikur Ambessa Specialized Hospital | retro | 303 | N/42.1/NS |
| Dauda, et al. [36] | 2011 | Nigeria | WA | NS | 2000-2007 | FMC Gombe | retro | 172 | 21-80/43.9/NS |
| Dedey, et al. [37] | 2016 | Ghana | WA | NS | 2013 | National Center for Radiotherapy and Nuclear Medicine Korle Bu Teaching Hospital | survey | 265 | NS/51.1/NS |
| Deressa, et al. [16] | 2019 | Ethiopia | EA | NS | 2016-2017 | University of Gondor Hospital Cancer Center | cross | 82 | 25-82/NS/45 |
| Dickens, et al. [8] | 2014 | South Africa | SNA | blk (964) nblk (107) | 2006-2016 | CHBAH breast Clinic | retro | 1071 | NS/55.4/NS |
| Effi, et al. [38] | 2017 | Ivory Coast | WA | NS | 2013-2015 | Central Laboratory Abidjan | pros | 302 | 24-84/48/NS |
| Engbang, et al. [14] | 2015 | Cameroon | CA | NS | 2004-2013 | Multicenter | retro | 3044 | 13-95/46/NS |
| Eniojukan, et al. [39] | 2015 | Nigeria | WA | NS | 2008-2012 | University College Hospital Ibadan | retro | 583 | NS/44.9/NS |
| Ermiah, et al. [40] | 2012 | Libya | NA | NS | 2008-2009 | National Oncology Institute Sabratha | survey | 200 | 22-75/45.4/NS |
| Errahhali, et al. [41] | 2017 | Morocco | NA | NS | 2005-2012 | Hassan II regional oncology center | retro | 2406 | NS/48.7/NS |
| Fatiregun, et al. [42] | 2016 | Nigeria | WA | NS | NS | Lagos State University Teaching Hospital Ikeja | survey | 200 | NS/49.6/NS |
| Fessahaye, et al. [43] | 2017 | Eritrea | EA | NS | 2013-2014 | National Health Laboratory Ministry of Health Amara | retro | 144 | 19-91/51.5/NS |
| Fitzpatrick, et al. [44] | 2018 | Senegal | WA | NS | 2001-2016 | Dantec Hospital | retro | 197 | NS/47/NS |
| Gabremariam, et al. [45] | 2019 | Ethiopia | EA | NS | 2017-2018 | Multicenter | cross | 441 | NS/44.4/NS |
| Galukande, et al. [46] | 2014 | Uganda | EA | NS | 2008-2011 | Mulago Hospital Kampala | retro | 201 | 22-87/46.5/45 |
| Galukande, et al. [47] | 2015 | Uganda | EA | NS | 2004-2012 | National Institute Oncology Sabratha | 200 | 22-75/NS/NS | |
| Gross-frie, et al. [48] | 2018 | Mali | WA | NS | 2016 | University Hospital Bamako | survey | 64 | NS/45/NS |
| Hussein, et al. [49] | 2013 | Egypt | NA | NS | 2006-2011 | Mansoura University Oncology center | retro | 263 | NS/52/NS |
| Jedy-Agba, et al. [4] | 2017 | Nigeria | WA | NS | 2014-2016 | Multicenter | pros | 316 | 24-86/45.4/NS |
| Joffe, et al. [50] | 2018 | South Africa | SNA | NS | 2015-2016 | Chris Hani Baragwanath Academic Hospital Hospital Soweto | survey | 499 | NS |
| Kene, et al. [51] | 2010 | Nigeria | WA | NS | 2001-2005 | ABUTH Zaria | retro | 103 | NS/44.5/NS |
| Khaial, et al. [52] | 2015 | Libya | NA | NS | 2007-2008 | Al-Jamhouria Hospital | pros | 301 | NS/49/NS |
| Kholer, et al.[53] | 2015 | Malawi | EA | NS | 2011-2013 | Kamuzu Central Hospital Lilongwe | retro | 198 | 12-89/NS/34 |
| Kone, et al. [54] | 2019 | Mali | WA | NS | 2014-2016 | Bamako Radiotherapy center | retro | 134 | 18-88/47.1/N |
| Lopes, et al. [55] | 2015 | Angola | CA | NS | 2006-2014 | Angola Institute of Cancer Control Luanda | retro | 1843 | 16-87/NS/47 |
| Mabula, et al. [56] | 2012 | Tanzania | EA | NS | 2002-2011 | Bugando Medical Centre, Mwanza | retro | 384 | 21-78/NS/NS |
| Medhin, et al. [57] | Eritrea | EA | |||||||
| Mensah, et al. [58] | 2016 | Ghana | WA | NS | 2002-2008 | Korle Bu Teaching Hospital & National center for radiology and Nuclear medicine | pros | 1022 | 20-92/47.9/NS |
| Miguel, et al. [59] | 2017 | Angola | CA | NS | 2011-2014 | Angola Institute of Cancer Control Luanda & clinica Sagrade esperanca | pros | 140 | 24-84/47/NS |
| Mokone- Fatunla, et al. [15] | 2019 | South Africa | SNA | blk (1461) others 25 | 2000-2016 | Dr George Mukhari Academic Hospital | retro | 1482 | 21-96/54.9/NS |
| Moodley, et al. [60] | 2018 | South Africa | SNA | NS | 2015-2016 | Breast Clinic Western Province | cross | 201 | NS/NS/54 |
| Mousa, et al. [61] | 2011 | Egypt | NA | NS | 2009-2010 | Tanta Cancer Center Gharbiah Province | survey | 163 | NS |
| Muchuweti, et al. [19] | 2017 | Zimbabwe | EA | NS | 2010-2013 | Parirenyatwa Group of Hospital Harare | pros | 73 | NS |
| Murugan, et al. [9] | 2014 | South Africa | SNA | blk (964) | 2006-2012 | CHBAH Soweto | database | 1071 | N/55/N |
| Mechita, et al. [62] | 2016 | Morocco | NA | NS | 2005-2008 | National Institute of Oncology Rabat | database | 626 | NS/51.1/NS |
| Nasiru, et al. | 2011 | Nigeria | WA | NS | 2006-2009 | LASUTH Ikeja | pros | 350 | 23- 104/48.9/55.4 |
| Nguefack, et al.[13] | 2012 | Cameroon | CA | NS | 2006-2009 | Duala General Hosptial | pros | 42 | 29-73/46/NS |
| Nwafor, et al. [63] | 2012 | Nigeria | WA | NS | 2009-2013 | MeCure Health Limited Lagos | retro | 48 | 29-78/49.5/NS |
| O neil, et al. [64] | 2017 | Rwanda | EA | NS | 2012-2013 | Butaro Cancer Center of Excellence | retros | 150 | 26-84/48.3/N |
| Oguntunde, et al. [20] | 2016 | Nigeria | WA | NS | 2011-2016 | University of Ilorin Teaching Hospital Oke Oyi | database | 300 | 20-96/49.7/NS |
| Ohene-yeboah, et al. [65] | 2012 | Ghana | WA | NS | 2004-2009 | Komfo Anokye Teaching Hospital Kumasi | pros | 330 | N/49.1/N |
| Okoye, et al. [66] | 2016 | Nigeria | WA | NS | 2012-2016 | Multicentered | retro | 334 | 23-95/50.3/N |
| Omoniyi-esan, et al. [67] | 2015 | Nigeria | WA | NS | 2007-2012 | OAUTHC Ile-Ife | retro | 136 | 23-92/50.7/NS |
| Otieno, et al. [68] | 2010 | Kenya | EA | NS | 2003-2006 | Kenyatta National Hospital | Pros | 166 | 17-88/47/NS |
| Otieno, et al. [69] | 2010 | Kenya | EA | NS | 2000-2004 | Kenyatta National Hospital | retro | 389 | 17-99/44/NS |
| Pace, et al. [70] | 2015 | Rwanda | EA | NS | 2012-2014 | Butaro & Rwnkwavu Hosptial | survey | 144 | NS/NS/49 |
| Popoola, et al. [71] | 2013 | Nigeria | WA | NS | NS | Lagos State University Teaching Hospital Ikeja | Pros | 190 | NS/32/NS |
| Popoola, et al. [18] | 2012 | Nigeria | WA | NS | |||||
| Quayson, et al. [72] | 2014 | Ghana | WA | NS | 2000-2004 | Korle Bu Teaching Hospital | retro | 821 | 14-98/48/NS |
| Rahman, et al. [73] | 2014 | Nigeria | WA | NS | 2003-2008 | University of Ilorin Teaching Hospital Oke Oyi [68] | retro | 82 | 29-75/48.9/NS |
| Rambau, et al. [74] | 2014 | Tanzania | EA | NS | NS | Bugando Medical Centre, Mwanza | retro | 52 | NS/49/NS |
| Rayne, et al. [75] | 2017 | South Africa | SNA | blk (85) nblk (170) | 2011-2013 | Johannesburg | survey | 263 | 18-86/NS/52 |
| Salih, et al. [76] | 2016 | Sudan | NA | NS | 2014-2018 | Bashaier University Hospital & Khartoum Center for Radiation and Isotopes | pros | 63 | 22-91/46.8/NS |
| Sayed, et al. [77] | 2018 | Kenya | EA | NS | 2012-2015 | Multicenter | survey | 846 | NS/48/NS |
| Sengal, et al. [10] | 2017 | Sudan | NA | NS | 2010-2015 | retro | 560 | 20-94/48.8/NS | |
| Sengal, et al. [7] | 2017 | Eritrea | EA | NS | 2011-2015 | University of Gezira | retro | 562 | NS/NS/NS |
| Sengal, et al. [7] | 2017 | Sudan | NA | NS | 2011-2015 | Orotta School of Medicine and Dentistry Amaru | retro | 116 | NS/NS/NS |
| Ssemanda, et al. [78] | 2018 | Uganda | EA | NS | 2005-2014 | MaKCHS Lab Kampala | retro | 599 | NS/NS/NS |
| Stapleton, et al. [79] | 2011 | Egypt | NA | NS | 2007-2008 | National Cancer Institute of Cairo University & Tanta Cancer Center Nile Delta | cross | 343 | NS/NS/NS |
| Tazzite, et al. [80] | 2013 | Morocco | NA | NS | 2009 | Oncology Centre, Ibn Rochd University Hospital Casablanca | retro | 570 | NS/47.07/NS |
| Tesfamariam, et al. [81] | 2013 | Eritrea | EA | NS | 2007-2008 | Multicenter | retro | 82 | 26-80/48.4/NS |
| Titiloye, et al. [82] | 2013 | Nigeria | WA | 2004-2006 | retro | 89 | |||
| Traore, et al. [83] | 2015 | Guinea | WA | NS | 2007-2012 | Donka Chu Conakry | retro | 278 | 20-85 |
| Usman, et al.[84] | 2019 | Nigeria | WA | NS | 2011-2015 | Amino Kano Teaching Hospital Kano | retro | 478 | 20-80/46.9/N |
| Wondima -gagnehu, et al. [85] | 2019 | Ethiopia | EA | NS | 2017-2018 | Multicentered | cross | 428 | NS/40/NS |
Result of article request; Four authors contacted for full-text responded [42, 86-88] yielding one eligible article. One [54] of 20 authors [7, 12, 31, 32, 36, 40, 49, 54, 60-62, 64, 74, 75, 77, 89-93] contacted for data on age distribution responded. Three authors [30, 39, 61] could not be reached to clarify data on age distribution, two could not be reached for data on educational status [39, 94], and one could not be reached for data on time to presentation [94]. Five authors contacted for data on stage distribution did not respond [48, 58, 60, 64, 70], and one could not be reached [95]. Three authors could not be reached to clarify data on tumor stage [30, 39, 61]. Two authors contacted for data on tumor size did not respond. One [37] of four authors contacted for data on marital status [37, 40, 70, 75] responded with usable data and one could not be reached [31, 74, 95]. Regional representation and summary of design; Twenty-four countries were represented from all five regions of Africa; CA-6, EA-19, NA-13, SNA-7, and WA-35. Three publications were in french [14, 62, 83]; all others were in English. Abbreviations, CA- Central Africa; EA- East Africa; WA-West Africa; NA- North Africa; WA-West Africa; SNA- Southern Africa; udy, retro- retrospective study’ survey- questionnaire-based survey
Each article contributed data for one country except Sengal et al. [7], which provided data for Sudan and Eritrea in one article. Two articles from South Africa [8, 9], Sudan [7, 10], and Central African Republic [11, 12] shared the same population of subjects but provided different data points (Table 2). Attempts to clarify incomplete or obscured data via email communications with authors yielded variable results as detailed in Table 2 footnote.
Twenty-three countries from all five regions of Africa contributed articles. WA contributed the largest number of articles (35 articles), followed by EA-19, NA-13, SNA-7, and CA 6. Nigeria contributed 23 articles, the largest from a single country (Figure 2).
Figure 2. Map of Africa Showing the Distribution of Study Subjects from each Country. The deeper blue shading represents a higher contribution, and lighter blue shading represents a lower contribution. The gray shadings represent no contribution.
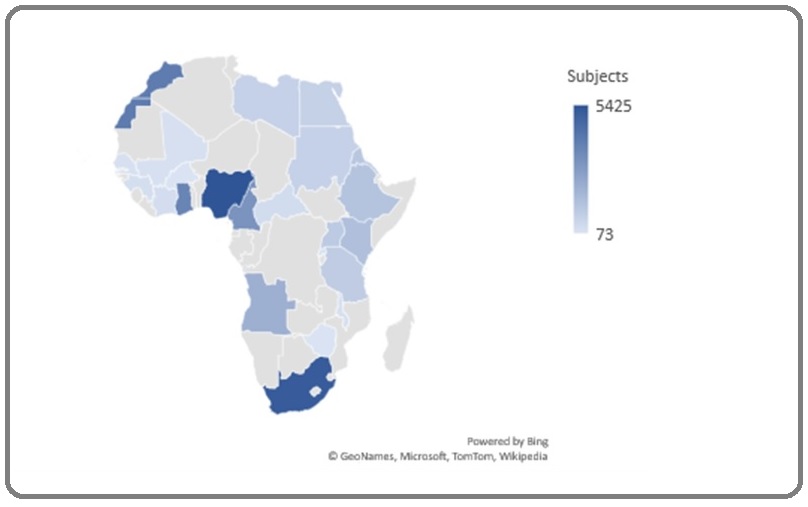
There were 33,199 subjects in total. The minimum number of subjects in a study was 42 [13], and the maximum was 3044 [14]. The maximum number of subjects from one country was 5425, contributed by Nigeria (Figure 2). The majority of studies (n=39) were in the B quality assessment category; the study rationale was well stated in 84%, the design was adequate in 85%, and the participants were adequately stated in all studies. The study outcomes were adequately described in 77%, but the ease of data extraction was present in only 36% (Supplementary File). There was marked heterogeneity (>75%) in the overall summary estimates of the continent- wide analysis. The heterogeneity was significantly reduced or eliminated in most by-region and by-country analyses (Supplementary File).
Sex distribution
Twenty-five articles, including 11,476 subjects, contributed to the analysis of sex distribution. Ninety-seven per-cent (95% CI 97-98, I2 77%) of patients were female and 3% (95% CI 2.0-4.0%, I2 77) were male. EA had prevalence of male BC at 5% (95% CI 2.0-476%), more than double the prevalence in WA (2% (95% CI 2.0-2.0, I2-80%). Regional analysis was not feasible for NA, CA and SNA. By-country analysis showed a similar distribution of 2-3% in Nigeria, Tanzania, Eritrea, and Ghana. Single studies reported 2% male prevalence in Cameroon [14] and South Africa [15]. A single study from Ethiopia recorded male BC prevalence of 18% [16]. Subgroup analysis showed a rising trend in male BC incidence in the last ten years compared to the decade before. (Supplementary file).
Age distribution
Thirty-three articles (14,545 subjects) contributed to age distribution analysis. Overall, more than half of patients (58%) were diagnosed before the age of 50. Twenty-eight per-cent of patients (95% CI 24-31) were diagnosed before the age of 40, and 6.0% (95% CI 5.0-8.0, I2=90%) were diagnosed at 30 years or younger. The youngest patients were in EA, where 8% (95%CI 6.0-11, I2=82%) were diagnosed under the age of 30, 38% (95% CI 31-45, I2=85%) were diagnosed under the age of 40, and 64% were diagnosed before the age of 50. Conversely, in SNA, over 60% were diagnosed at the age of 50 or above, and 37% (95% CI 35-39, I2=0%) were diagnosed at the age of 60 or above. The age distribution analysis was possible for NA only in the 50-year cutoff, showing that the majority were also younger than 50 years (58%, (95% CI 44-72, I2=94%). (Figure 3, Supplementary file)
Figure 3. Age of Breast Cancer Patients in Africa.
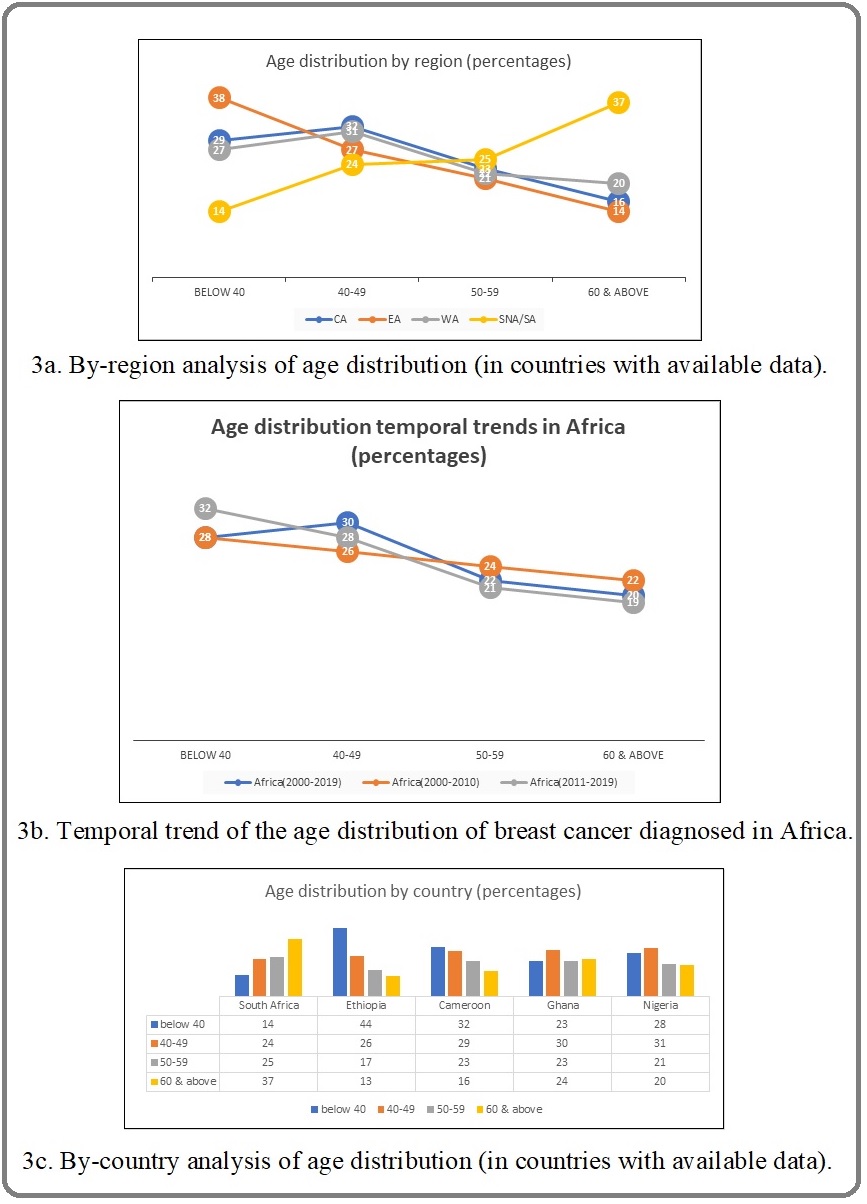
By-country analysis showed Ethiopia had the youngest patients with 10% (95% CI 8.0-13, I2=63%) being younger than 30 years and 73% (95% CI 61-80, I2=87%) younger than 50 years (Figure 3 and Supplementary File). Temporal analysis revealed declining age of BC patients over time. Sixty per-cent of patients from 2010-2019 were less than 50 years of age, compared to 55% in 2000-2010 period. Eight per-cent of patients were less than 30 years of age from 2010-2019 compared to 4% in the 2000-2010 period (Figure 2, Supplementary file). Overall, 57% (95% CI 54-61, I2 87%) of patients were premenopausal. This was similar throughout regions with available data (56-60%). By-country analysis showed that Ghana had the highest prevalence of premenopausal patients (64% (95 % CI 42-83, I2=92%)) (Supplementary file).
Educational and marital status
Eleven publications (3747 subjects) contributed to the marital status analysis. Overall, 61% were married and 39% were unmarried, including 20% single. One study from CA [12] reported the highest proportion (74%) of unmarried singles; sensitivity analysis excluding this study saw the proportion of singles overall drop to 15%. The regional distribution of single patients was 11% EA, 14% WA, and 24% SNA. The largest unmarried population was in South Africa (57%) while the smallest was in Nigeria (27%). The temporal trend showed a slight increase in unmarried women diagnosed with BC in the last decade (41%) compared to the period between 2000-2010 (35%) (Table 3). Twelve articles (3,103 subjects) contributed to the educational status analysis. Thirty-eight per-cent of patients had none or primary education, 36% completed secondary education, and 26% completed tertiary education overall. The proportion of patients with secondary or tertiary level education was highest in SNA (75%) than EA (62%) and WA (62%). The proportion of patients with secondary or tertiary level education in the last decade (64%) was slightly higher than the overall analysis (62%) (Table 3). Subgroup analysis for 2000-2010 was not feasible. However, a single study from Nigeria in the 2000-2010 period recorded 52% secondary and tertiary education [17], another study from Nigeria with data between 2010 and 2012 recorded 66%, [18], and a separate study in Uganda including data between 2010 and 2013 [19] recorded 62%. (Supplementary file).
Laterality
Eight articles (2,947 subjects) contributed to the analysis of BC laterality in the continent. Most patients (97%) had unilateral BC, compared to 3% (95% CI 2.0-6.0 I2=80%) who had bilateral BC. The highest prevalence of bilateral BC was in Nigeria, 8% (95% CI 6.0-12 ) [20] and Tanzania (6%, 95%CI 4-8) [21]. A slightly higher proportion (51% (95% CI 46-55) were left-sided tumors (Table 3).
| Sex | Female % (95 % CI) | Male % (95 % CI) | I-squared (%) | |
| Africa Overall | 97 (96-98) | 3 (2-4) | 77 | |
| By-region | ||||
| EA | 96 (93-97) | 5 (3-7) | 76 | |
| WA | 98 (96-99) | 2 (1-4) | 80 | |
| By-country | ||||
| Eritrea | 96 (93-96) | 4 (2-7) | 23 | |
| Ghana | 98 (97-99) | 2 (1-3) | 23 | |
| Nigeria | 97 (96-99) | 3 (1-4) | 85 | |
| Tanzania | 97 (95-98) | 3 (2-5) | 16 | |
| Educational Status | None/Primary % (95 % CI) | Secondary % (95 % CI) | Tertiary % (95 % CI) | |
| Africa Overall | 38 (29-47) | 36 (28-41) | 26 (19-34) | 95 |
| Africa (2011-2019) | 35 (25-46) | 39 (27-49) | 26 (17-36) | 90 |
| By-region | ||||
| EA | 36 (19-56) | 38 (18-56) | 26 (9-43) | 57 |
| SNA | 25 (21-29) | 48 (43-53) | 27 (23-31) | 0 |
| WA | 38 (27-49) | 33 (22-44) | 29 (19-40) | 52 |
| Marital Status | Married % (9 5% CI) | Unmarried % (95 % CI) | Single% (95 % CI) | |
| Africa Overall | 62 (50-69) | 19 (11-26) | 20 (12-27) | 97 |
| Africa (2011-2019) | 59 (39-72) | 19 (7-33) | 22 (9-36) | 98 |
| Africa (2000-2010) | 65 (47-77) | 20 (8-33) | 16 (6-28) | 96 |
| By-region | ||||
| EA | 62 (49-72) | 27 (17-37) | 11 (5-19) | 91 |
| SNA | 43 (39-47) | 33 (30-37) | 24 (21-27) | 0 |
| WA | 71 (64-78) | 15 (9-20) | 14 (9-20) | 90 |
| By-country | ||||
| Ghana | 67 (47-82) | 17 (5.0- 32) | 16 (4-32) | 96 |
| Nigeria | 73 (59-84) | 15 (6-26) | 12 (4.0-22) | 91 |
| Laterality | Right % (95 % CI) | Left % (95 % CI) | Bilateral% (95 % CI) | |
| Africa Overall | 46 (41-51) | 51 (40-55) | 3 (2-6) | 80 |
| By-region | ||||
| WA | 44 (32-56) | 53 (40-64) | 3 (0-9) | 84 |
| EA | 52 (43-61) | 45 (35-53) | 3 (0-9) | 85 |
Stage distribution
Fifteen articles (9,185) contributed to carcinoma-in- situ analysis. Prevalence of carcinoma in-situ was generally low in all regions (CA- 4%, EA-2%, and NA-1%, SNA, and WA-1%). The highest proportion of carcinoma-in-situ in individual publications was 6% reported in Central African Republic and 5% in Malawi.
Overall, 98% of BCs were invasive based on analysis of 30 articles (10,352 subjects). Advanced BC (AJCC stage III or IV) accounted for 67%. Overall, 7% (95% CI 4.0-9.0, I2= 98%) of patients were diagnosed stage I disease, ranging from 2-10% in each region. Twenty-six per-cent of disease was stage II, ranging from 21-35% in each region, 50% of disease was stage III, ranging from 39-74% in each region, and 17% of disease was stage IV, ranging from 3-21% in each region. The earliest tumors were in NA; 74%, and 81% were Stage II or III in SNA and NA, respectively, while 70% or above were Stage III or IV in other regions (Figure 4 and Supplementary File).
Figure 4. AAA.
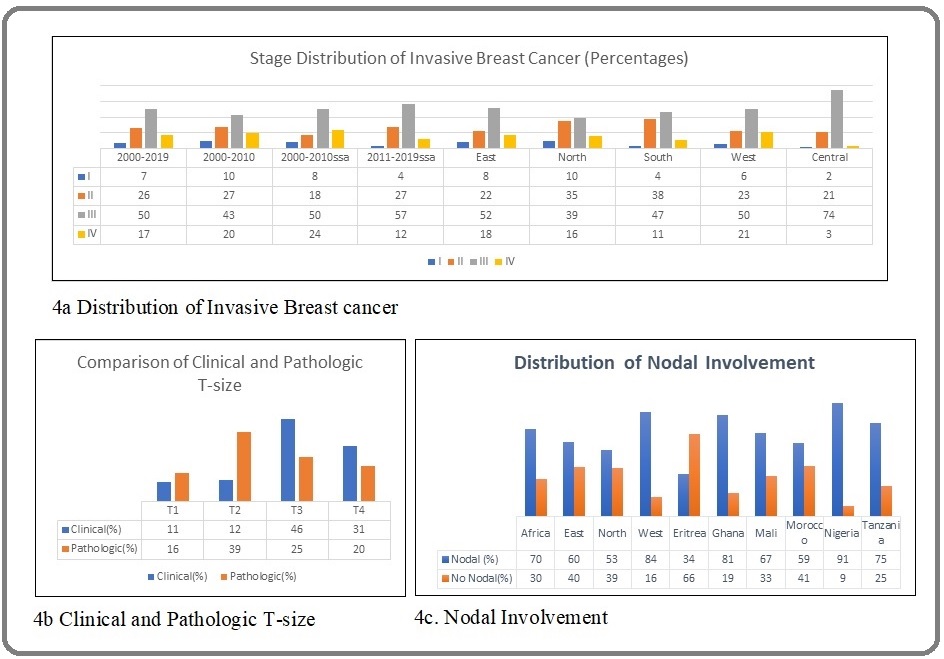
Trend analysis showed a decreasing prevalence of stage I (from 8% to 4%) and stage IV (from 24% to 12%) disease, with an increasing prevalence of stage II and III disease in the last decade (Figure 4).
Two articles (577 subjects) contributed to the clinical T-stage analysis, and seven articles (2151 subjects) contributed to the pathologic T-stage analysis. The majority of tumors were clinical T3 or T4, whereas the majority were pathologic T2 or T3. The prevalence of pathologic or clinical nodal positivity was 70% (99% CI 60-80), based on the analysis of 21 articles (8,357 subjects). WA had the highest prevalence of nodal disease (84%, 95% CI 71-94, I2=99%). By-country analysis showed that Nigeria (91%, 95% CI 75-100, I2=98) had the highest prevalence of lymph node positivity, and Eritrea (34%, 95% CI 28-41, I2=21) had the lowest.
Treatment modalities
Post hoc analysis of the treatment modalities in the continent showed that 72% of BC patients overall underwent surgery. Overall mastectomy prevalence was 71% (95% CI 51-88, I2=99%) while prevalence of breast-conserving surgery was 1.0% (95% CI 0-2, I2=0%). Eighty-three per-cent (64-96 I2=98%) of patients received chemotherapy, 18% (95% CI 4-33, I2 =94%) received radiotherapy, and 77% (95% CI 42-100 I2= 99) received hormonal therapy. Three studies reported routine hormonal therapy in all patients (see Supplementary File). There was no data on targeted-therapy.
Discussion
The African continent has a total population of approximately 1.34 billion inhabitants, accounting for 17% of the world’s population. The United Nations recognizes five African regions comprising 64 territories/ countries: EA (22 countries) 0.45 billion, WA (17 countries) 0.40 billion, NA (11 countries) 0.25 billion, CA (9 countries) 0.18 billion and SNA (5 countries) 0.07 billion. Together WA and EA account for more than 60% of Africa’s population, and South Africa (SA) alone accounts for 88% of the population of SNA.
We aggregated data from 80 articles published within the last decade, from 23 countries representing Africa’s regions. Our findings corroborated previous evidence that African BC patients are younger than those from Europe and the US. In this study, 6% of patients were <30 years of age compared to 0.43% in the UK, 28% were <40 years compared to 6.6% in the US [96, 97], and 58% were <50 years compared to 20% in Europe [98].
The age distribution of BC in South Africa showed a reverse pattern, mirroring the Caucasian age distribution seen in Europe. One explanation might be the proportion of Caucasian inhabitants in SA. Nonetheless, in four of the seven studies included from SA where the race was reported, 90% were Black patients (Table 1). However, it was not reported whether these patients might have been mixed-race Black patients. Even then, previous report suggests that black BC patients in SA are older than other races with BC in SA [99], though this may be partially- attributable to under-reporting.
The declining age of BC found in this study in the setting of the increasing age of Africa’s population overall [100] contradicts the view that the earlier age of BC onset can be entirely attributed to the younger population in Africa. Additionally, the elevated proportion of male breast cancer, 3% overall and 4% in the last decade, compared to approximately 1% reported globally [101, 102], and previously reported increased rates of triple negative disease raise questions regarding potential genetic predisposition and merit further investigation.
The early age of BC onset in Africa brings numerous challenges regarding screening, early diagnosis, and treatment compliance [103]. Young women may be less likely to complete the diagnostic process or treatment for BC due to social reasons, such as fertility issues and socio- cultural isolation. A report in Nigeria found that 31% of young women outrightly declined the diagnostic biopsy procedure, 60% of those who did not decline failed to return for the result of the biopsy, and only 45% of those offered mastectomy accepted treatment [103]. Future intervention should be directed toward improving early diagnosis and compliance with treatment in this patient population.
Even in high-income countries where screening is ubiquitous, it is recommended to begin after 40 years (or 50 according to some guidelines). A third of BC patients in Africa were <40 and would be missed by applying the same screening age guidelines as in the US. While population-based mammographic screening programs are not feasible in most African countries due to resource constraints, education of the general population, paired with clinical breast examination (CBE) has the ability to downstage clinically apparent disease, and age range recommendations should be based on available data.
Thirty-eight per-cent of breast cancer patients in this analysis had none or primary education, and education level varied widely by region. This underscores the importance of tailoring breast cancer education and breast health awareness for both patients and the general population to the local context, taking into account educational and cultural background.
Although unmarried BC patients’ population appears to be increasing in Africa, perhaps due to delaying marriage for education, the current predominantly married BC patient population still provides opportunities to include men as potential intervention targets. Reports suggest men are willing to support women in BC control [104, 105].
In this analysis, nearly 90% of tumors were greater than 2cm, half were greater than 5cm, and one-quarter had skin or chest wall involvement. This preponderance of clinically-detectable disease means that the vast majority of patients have the potential for earlier detection by CBE. Marked regional variations in tumor size and stage across regions, might be explained in part by differences in health systems. Coordinating and centralizing local resources provided affordable, comprehensive health financing, and helped to downstage BC in NA [106]. Increasing awareness and reducing the distance and bottlenecks between BC patients and specialists aided downstaging in SA [9]. Countries in SSA could benefit from the experience in NA and SA to attain the goals of downstaging invasive BC. The smaller pathologic T staging compared to the clinical T staging in our analysis might be linked to the widespread use of neoadjuvant chemotherapy or errors of clinical or pathologic measurement and requires further investigation.
The decreasing rate of stage IV disease over time (24% to 12% in the first decade vs. the second decade of this analysis) shows promise for a slow, but positive trend toward earlier diagnosis, which is one of the most important factors in improving outcomes. The generally low prevalence of carcinoma-in-situ, 2-4% in all regions, can be explained by the common lack of population- based screening. Population-based screening should not be considered until a health system has the resources and ability to effectively diagnose and treat clinically apparent disease. Nonetheless, it is important to note that the rise in the prevalence of small tumors (<1cm ) and carcinoma-in- situ (from 2% to >20% ) in developed countries was linked to screening [107]. In light of the emerging evidence supporting the use of ultrasound in detecting early BC in young women [96, 108, 109], when a health system is ready to consider targeted screening, ultrasound may be considered along with CBE.
The heterogeneity, narrow spread, and paucity of articles capturing some of the variables analyzed limit our findings. Notably, only SA contributed to the findings for SNA. Similarly, in a previous review [110], only SA contributed to the meta-analytical review of the stage at presentation in SNA because the literature on BC is scarce from other SNA countries. Also, the reporting of demographic variables was influenced by the region as articles from the same region reported similar data points in similar formats. The countries of WA and EA reported more information on patient demographics than in the other regions. Availability of data limited our ability to draw conclusions regarding certain critical variables of interest, such as immunohistochemistry or treatment modality by stage.
In conclusion, Africa has a common goal of downsizing and downstaging BC, which is achievable through the early diagnosis of clinically detectable disease. There is marked regional variation in the clinical pattern and patient demographics of BC in Africa, and the interventions developed should be tailored to the local context in each area, while allowing for countries and regions to benefit from shared knowledge and experiences.
Acknowledgements
Declaration Funding
The authors received no funding for this research
Conflict of interest/Competing interests
The authors declare no conflict of interest.
Availability of data and Materials:
All data used in this article are freely available
Authors Contribution
Agodirin Contributed to all aspect of the research, all authors contributed to approval. Aremu contributed to conception, data acquisition, extraction , and review. Rahman contributed to conception, data interpretation, drafting, review. Olatoke contributed to conception, data interpretation and review. Olaogun contributed to draft, review and data interpretation, Akande contributed to data interpretation, drafting and review. Romanoff contributed to data interpretation, drafting and review.
References
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray Freddie, Ferlay Jacques, Soerjomataram Isabelle, Siegel Rebecca L., Torre Lindsey A., Jemal Ahmedin. CA: A Cancer Journal for Clinicians.2018;68(6). CrossRef
- Rising global burden of breast cancer: the case of sub-Saharan Africa (with emphasis on Nigeria) and implications for regional development: a review Azubuike Samuel O., Muirhead Colin, Hayes Louise, McNally Richard. World Journal of Surgical Oncology.2018;16(1). CrossRef
- Downstaging Breast Cancer in sub-Saharan Africa: A realistic target? dos Santos Silva I, McCormack V, Jedy-Agba E, Adebamowo C. Cancer Control.2017;:46-52.
- Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis Jedy-Agba Elima, McCormack Valerie, Adebamowo Clement, dos-Santos-Silva Isabel. The Lancet Global Health.2016(b);4(12). CrossRef
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement Moher David, Liberati Alessandro, Tetzlaff Jennifer, Altman Douglas G.. PLoS Medicine.2009;6(7). CrossRef
- The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies von Elm Erik, Altman Douglas G., Egger Matthias, Pocock Stuart J., Gøtzsche Peter C., Vandenbroucke Jan P.. International Journal of Surgery.2014;12(12). CrossRef
- Immunohistochemistry defined subtypes of breast cancer in 678 Sudanese and Eritrean women; hospitals based case series Sengal Asmerom Tesfamariam, Haj-Mukhtar Nada Suliman, Elhaj Ahmed Mohammed, Bedri Shahinaz, Kantelhardt Eva Johanna, Mohamedani Ahmed A.. BMC Cancer.2017;17(1). CrossRef
- Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: A South African public hospital case series of over 1,000 women Dickens Caroline, Joffe Maureen, Jacobson Judith, Venter Francois, Schüz Joachim, Cubasch Herbert, McCormack Valerie. International Journal of Cancer.2014;135(9). CrossRef
- Down-staging of breast cancer in the pre-screening era: Experiences from Chris Hani Baragwaneth Academic Hospital, Soweto, South Africa Murugan N, Dickens C, Pisa P, McCormack V, Joffe M, Jacobson J, Cubasch H. South African Medical Journal.2014;104(5). CrossRef
- Comparison of Receptor-Defined Breast Cancer Subtypes Between German and Sudanese Women: A Facility-Based Cohort Study Sengal Asmerom Tesfamariam, Haj Mukhtar Nada Suliman, Vetter Martina, Elhaj Ahmed Mohammed, Bedri Shahinaz, Hauptmann Steffen, Thomssen Christoph, Mohamedani Ahmed Abdalla, Wickenhauser Claudia, Kantelhardt Eva Johanna. Journal of Global Oncology.2018;(4). CrossRef
- Histo-epidemiological profile of breast cancers among women in the Central African Republic: about 174 cases Balekouzou Augustin, Yin Ping, Bekolo Cavin Epi, Pamatika Christian Maucler, Djeintote Marceline, Nambei Sylvain Wilfrid, Ba-Mpoutou Bertrand, Mandjiza Dieubeni Rawago, Shu Chang, Yin Minghui, Qing Tingting, Koffi Boniface. BMC Cancer.2018;18(1). CrossRef
- Epidemiology of breast cancer: retrospective study in the Central African Republic Balekouzou Augustin, Yin Ping, Pamatika Christian Maucler, Bishwajit Ghose, Nambei Sylvain Wilfrid, Djeintote Marceline, Ouansaba Barbara Esther, Shu Chang, Yin Minghui, Fu Zhen, Qing Tingting, Yan Mingming, Chen Yuanli, Li Hongyu, Xu Zhongyu, Koffi Boniface. BMC Public Health.2016;16(1). CrossRef
- Epidemiology and surgical management of breast cancer in gynecological department of Douala General Hospital Nguefack CT, Biwole ME, Massom A, Kamgaing JT, Njamen TN, Ekane GH, et al . The Pan African medical journal.2012;13:35.
- Breast cancer in Cameroon, histo-epidemiological profile: about 3044 cases Engbang JP, Essome H, Koh V M, Simo G, Essam J D, Mouelle A S, et al . Pan Afr Med J.2015;21:242.
- Laterality of breast cancer at Dr George Mukhari Academic Hospital Mokone-Fatunla DH, Koto MZ, Becker JHR, Bondo M, Mundawarara S. S Afr J Surg.2019;57(3):56.
- Breast cancer care in northern Ethiopia – cross-sectional analysis Deressa Biniyam Tefera, Cihoric Nikola, Badra Eugenia Vlaskou, Tsikkinis Alexandros, Rauch Daniel. BMC Cancer.2019;19(1). CrossRef
- Pattern of Spread of Breast Cancer among Patients attending Cancer Unit of Lagos State University Teaching Hospital2012(b) Popoola A, Ibrahim N, Omodele F, Oludara M, Adebowale S, Igwilo AI, editors . .
- Pattern of spread of breast cancer among patients attending cancer units of Lagos state university Teaching Hospital Asian Journal of Medical Science Popoola A, Ibrahim A, Omodele F, Oludara MA, Adebowale A, Igwilo A. 2012 (a);4(3):89-94.
- Factors Contributing to Delayed Breast Cancer Presentation: A Prospective Study at Parirenyatwa Group of Hospitals, Harare, Zimbabwe 2010-2013 Muchuweti D, Nyandoro G, Muguti E, Muchaziwepi T. Journal of Cancer and Tumor International.2017;5(1). CrossRef
- Breast cancer patients in Nigeria: Data exploration approach Oguntunde Pelumi E., Adejumo Adebowale O., Okagbue Hilary I.. Data in Brief.2017;15. CrossRef
- Clinical and Epidemiologic Profile of Breast Cancer in Tanzania Burson Ashley M., Soliman Amr S., Ngoma Twalib A., Mwaiselage Julius, Ogweyo P., Eissa Mohab S., Dey Subhojit, Merajver Sofia D.. Breast Disease.2010;31(1). CrossRef
- Epidemiology and challenges of managing breast cancer in Keffi, North-Central Nigeria: A preliminary report Adejumo AdeyinkaA, Ajamu OlusolaJ, Akanbi OlusolaO, Onwukwe JohnC, Adeosun OluseyiA, Omoregie PaulO, Amos Aaron, Garba Yakubu, Koroye OyintobraF, Garba StephenE. Nigerian Medical Journal.2019;60(4). CrossRef
- Survivorship Patterns of Histopathological Varaints and Molecular subtypes of breast cancer in Teaching Hospital In Nigeria East African medical journal Adeniji A, Dezheng R, Rahman G, Akande T, Olatoke S, Akande H, et al . 2016;:41-47.
- Clinical Presentation, Prevalence and Managementn of breast cancer in Sokoto, Nigeria Agbo SP, Khalid A, Oboirien M. J Women's Health Care.;3:149.
- Delay between Breast Cancer Detection and Arrival at Specialist Clinic Preliminary Revelations of Multicentered Survey in Nigeria A. TEXILA INTERNATIONAL JOURNAL OF PUBLIC HEALTH.2017;5(4). CrossRef
- Delay presentation of breast cancer: a study among south western Nigerian women Akanbi O, Oguntola S, Adeoti M, Aderounmu A, Idris O, Abayomi O. International Journal of Current Research.2015;7(8):1-5.
- Breast cancer patients’ presentation for oncological treatment: a single centre study Akinkuolie Akinbolaji Andrew, Etonyeaku Amarachukwu Chiduziem, Olasehinde Olalekan, Arowolo Olukayode Adeolu, Babalola Rereloluwa Nicodemus. Pan African Medical Journal.2016;24. CrossRef
- Acceptance and adherence to treatment among breast cancer patients in Eastern Nigeria Anyanwu Stanley N.C., Egwuonwu Ochonma A., Ihekwoaba Eric C.. The Breast.2011;20. CrossRef
- Beliefs and practices associated with late presentation in patients with breast cancer; an observational study of patient presenting in a tertiary care facility in Southwest Nigeria Ayoade B. A., Salami B. A., Agboola A. J., Tade A. O., Adekoya A. O., Olatunji A. A., Nwokoro C. C.. Journal Africain du Cancer / African Journal of Cancer.2015;7(4). CrossRef
- Breast cancer: descriptive profile of 80 women attending breast cancer care in the Department of General and Digestive Surgery of CHU-YO Bambara Hierrhum Aboubacar, Zouré Abdou Azaque, Sawadogo Alexis Yobi, Ouattara Abdoul Karim, Marie Nabonswindé Lamoussa, Traoré Si Simon, Bakri Youssef, Simpore Jacques. Pan African Medical Journal.2017;28. CrossRef
- Prevalence of molecular subtypes and prognosis of invasive breast cancer in north-east of Morocco: retrospective study Bennis Sanae, Abbass Fouad, Akasbi Yousra, Znati Kaoutar, Joutei Khalid Amrani, El Mesbahi Omar, Amarti Afaf. BMC Research Notes.2012;5(1). CrossRef
- Breast cancer patients in Libya: Comparison with European and central African patients Boder Jme, Elmabrouk Abdalla Fb, Elfageih Ma, Abusaa A, Buhmeida A, Collan Y. Oncology Letters.2011;2(2). CrossRef
- Factors contributing to delays in diagnosis of breast cancers in Ghana, West Africa Brinton Louise, Figueroa Jonine, Adjei Ernest, Ansong Daniel, Biritwum Richard, Edusei Lawrence, Nyarko Kofi M., Wiafe Seth, Yarney Joel, Addai Beatrice Wiafe, Awuah Baffour, Clegg-Lamptey Joe Nat. Breast Cancer Research and Treatment.2016 (b);162(1). CrossRef
- Breast cancer survival in Soweto, Johannesburg, South Africa: A receptor-defined cohort of women diagnosed from 2009 to 11 Cubasch Herbert, Dickens Caroline, Joffe Maureen, Duarte Raquel, Murugan Nivashni, Tsai Chih Ming, Moodley Kiashanee, Sharma Vinay, Ayeni Oluwatosin, Jacobson Judith S., Neugut Alfred I, McCormack Valerie, Ruff Paul. Cancer Epidemiology.2018;52. CrossRef
- Assessment of breast cancer treatment outcome at Tikur Anbessa Specialized Hospital Adult Oncology Unit, Addis Ababa, Ethiopia Dagne Selamawit, Abate Sefinew Migbaru, Tigeneh Wondemagegnhu, Engidawork Ephrem. European Journal of Oncology Pharmacy.2019;2(2). CrossRef
- Histopathological types of breast cancer in Gombe, North Eastern Nigeria: A seven Year review Dauda A, Misauno M, Ojo E. African Journal of Reproductive Health.2011;15(1):107-109.
- Factors Associated With Waiting Time for Breast Cancer Treatment in a Teaching Hospital in Ghana Dedey Florence, Wu Lily, Ayettey Hannah, Sanuade Olutobi A., Akingbola Titilola S., Hewlett Sandra A., Tayo Bamidele O., Cole Helen V., de-Graft Aikins Ama, Ogedegbe Gbenga, Adanu Richard. Health Education & Behavior.2016;43(4). CrossRef
- Immunohistochemical determination of estrogen and progesterone receptors in breast cancer: relationship with clinicopathologic factors in 302 patients in Ivory Coast Effi Ahoua Benjamin, Aman Nguiessan Alphonse, Koui Baumaney Sylvanus, Koffi Kouadio Donatien, Traoré Zie Cheick, Kouyate Mohamed. BMC Cancer.2017;17(1). CrossRef
- An audit of the management and associaed contextual correlates of clinical presentations of breast cancer in a tertiary hospital in south west Nigeria IOSR Journal of Pharmacy Eniojukan J, Adepoju T. 2015;5(6):11-21.
- Diagnosis delay in Libyan female breast cancer Ermiah Eramah, Abdalla Fathi, Buhmeida Abdelbaset, Larbesh Entesar, Pyrhönen Seppo, Collan Yrjö. BMC Research Notes.2012;5(1). CrossRef
- First report on molecular breast cancer subtypes and their clinico-pathological characteristics in Eastern Morocco: series of 2260 cases Elidrissi Errahhali Manal, Elidrissi Errahhali Mounia, Ouarzane Meryem, El Harroudi Tijani, Afqir Said, Bellaoui Mohammed. BMC Women's Health.2017;17(1). CrossRef
- Anxiety disorders in breast cancer: Prevalence, types, and determinants Fatiregun Olamijulo A., Olagunju Andrew T., Erinfolami Adebayo R., Fatiregun Omolara A., Arogunmati Olubunmi A., Adeyemi Joseph D.. Journal of Psychosocial Oncology.2016;34(5). CrossRef
- Association of Epstein - Barr virus and breast cancer in Eritrea Fessahaye Ghimja, Elhassan Ahmed M., Elamin Elwaleed M., Adam Ameera A. M., Ghebremedhin Anghesom, Ibrahim Muntaser E.. Infectious Agents and Cancer.2017;12(1). CrossRef
- Pathology of Senegalese breast cancers Fitzpatrick Megan Burke, Rendi Mara Hester, Kiviat Nancy Barbara, Toure Pape, Dem Amadou, Sow Papa Salif, Hawes Stephen Edward, Feng Qinghua, Allison Kimberly Heller. Pan African Medical Journal.2019;34. CrossRef
- Time intervals experienced between first symptom recognition and pathologic diagnosis of breast cancer in Addis Ababa, Ethiopia: a cross-sectional study Gebremariam Alem, Addissie Adamu, Worku Alemayehu, Assefa Mathewos, Pace Lydia E, Kantelhardt Eva Johanna, Jemal Ahmedin. BMJ Open.2019;9(11). CrossRef
- Patient Delay in Accessing Breast Cancer Care in a Sub Saharan African Country: Uganda Galukande Moses. British Journal of Medicine and Medical Research.2014;4(13). CrossRef
- Breast cancer survival experiences at a tertiary hospital in sub-Saharan Africa: a cohort study Galukande Moses, Wabinga Henry, Mirembe Florence. World Journal of Surgical Oncology.2015;13(1). CrossRef
- Factors associated with time to first healthcare visit, diagnosis and treatment, and their impact on survival among breast cancer patients in Mali Grosse Frie Kirstin, Kamaté Bakarou, Traoré Cheick Boudagari, Ly Madani, Mallé Brahima, Coulibaly Bourama, Wienke Andreas, Kantelhardt Eva Johanna. PLOS ONE.2018;13(11). CrossRef
- Hormone Receptors and Age Distribution in Breast Cancer Patients at a University Hospital in Northern Egypt Hussein Osama, Mosbah Mahmoud, Farouk Omar, Farag Kamel, El-Saed Aiman, Arafa Mohammad, Abdallah Ahmed. Breast Cancer: Basic and Clinical Research.2013;7. CrossRef
- Barriers to early presentation of breast cancer among women in Soweto, South Africa Joffe Maureen, Ayeni Oluwatosin, Norris Shane Anthony, McCormack Valerie Ann, Ruff Paul, Das Ishani, Neugut Alfred I., Jacobson Judith S., Cubasch Herbert. PLOS ONE.2018;13(2). CrossRef
- Pattern of Presentation and Survival of Breast Cancer in a Teaching Hospital in North Western Nigeria Kene Terfa, Odigie Vincent, Yusufu Lazarus, Yusuf Bidemi, Shehu Sani, Kase John. Oman Medical Journal.2010;25(2). CrossRef
- A Study of Risk Factors for Breast Cancer in a Primary Oncology Clinic in Benghazi-Libya Bodalal Zuhir, Khaial Fatma, Elramli Amal, Elkhwsky Fayek, Eltaguri Adel, Bendardaf Riyad. International Journal of Statistics in Medical Research.2015;4(1). CrossRef
- Pathologically confirmed breast cancer in Malawi: a descriptive study: Clinical profile of breast cancer Kohler RE, Moses A, Krysiak R, Liomba NG, Gopal S. Malawi Medical Journal.2015;27(1). CrossRef
- Epidemiological and Clinical Profile of Breast Cancer at Bamako Radiotherapy Center Kone A. S., Diakite A., Diarra I. M., Diabate K., Camara M. A., Diallo Y. L., Sidibe S.. Journal of Cancer Therapy.2019;10(09). CrossRef
- Stage at presentation of breast cancer in Luanda, Angola - a retrospective study Lopes Lygia Vieira, Miguel Fernando, Freitas Helga, Tavares António, Pangui Salvador, Castro Clara, Lacerda Gonçalo Forjaz, Longatto-Filho Adhemar, Weiderpass Elisabete, Santos Lúcio Lara. BMC Health Services Research.2015;15(1). CrossRef
- Stage at diagnosis, clinicopathological and treatment patterns of breast cancer at Bugando Medical Centre in north-western Tanzania Mabula J, Mchembe M, Chalya P, Giiti G, Chandika A, Rambau P, et al . Tanzania Journal of Health Research.2012;14(2).
- Incidence of Breast Cancer in Eritrea: A Retrospective Study from 2011 to 2017 Medhin Lidia B., Tekle Lia A., Fikadu Daniel T., Sibhatu Danait B., Gebreyohans Samson F., Gebremichael Kibrom H., Halki Tesfamariam M., Said Saleh M., Ghidei Yosief T., Lobeck Hartmut. International Journal of Breast Cancer.2019;2019. CrossRef
- Survival Outcomes of Breast Cancer in Ghana: An Analysis of Clinicopathological Features Mensah Alice C., Yarney Joel, Nokoe Sagary Kaku, Opoku Samuel, Clegg-Lamptey J. N.. OALib.2016;03(01). CrossRef
- Breast cancer in Angola, molecular subtypes: a first glance Miguel Fernando, Vieira Lopes Lygia, Ferreira Eduardo, Ribas Emília, Fuentes Pelaez Alexis, Leal Conceição, Amaro Teresina, Lopes Paula, Mendes Santos Cristina, Lopes Carlos, Lara Santos Lúcio. ecancermedicalscience.2017;11. CrossRef
- From symptom discovery to treatment - women's pathways to breast cancer care: a cross-sectional study Moodley Jennifer, Cairncross Lydia, Naiker Thurandrie, Constant Deborah. BMC Cancer.2018;18(1). CrossRef
- Patterns of seeking medical care among Egyptian breast cancer patients: Relationship to late-stage presentation Mousa Shimaa M., Seifeldin Ibrahim A., Hablas Ahmed, Elbana Eman S., Soliman Amr S.. The Breast.2011;20(6). CrossRef
- Survie au cancer du sein à Rabat (Maroc) 2005-2008 Mechita Nada Bennani, Tazi Mohammed Adnane, Er-Raki Abdelouahed, Mrabet Mustapha, Saadi Asma, Benjaafar Noureddine, Razine Rachid. Pan African Medical Journal.2016;25. CrossRef
- Pattern of hormone receptors and human epidermal growth factor receptor 2 status in sub-Saharan breast cancer cases: Private practice experience Nwafor CC, Keshinro SO. Nigerian Journal of Clinical Practice.2015;18(4). CrossRef
- Quality of Breast Cancer Treatment at a Rural Cancer Center in Rwanda O’Neil Daniel S., Keating Nancy L., Dusengimana Jean Marie V., Hategekimana Vedaste, Umwizera Aline, Mpunga Tharcisse, Shulman Lawrence N., Pace Lydia E.. Journal of Global Oncology.2018;(4). CrossRef
- Breast cancer in Kumasi, Ghana Ohene-Yeboah M, Adjei E. Ghana medical journal.2012;46(1):8-13.
- Epidemiology of Female Breast Cancer in Ogun State: Intra- and Inter-regional Discuss Okoye Jude Ogechukwu, Erinle Charles, Atulomah Nnodimele Onuigbo, Adeleke Oluwaseun Kelechi. Universal Journal of Clinical Medicine.2017;5(2). CrossRef
- Hormonal and Her2 receptor Immunohistochemistry of breast cancers in Ile-Ife, Nigeria Omoniyi-Esan G, Olaofe O, Omonisi A, Olasode B, Adis A. Austin J Womens Health.2015;2(1):1009.
- Delayed presentation of breast cancer patients Otieno ES, Micheni JN, Kimende SK, Mutai KK. East African Medical Journal.2010;87(4). CrossRef
- Provider delay in the diagnosis and initiation of definitive treatment for breast cancer patients Otieno ES, Micheni JN, Kimende SK, Mutai KK. East African Medical Journal.2010;87(4). CrossRef
- Delays in Breast Cancer Presentation and Diagnosis at Two Rural Cancer Referral Centers in Rwanda Pace Lydia E., Mpunga Tharcisse, Hategekimana Vedaste, Dusengimana Jean‐Marie Vianney, Habineza Hamissy, Bigirimana Jean Bosco, Mutumbira Cadet, Mpanumusingo Egide, Ngiruwera Jean Paul, Tapela Neo, Amoroso Cheryl, Shulman Lawrence N., Keating Nancy L.. The Oncologist.2015;20(7). CrossRef
- Literacy and breast cancer diagnosis and treatment among patients in a tertiary health institution of lagos Nigeria Popoola A, Wright K, Igwilo A, Sowunmi A, Kuyinu Y. Journal of Dental and Medical Sciences.2013(b);5(4):49-54.
- Breast cancer in Accra, Ghana Quayson SE, Wiredu EK, Adjei DN, Anim JT. Journal of Medical and Biomedical Sciences.2015;3(3). CrossRef
- Socio-demographic and clinical profile of immuno-histochemically confirmed breast cancer in a resource limited country Rahman Ganiyu Adebisi, Olatoke Samuel Adegboyega, Agodirin Suleiman Olayide, Adeniji Kayode Adebanji. Pan African Medical Journal.2014;17. CrossRef
- Triple negative breast cancer in a poor resource setting in North-Western Tanzania: a preliminary study of 52 patients Rambau Peter, Masalu Nestory, Jackson Kahima, Chalya Philipo, Serra Patrizia, Bravaccini Sara. BMC Research Notes.2014;7(1). CrossRef
- Fear of Treatments Surpasses Demographic and Socioeconomic Factors in Affecting Patients With Breast Cancer in Urban South Africa Rayne Sarah, Schnippel Kathryn, Firnhaber Cynthia, Wright Kathryne, Kruger Deirdre, Benn Carol-Ann. Journal of Global Oncology.2017;3(2). CrossRef
- Factors Delaying Presentation of Sudanese Breast Cancer Patients: an Analysis Using Andersen's Model Salih Alaaddin M, Alfaki Musab M, Alam-Elhuda Dafallah M, Nouradyem Momin M. Asian Pacific Journal of Cancer Prevention.2016;17(4). CrossRef
- Ethnicity and breast cancer characteristics in Kenya Sayed Shahin, Moloo Zahir, Wasike Ronald, Bird Peter, Oigara Raymond, Njoroge Faith Wambui, Shaikh Asim Jamal, Prasad Satya Vara, Vinayak Sudhir, Gierach Gretchen L., Dawsey Sanford M., Palakal Maya, Fan Shaoqi, Mullooly Maeve, Chauhan Rajendra, Okiro Patricia, Gakinya Samuel, Nzioka Ancent, Kyobutungi Catherine, Mohamed Shukri, Haregu Tilahun, Mussajee Mustafa, Bonass Betty, Mariwa Costa, Sherman Omar Ali, Mohammed Abdihakim, Gachii Andrew, Githaiga Joseph, Karanu Joseph, Nyagah Robert, Njoroge Richard, Muramba Irene, Otieno James Obondi, Raburu Dan Omondi, Mwachiro Elizabeth B., Abayo Innocent, Saleh Mansoor. Breast Cancer Research and Treatment.2017;167(2). CrossRef
- Breast diseases histologically diagnosed at a tertiary facility in Uganda (2005–2014) Ssemmanda Salvatore, Katagirya Eric, Bukirwa Phiona, Alele David, Lukande Robert, Kalungi Samuel. BMC Cancer.2018;18(1). CrossRef
- Patient-mediated factors predicting early- and late-stage presentation of breast cancer in Egypt Stapleton Jaye M., Mullan Patricia B., Dey Subhojit, Hablas Ahmed, Gaafar Rabab, Seifeldin Ibrahim A., Banerjee Mousumi, Soliman Amr S.. Psycho-Oncology.2011;20(5). CrossRef
- Relationship between family history of breast cancer and clinicopathological features in Moroccan patients Tazzite A, Jouhadi H, Saiss K, Benider A, Nadifi S. Ethiopian journal of health sciences.2013;23(2):150-157.
- Breast cancer clinicopathological presentation, gravity and challenges in Eritrea, East Africa: Management practice in a resource-poor setting Tesfamariam Asmerom, Gebremichael Andemariam, Mufunda Jacob. South African Medical Journal.2013;103(8). CrossRef
- Histological Features and Tissue Microarray Taxonomy of Nigerian Breast Cancer Reveal Predominance of the High-Grade Triple-Negative Phenotype Titloye N.A., Foster A., Omoniyi-Esan G.O., Komolafe A.O., Daramola A.O., Adeoye O.A., Adisa A.O., Manoharan A., Pathak D., D''Cruz M.N., Alizadeh Y., Lewis P.D., Shaaban A.M.. Pathobiology.2016;83(1). CrossRef
- HIV infection in patients with breast cancer in Guinea (West Africa) Traore Bangaly, Diane Solomana, Sow Mamadou Saliou, Keita Mamady, Conde Mamoudou, Traore Fodé Amara, et al . The Pan African medical journal.2015;21:261.
- Molecular subtyping of carcinoma of the female breast in a tertiary teaching hospital in Northern Nigeria Usman Asma'u, Iliyasu Yawale, Atanda AkinfenwaTaoheed. Annals of Tropical Pathology.2019;10(1). CrossRef
- Depression and social support among breast cancer patients in Addis Ababa, Ethiopia Wondimagegnehu Abigiya, Abebe Workeabeba, Abraha Aynalem, Teferra Solomon. BMC Cancer.2019;19(1). CrossRef
- Tumor size and stage of breast cancer in Côte d'Ivoire and Republic of Congo – Results from population-based cancer registries Islami Farhad, Lortet-Tieulent Joannie, Okello Catherine, Adoubi Innocent, Mbalawa Charles Gombé, Ward Elizabeth M., Parkin D. Maxwell, Jemal Ahmedin. The Breast.2015;24(6). CrossRef
- Unraveling the South African Breast Cancer Story: The Relationship of Patients, Delay to Diagnosis, and Tumor Biology With Stage at Presentation in an Urban Setting Rayne Sarah, Schnippel Kathryn, Grover Surbhi, Fearnhead Kirstin, Kruger Deirdre, Benn Carol, Firnhaber Cynthia. Journal of Surgical Research.2019;235. CrossRef
- Female Malignant Breast Lesions: The Lagos University Teaching Hospital Experience (1999–2013) Daramola A, Obiajulu F, Banjo AA, Abdulkareem FB, Shaaban A. Nig Qt J Hosp Med.2016;26(2):395-398.
- Breast cancer burden in central Sudan Mohammed . International Journal of Women's Health.2010. CrossRef
- Breast Cancer Survival in Cameroon: Analysis of a Cohort of 404 Patients at the Yaoundé General Hospital Ngowa Jean Dupont Kemfang, Kasia Jean Marie, Yomi Jean, Nana Achille Nkigoum, Ngassam Anny, Domkam Irenée, Sando Zacharie, Ndom Paul. Advances in Breast Cancer Research.2015;04(02). CrossRef
- Patterns of breast cancer among Ethiopian patients: Presentation and Histopathological Features Gemta E, Bekele A, Mekonen W, Seifu D, Bekuretsion Y, Kantelhardt EJ. J Cancer Sci Ther.2019;11(2).
- Epidemiology and Histology Aspects of Breast Cancers of Women in Ivory Coast N’Dah Kouame Justin, Troh Emile, Koffi Kouakou Emmanuel, Doukouré Brahima, Didier Kouame Arthur, Didier Abouna Alain, Ahoua Effi Benjamin, I Diomandé Mohenou. Journal of Cancer Therapy.2012;03(05). CrossRef
- Breast cancer laterality among Egyptian patients and its association with treatments and survival Zeeneldin Ahmed A., Ramadan Mohamed, Elmashad Nehal, Fakhr Ibrahim, Diaa Amira, Mosaad Ehab. Journal of the Egyptian National Cancer Institute.2013;25(4). CrossRef
- Factors contributing to delays in diagnosis of breast cancers in Ghana, West Africa Brinton Louise, Figueroa Jonine, Adjei Ernest, Ansong Daniel, Biritwum Richard, Edusei Lawrence, Nyarko Kofi M., Wiafe Seth, Yarney Joel, Addai Beatrice Wiafe, Awuah Baffour, Clegg-Lamptey Joe Nat. Breast Cancer Research and Treatment.2016(a);162(1). CrossRef
- Factors related to incomplete treatment of breast cancer in Kumasi, Ghana Obrist Mark, Osei-Bonsu Ernest, Awuah Baffour, Watanabe-Galloway Shinobu, Merajver Sofia D., Schmid Kendra, Soliman Amr S.. The Breast.2014;23(6). CrossRef
- The Role of Ultrasound as a Diagnostic Tool for Breast Cancer in the Screening of Younger Women (Age 25-38) in Guyana Nandan D, Alladin A. J Med Diagn Meth.2018;7(2).
- Breast Cancer Before Age 40 Years Anders Carey K., Johnson Rebecca, Litton Jennifer, Phillips Marianne, Bleyer Archie. Seminars in Oncology.2009;36(3). CrossRef
- Breast Cancer in Europe: Epidemiology, Risk Factors, Policies and Strategies. A Literature Review Aljohar Bashaier Abdullah, Kilani Mohammed Ahmedhani. Global Journal of Health Science.2018;10(11). CrossRef
- Breast cancer trends differ by ethnicity: a report from the South African National Cancer Registry (1994–2009) Singh E., Joffe M., Cubasch H., Ruff P., Norris S. A., Pisa P. T.. The European Journal of Public Health.2016. CrossRef
- Aging in Sub-Saharan Africa: The Changing Demography of the Region. In: National Research Council (US) Committee on Population; Cohen B, Menken J, editors. Aging in Sub-Saharan Africa: Recommendation for Furthering Research. Washington (DC): National Academies Press (US) Velkoff V, Kowal P. Available from: https://www.ncbi.nlm.nih.gov/books/NBK20301/.2006;2.
- 10.1002/ijc.27841Breast carcinoma in men Giordano Sharon H., Cohen Deborah S., Buzdar Aman U., Perkins George, Hortobagyi Gabriel N.. Cancer.2004;101(1). CrossRef
- An international comparison of male and female breast cancer incidence rates Ly Diana, Forman David, Ferlay Jacques, Brinton Louise A., Cook Michael B.. International Journal of Cancer.2012;132(8). CrossRef
- Default from neoadjuvant chemotherapy in premenopausal female breast cancer patients: What is to blame? Egwuonwu OA, Nwofor AME, Anyanwu SNC. Nigerian Journal of Clinical Practice.2012;15(3). CrossRef
- The role of men in early detection of their spouses' breast lump(s)/cancer Moses A, Olayide A, Olusola O, Adetunji O, Temitope B, Atilola A. Nigerian Journal of General Practice.2011;9(2). CrossRef
- Perceptions of Arab men regarding female breast cancer screening examinations—Findings from a Middle East study Donnelly Tam Truong, Al-Khater Al-Hareth, Al-Bader Salha Bujassoum, Al-Kuwari Mohamed Ghaith, Abdul Malik Mariam Ali, Al-Meer Nabila, Singh Rajvir. PLOS ONE.2017;12(7). CrossRef
- Delay in Seeking Medical Advice and Late Presentation of Female Breast Cancer Patients in Most of the World. Could We Make Changes? The Experience of 23 Years in Port Said, Egypt Elzawawy Ahmed M., Elbahaie Alaadeen M., Dawood Salah M., Elbahaie Hussaam M., Badran Atef. Breast Care.2008;3(1). CrossRef
- Prognostic factors for patients with breast cancers 1cm and smaller Chen Yunn-Yi, Schnitt Stuart J.. Breast Cancer Research and Treatment.1998;51(3). CrossRef
- Breast cancer screening in a resource poor country: Ultrasound versus mammography Omidiji Olubukola A.T., Campbell Princess C., Irurhe Nicholas K., Atalabi Omolola M., Toyobo Oluyemisi O.. Ghana Medical Journal.2017;51(1). CrossRef
- Are Both Ultrasonography and Mammography Necessary for Cancer Investigation of Breast Lumps in Resource-Limited Countries? Chairat Rungnapa, Puttisri Adisorn, Pamarapa Asani, Samintharapanya Sahatham, Tawichasri Chamaiporn, Patumanond Jayanton. ISRN Oncology.2013;2013. CrossRef
- Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis Jedy-Agba Elima, McCormack Valerie, Adebamowo Clement, dos-Santos-Silva Isabel. The Lancet Global Health.2016(a);4(12). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2021
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times
- Supplementary file downloaded - 0 times