The Role of Immunohistochemistry in the Workup of Malignant Neoplasms of Unknown Primary Origin at Khartoum Oncology Hospital
Download
Abstract
Background: Carcinoma of unknown primary origin (CUP) comprises various malignancies classified by detection of tissue-specific genes through immunohistochemistry (IHC). We aimed to explore the role of available immunohistochemical markers in diagnosing and classifying malignant neoplasms of unknown primary origin.
Methods: A cross-sectional study included 141 patients diagnosed histologically as CUP and referred to the Histopathology and Immunohistochemistry Department, Khartoum Oncology Hospital, from 2012 to 2017. Hematoxylin and Eosin (H&E) and immune stained slides used in the workup were reviewed and classified into the main histologic types of CUP. Data were -analyzed by SPSS.
Results: Out of 4436 cases, CUP represents (3.2%). The age group (60-69) years have the highest percentage (20.13%), with male predominance (51.77%). Lymph nodes represent (41.84%) followed by the liver (12.77%), spine (3.55%), and lungs (2.13%). Adenocarcinoma (75.89%) was the most common subtype, followed by undifferentiated neoplasm (14.18%), squamous cell carcinoma (7.09%), and carcinoma with neuroendocrine differentiation (2.84%). In 70 cases (49.6%) of the study cases, the primary site was determined, (17.7%) were given an only differential diagnosis, and in (32.6%) the origin remains unknown.
Conclusions: CUP cases during the study period are infrequent (3.2%), and the primary origin was determined in nearly half of patients by the available immune markers. CUP’s common histological types were adenocarcinoma, undifferentiated neoplasm, squamous cell carcinoma, and carcinoma with neuroendocrine differentiation. The most common presenting sites were lymph node, liver, spine, and lungs.
Introduction
Cancer of unknown primary origin (CUP) is a group of malignant neoplasms defined by a metastatic disease with no primary tumor at presentation. It represents 2 to 5% of all cancers, and it is the fourth most common cause of cancer death. It represents 2-6% of all cancers diagnosed in the United States accounting for 2-9% of cancers diagnosed worldwide [1, 2]. Equal incidence for men and women was reported, with the median age of presentation for men and women ranges from 59-66 years [3, 4].Reported life expectancy is very short, about 6-9 months. Median survival ranges between 11 months and 11 weeks with 11%five-year survival rates [3, 4].
The diagnosis starts by biopsy with malignancy for which the anatomical origin is unknown after a medical history, physical examination, and several investigations including liver, kidney function test, blood test, stool for occult blood, chest radiograph, abdominal, chest CT scan and mammography for female, prostate-specific antigen test for male. Hematoxylin and Eosin (H/E) stainidentifiesthe general histology by, and it can be divided into adenocarcinoma well, moderately or poorly differentiated carcinomas, Squamous cell carcinomas, undifferentiated neoplasms, and carcinomas with neuroendocrine differentiation [5, 6].
All biomarkers used in pathology for CUP targeting the diagnosis and establishment of cancer type, subtype, and site, including IHC and ancillary molecular tests.
As more specific therapies emerge, prognostic and predictive biomarkers may also become necessary in CUP. However, the best method for identifying CUP origin is immunohistochemistry, and it remains the gold standard method, especially in countries with limited resources and unable to pay for other ancillary techniques.
The early diagnosis and proper treatment of patients with cancer of unknown primary is an important prognostic factor that may increase the survival rate and improve outcome [5, 6]. The biology of CUP is not clearly understood, but generally, CUP tumors have common biological features. Their main characteristics are early metastasis in the clinical undetectable primary tumor, the unpredictability of metastatic pattern, aggressive biology, and clinical behavior [1, 2].
Histopathological examination and clinical correlation remain the cornerstone in morphologic diagnosis; IHC supports or rules out possible differential diagnoses. Immunohistochemistry combines anatomic and immunologic techniques to identify tissue components using a specific antigen-antibody reaction that can be detected through enzyme reactions with the antibodies being used. The enzyme reaction appears as a color at the site of antibody-antigen binding, so it permits the visualization and localization of specific cellular components within a cell or tissue [7].
After the biopsy, morphology is examined; first, the pathologist must prove cancer’sexistence. A stepwise approach using IHC markers panels is followed to identify the tumor type, and subtype’sbroad likely site of origin is recognized using organ-specific markers.
As CUP is a common and challenging clinical problem, the role of IHC workup in CUP is classification according to the broad tumor type, subtype and, site of origin if possible, and this provides excellent benefits in CUP patients’ diagnosis and management. IHC has an essential role in the diagnosis of metastatic tumors. It is done in paraffin-embedded tissue and has a low cost compared to advanced imaging studies and molecular genetic analysis. The identification of patients with the favorable disease is essential since they may benefit from directed treatment. After a thorough review of the literature, we could not find regional or local publications covering this issue. We conducted this study to assess the CUP cases’ frequency, classification, and workup using available IHC markers from January 2012 to December 2017.
Materials and Methods
In this cross-sectional study, the H&E-stained and immune-stained slides panels used in the workup were retrieved from Khartoum Oncology Hospital (KOH) archives then examined under the light microscope by the supervisor histopathologist and the researcher. The clinical information of the patients was obtained from the request forms in the records of the laboratory. The stepwise approach was followed in the workup of CUP in the Histopathology Department, KOH. Briefly, once a biopsy was obtained, the presence of malignancy has been confirmed on the H&E slides. After that, a stepwise approach using IHC markers panels was taken to identify the broad tumor type, then tumor subtype. Cytokeratin is an essential marker in CUP’s workup first steps with specific distribution in different organs using mainly CK7 & CK20. If adenocarcinoma was diagnosed, the likely site of origin was identified with organ-specific markers. The collected data were analyzed by SPSS and Excel analytical system. The P-value calculated and 0.05 or less was the cut-off value.
The study was approved by the Ethical Committees of Faculty of Medicine, University of Khartoum and Ministry of Health, Khartoum State. Permission was taken from KOH and the laboratory director. The research purpose and objectives were explained to the laboratory director. Informed consent was waived since there was no contact with patients, and the paraffin-embedded blocks and data were anonymized.
Results
This study covered 141 CUP patients out of 4436 cases presented to the histopathology laboratory at Khartoum Oncology Hospital during the study period. Their distribution by year is shown in (Figure 1).
Figure 1. Percentage of CUP Cases Per Year.
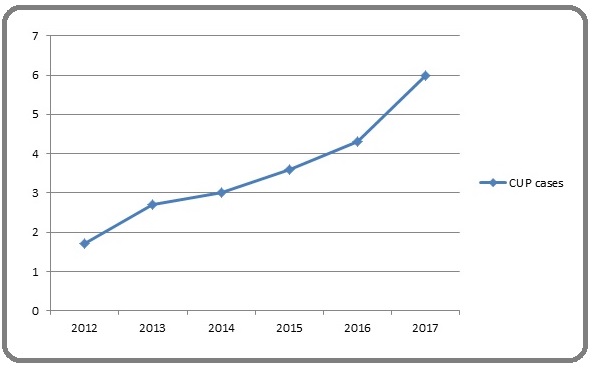
Males represented 73 (51.77%) and females 68(48.23%). Patients’ age ranged between 5years and 85years with a mean age of 49.66 ±19.069. The highest percentage (39%) was for the age group between (60-89) years (Table1).
| Character | Number of cases | Percentage (%) |
| Age | ||
| Less than 20 | 13 | 9 |
| 20-29 | 11 | 7.80 |
| 30-59 | 62 | 44.10 |
| 60-89 | 55 | 39 |
| Sex | ||
| Male | 73 | 52 |
| Female | 68 | 48 |
| Site of presentation | ||
| Lymph node | 59 | 41.84 |
| Liver | 18 | 12.77 |
| spine | 5 | 3.55 |
| Lung | 3 | 2.13 |
| Others * | 56 | 39.72 |
| Histopathological variant | ||
| Adenocarcinoma | 107 | (76%) |
| (14%) | ||
| Undifferentiated carcinoma | 20 | 7 |
| Squamous cell carcinoma | 10 | 3 |
| Carcinoma with neuroendocrine differentiation | 4 |
*others; peritoneal cavity, soft tissue, umbilicus, ovary and bowel.
Lymph nodes were the common presenting site 59 (41.84%), followed by liver 18 (12.77%), spine 5 (3.55%), lung 3 (2.13%), and others 56 (39.72%) like a peritoneal cavity, soft tissue, umbilicus, ovary, and bowel (Table 1). Out of the 59 (41.84%)metastatic CUP in the lymph nodes, 38 (64.4%) were cervical, 8 (13.6%) axillary, 6 (10.2%) inguinal,5 (8.47%) supraclavicular, and 1 (1.7%) for para- aortic and submandibular lymph nodes. Lymph nodes work up in the study exposed tumor origin in 38(64%) cases. In contrast, the only differential diagnosis was reported in 12 (21%), and in 9 (15%) cases,the origin was not determined. In cervical lymph nodes, 32 (84%) originated from the nasopharynx, 3 (8%) metastasized from the lung and 2 cases from each pancreatic and ovarian tissue. The histological types identified in the studied cases were adenocarcinoma 107 (75.89%), undifferentiated neoplasms 20 (14.18%), squamous cell carcinoma 10 (7.09%), and carcinoma with neuroendocrine differentiation 4 (2.84%).The used markers in the workup for each case ranged from 1-17 with a mean of eightimmunohistochemical markers. An example case workup is showed in Figures 2-5.
Figure 2. Case 1. Mesenteric Mass Biopsy with Metastatic Pancreatic Adenocarcinoma. (A) H&E(B) CK7+ (C) CK19+ (D) CEA focal strong positivity, not shown CK20-, CDX2-, CA125-, WT-1-, ER-. (Original magnification: A and D X10, B and C X20).
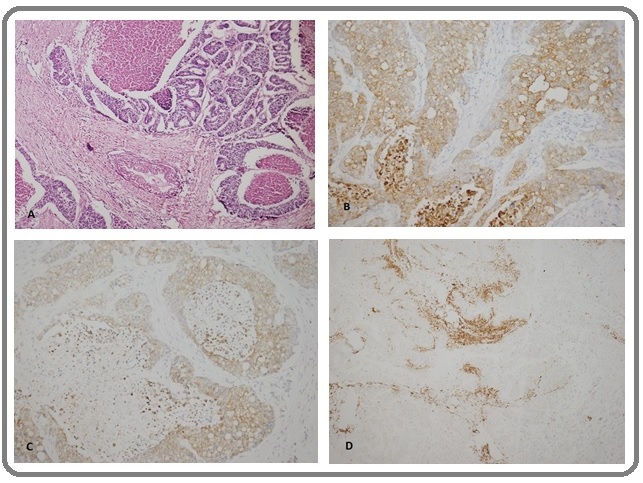
Figure 3. Case 2. Right Humorous Mass Biopsy with Metastatic Thyroid Carcinoma. (A) H&E, (B)CK7+, (C)TTF1+, and (D) thyroglobulin+. Not shown: vimentin+, WT1+, CK20-, CD10-, ER-, and CEA-. (Original magnification:H&E X10, B-D X20).
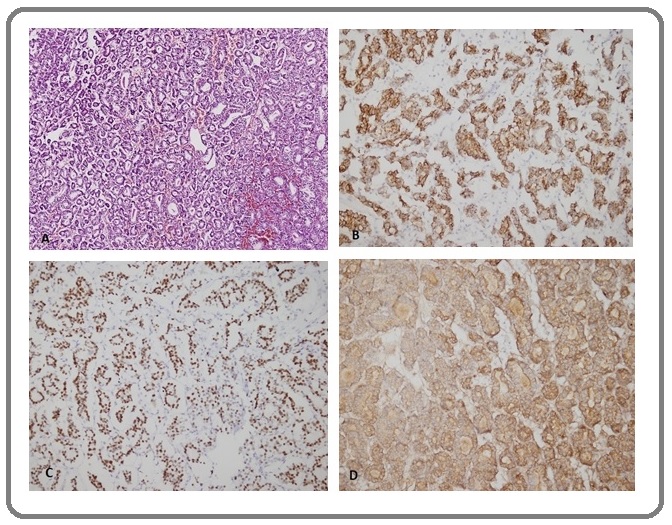
Figure 4. Case 3. Inguinal Lymph Nodes Biopsy with Metastatic Ovarian Carcinoma (A) H&E, (B) CK7+, (C) CA125+, (D) CEA+, (E) WT1+ and (F) PR+.(Original magnification: A-B-D-F X20,C X10, E X40).
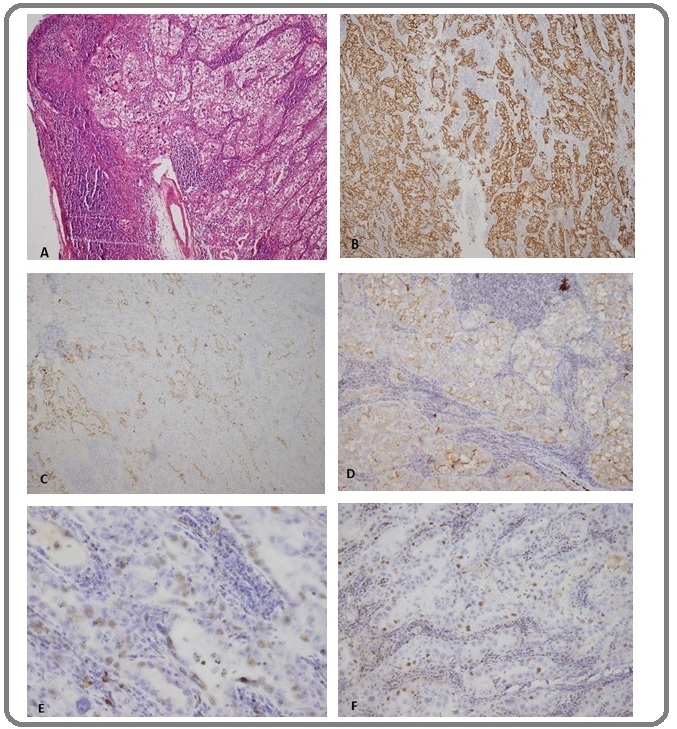
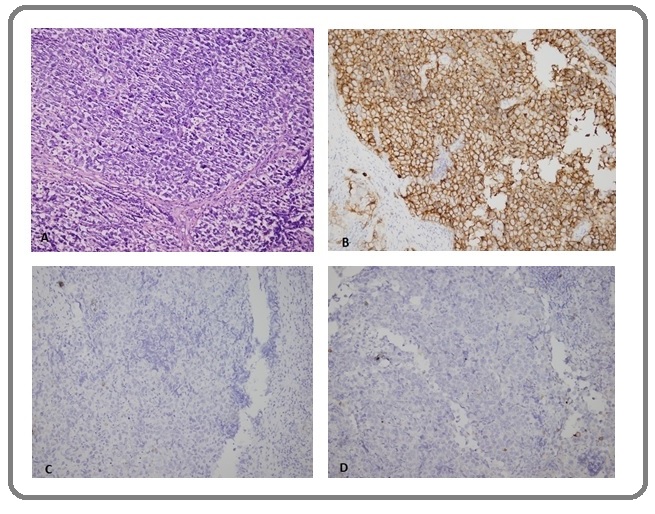
Figure 5. Case 4. Supraclavicular Lymph Node Biopsy with Metastatic seminoma (A) H&E, (B) PLAP+, (C) PAN CK- and (D) CD30-. (Not shown: LCA-, S100-, and CD117-). (Original magnification A-D X20).
Discussion
This research aimed to study the role of available immunohistochemical markers in detecting malignant neoplasms of unknown primary origin (CUP). This study covers 141 CUP cases out of 4436 cases diagnosed during the study period, representing 3.2%, comparable with the literature reported incidences of CUP ranging between 3-5% [1],[4],[8].
The study showed an increasing percentage of cases from 1.7% in 2012 to 6.5% in 2017.This rise may be due to an increase in the oncologist appreciation of the role of IHC in detecting the primary site of CUP and the availability of drugs that target specific biomarkers detected through IHC helps inpatient management with directed therapy. As more targeted anticancer agents are introduced, accurate and efficient classification of the tissue type becomes essential. Identification of tumor lineage and the primary site allows the pathologist to focus the search for biomarkers target by therapy in a particular tumor type [9].
The study shows that the age group 60-69 years has a high incidence (20.6%) of the study population, with slightly increased incidence in males (51.77%) to females (48.23%). A similar finding is found in the Royal College of Pathologists - Cancer datasets report, showing median age 60 with 53% M: 47% F[8].
CUP manifests itself as metastases,in the literature review studies,most commonly to lymph nodes, lungs, liver, and bone [3]. This study also indicates that the most common presenting site is lymph nodes (41.84%), liver (12.77%), spine (3.55%), and lung (2.13%).This study shows that the cervical lymph nodes are the most familiar presenting site in the study populationfor CUPlymph nodes. IHC workup for cervical lymph node with metastatic CUP identified nasopharynx as the common primary origin site (84%).
Most studies and review articles show that the most common histopathological entities are adenocarcinomas, followed by undifferentiated carcinomas, squamous carcinomas, and carcinoma with neuroendocrine differentiation [1], [4], and [8]. This study has a similar result that the common histological types are adenocarcinoma (75.89%), followed by undifferentiated neoplasm (14.18%), squamous cell carcinoma (7.09%), and carcinoma with neuroendocrine differentiation (2.84%),
Immunohistochemistry is a sensitive method for tumor diagnosis that requires practical training and good basic knowledge. The diagnosis begins with the proper processing of tissue samples with standard staining techniques, choice of appropriate immune markers panel, and finally interpretation of the stain by the pathologist. These steps minimize errors and so get benefit from IHC. In using IHC, the laboratory of IHC must be under the supervision of a well-trained pathologist with high skills in the methods and techniques of IHC and have the necessary knowledge in the interpretation of IHC stain results.The nature of target antigens is an essential factor in interpreting the results.The pattern of expression of the antigen, whether nuclear, cytoplasmic, membranous or extracellular, is also essential. New antibodies were introduced according to their sensitivity and specificity and after studying their properties. Standardization of staining methods using positive and negative controls is important [10].
Depending on the histopathological appearance of the lesion, different panels of antibodies are used,including epithelial markers like PAN CK, EMA, and other cytokeratins, CK7, CK20, CK5\6. Also, LCA, SI00, and melanocytic markers like Melan A and HMB45 exclude lymphoid, sarcomatous, and melanocytic origins.
Accordingly, a second panel is performed to include organ-specific markers like PSA, CDX2, TTF1 for prostatic, colonic, thyroid, or lung primary, respectively. Other markers used in the study cases workup includes synaptophysin and chromogranin, for neuroendocrine differentiation,ER, HER2, CA125, and EBV.
Keratins, a family of intermediate filament proteins expressed in epithelial cells, are used to confirm the epithelial origin mainly poorly differentiated neoplasms. Although the patterns are not entirely specific, expression patterns of cytokeratin in epithelial cells suggest possible primary sites in the setting of metastatic CUP.The CK7- positive, CK20-negative profile is most common in CUP, which does not helpful suggest of a specific anatomic site of origin. Therefore, these profiles must be used with additional morphologic and immunohistochemical support [10, 11]. Many different immune markers can be used as organ-specific markers. These markers show the reaction of specific antibody to either cytoplasmic (and/ or membranous) component or nuclear transcription factors [4].
Some valuable organ-specific markers in CUP workup include ER,GATA3,Mammoglobin A, GCDFP-15 for breast origin.Estrogen Receptor (ER) expression is more among the primary breast cancers than the metastatic cases [4][12], so it has a limited role in the workup of CUP. ER positivity is identifiedin other malignancies, such as endometrium and ovary carcinomas, thyroid papillary carcinomas, and adnexal tumors of the skin [13-16].
In recent times,GATA3 has been used as a very sensitive marker for breast carcinomas [4]. In ductal breast carcinoma, lobular, triple-negative and metaplastic cases, the expression of positivity is reported at 91%, 100%,43%, 54%, respectively. GATA3 is alsoexpressed in 90% of metastatic breast carcinoma [4][14].
The Thyroid Transcription Factor 1 (TTF-1) is expressed in thyroid and respiratory epithelium. TTF-1 sensitivity is seen mainly in lung adenocarcinomas and non-mucinous adenocarcinoma with a lepidic pattern; this sensitivity is maintained in metastatic lung carcinoma [15]. Co-expression of TTF-1 and Napsin A is present in pulmonary adenocarcinomas, so it is used in the workup of CUP when the pulmonary primary is suspected [4] [16]. CDX2 is positive in approximately all cases of colorectal adenocarcinoma. CDX2 expression is also seen in GI neuroendocrine tumors and those intestinal tumors such as carcinoid tumors [17]. As the liver is acommon site for metastatic tumor markers use in workup includes hepatocyte paraffin 1 (Hep-Par 1) antibody and arginase-1, they have excellent sensitivity and specificity [18].
Wilms tumor antibody (WT1), which is a nuclear transcription factor, specifies normal urogenital development. The major application of WT1 in CUP workout is in the identification of ovarian serous carcinomas, primary peritoneal adenocarcinomas, and fallopian tube serous carcinomas [19].Markers for prostatic cancer are specific and sensitive site-predictive markers and include prostatic specific antigen (PSA) and the more recently described homeobox gene NKX3.1 [4]. NKX3.1 is a sensitive marker for identifying metastatic prostatic adenocarcinoma, positive in 99%. Its sensitivity approaches 100%. Furthermore, it is maintained in high- grade prostatic carcinomas [20]. PSA is also used with GATA3 to distinguish between TCC and high-grade prostatic adenocarcinoma [4] [21].
In the renal system, there are five markers (PAX8, pVHL, RCCma, CD10, and KIM-1) useful in confirming the diagnosis of clear cell RCC. PAX8 is the best sensitive immune stain among those five markers.It is identified in the renal epithelial neoplasms, with conventional (clear cell) renal cell carcinoma expressing a sensitivity of 88% to 98%. Papillary renal cell carcinomas also showed high sensitivity, varying from 71% to 100% [22]. Squamous cell carcinomas, such as those arising in the lung or cervix and tumors that manifest a squamous immunophenotype such as thymus tumors are uniformly and strongly p63 positive and p40 positive [23]. In germ cell linage, the diagnosis of primary tumors of the testis has been assisted by IHC by assessing transcription factors including; octamer-binding transcription factor 4 (OCT4), Sal-like protein 4 (SALL4), SRY (sex-determining region Y)-box 2 (SOX2) and SOX17 that are confirmed to be significantly sensitive and specific for the differential diagnosis of germ cell tumors [24-26]. This study shows some limitations in the availability of these markers; this may be due to low financial resources. Despite that, 49.6% of CUP cases are finally diagnosed, and the primary sites for diagnosed CUP arenasopharynx (14.2%), colon (7.1%), ovary (6.4%), prostate (5%), pancreas (3.5%), stomach (1.4), breast (0.7), and others (8.5%) including thyroid, testis, and kidneys. In (17.7%), the IHC work ends by giving a differential diagnosis, and in (32.6 %) the origin remains unknown. Many studies also show that the primary origin remains unknown in some cases after thorough workup [27], and in some, the primary found at autopsy [8].
Immunohistochemistry is an efficient and cost- effective approach to identifying the site of origin in CUP. It is accessible to most anatomic pathologists and can be performed on formalin-fixed paraffin embedded tumor tissue [7].
Analysis of clinical, histopathological, and immunohistochemical data can help diagnose metastatic carcinoma of unknown primary origin. The initial histopathological interpretation provides valuable information for establishing the tumorlocation, complete with those obtained using immunohistochemical tests. As a result of the increasing number of available markers, the proportion of CUP cases in which pathologists can confidently assign primary sites will increase.
In conclusions, the study concluded that CUP cases represent 3.2% of all cases that refer to the Histopathology Department, Khartoum Oncology Hospital. The most common presenting sites were lymph nodes followed by liver, spine, and lung. Histological classification was in the following order:adenocarcinoma, undifferentiated neoplasm, squamous cell carcinoma, and carcinoma with neuroendocrine. Differentiation of the Primary site by IHC workup showed thatcervical lymph nodes are the commonest lymph node for metastatic CUP. Nasopharyngeal carcinoma is the most common primary for the metastatic cervical lymph nodes.The pathologist and lab managers are advised to use IHC in the proper histological context in panels rather than individually,and follow the introduction of new markers is considered a significant part of patient diagnosis and management.
Acknowledgments
Authors’ contribution
Eiman Omar, Nazik Emalika Husain: case review, data collection, interpretation, writing and reading of the manuscript. Eiman Omar, Nazik Emalika Husain, Amal Ismail: upgrading manuscript writing, scientific material review, intellectual editing and comments and approval of the manuscript.
Funding
No finding sources.
Ethical approval
This paper is prepared from a dissertation required in partial fulfillment of MD degree in clinical pathology, Faculty of medicine, University of Khartoum. The study approved from the ethical committee of Faculty of Medicine; University of Khartoum and Ministry of health, Khartoum state.
Conflict of interest
The authors declare no conflicts of interest
References
- Cancers of unknown primary origin: current perspectives and future therapeutic strategies Stella Giulia Maria, Senetta Rebecca, Cassenti Adele, Ronco Margherita, Cassoni Paola. Journal of Translational Medicine.2012;10(1). CrossRef
- Metastatic Carcinoma of Unknown Primary Conner James R., Hornick Jason L.. Advances in Anatomic Pathology.2015;22(3). CrossRef
- CUP Syndrome Zaun Gregor, Schuler Martin, Herrmann Ken, Tannapfel Andrea. Deutsches Aerzteblatt Online.2018. CrossRef
- Practical Applications in Immunohistochemistry: Carcinomas of Unknown Primary Site Kandalaft Patricia L., Gown Allen M.. Archives of Pathology & Laboratory Medicine.2015;140(6). CrossRef
- Carcinoma of Unknown Primary. Abeloff’s Clinical Oncology Varadhachary G, Abbruzzese JL. Elsevier.2020;:1694-1702. CrossRef
- Carcinoma of unknown primary with hepatic metastases: a need of judicious and contemplative diagnostic algorithm Shivaji Vikram Sai, Wilson Joseph Charles, Schmidt Noemi L., Kolokythas Orpheus, Lalwani Neeraj. Abdominal Radiology.2020;46(1). CrossRef
- Immunohistochemistry for Diagnosis of Metastatic Carcinomas of Unknown Primary Site Selves Janick, Long-Mira Elodie, Mathieu Marie-Christine, Rochaix Philippe, Ilié Marius. Cancers.2018;10(4). CrossRef
- Standards and datasets for reporting cancers Dataset for histopathological reporting of cancer of unknown primary (CUP) and malignancy of unknown primary origin (MUO) July 2018 Schofield JB, Oien K. https://www.rcpath.org/uploads/assets/555302b1-8b11-4d8a-a24431d0693d3287/G167-Dataset-for-histopathological-reporting-of-cancer-of-unknown-primary-CUP-and-malignancy-of-unknown-primary-origin-MUO.pdf..
- A Multicenter Study Directly Comparing the Diagnostic Accuracy of Gene Expression Profiling and Immunohistochemistry for Primary Site Identification in Metastatic Tumors Handorf Charles R., Kulkarni Anand, Grenert James P., Weiss Lawrence M., Rogers William M., Kim Oliver S., Monzon Federico A., Halks-Miller Meredith, Anderson Glenda G., Walker Michael G., Pillai Raji, Henner W. David. American Journal of Surgical Pathology.2013;37(7). CrossRef
- Recommendations for the Utility of Immunohistochemistry in Tumor Diagnosis. Immunohistochemistry in Tumor Diagnostics [Internet] Tuffaha MSA, Guski H, Kristiansen G. Springer International Publishing.2017;:257-258. CrossRef
- Diagnostic work-up of carcinoma of unknown primary: from immunohistochemistry to molecular profiling Oien K.A., Dennis J.L.. Annals of Oncology.2012;23. CrossRef
- Role of androgen receptor expression in early stage ER+/PgR−/HER2– breast cancer Tagliaferri Barbara, Quaquarini Erica, Palumbo Raffaella, Balletti Emanuela, Presti Daniele, Malovini Alberto, Agozzino Manuela, Teragni Cristina Maria, Terzoni Andrea, Bernardo Antonio, Villani Laura, Sottotetti Federico. Therapeutic Advances in Medical Oncology.2020;12. CrossRef
-
Can gata3 immunocytochemistry be utilized as a reliable diagnostic marker for metastatic breast carcinoma in cytological materials?
a comparative study with mammaglobin and gcdfp-15 expression Hafez Nesreen H., Shaaban Hebatallah M.. Turkish Journal of Pathology.2017. CrossRef - GATA-3 is superior to GCDFP-15 and mammaglobin to identify primary and metastatic breast cancer Ni Yun-Bi, Tsang Julia Y. S., Shao Mu-Min, Chan Siu-Ki, Cheung Sai-Yin, Tong Joanna, To Ka-Fai, Tse Gary M.. Breast Cancer Research and Treatment.2018;169(1). CrossRef
- Misleading Thyroid Transcription Factor-1 Staining in the Setting of Suspected Metastatic Thyroid Cancer Wodarcyk A. Chest.2020;158(4). CrossRef
- The Lung-Restricted Marker Napsin A Is Highly Expressed in Clear Cell Carcinomas of the Ovary Kandalaft Patricia L., Gown Allen M., Isacson Christina. American Journal of Clinical Pathology.2014;142(6). CrossRef
- Encyclopedia of Cancer [Internet]. Springer Berlin Heidelberg; 2014;865–9 Gross I, Hinkel I. CDX2 . . CrossRef
- The Histomorphological and Immunohistochemical Diagnosis of Hepatocellular Carcinoma Fanni D, Gerosa C, F G. Hepatocellular Carcinoma - Clinical Research .InTech.2012. CrossRef
- Contributors. Wilms Tumor [Internet]. Codon Publications; 2016 Mar 23;xvii–xxiii . CrossRef
- Comparative Analysis of Serum Prostate Specific Antigen Levels in Various Prostatic Lesions Parvathaneni Sowmya. Journal of Medical Science And clinical Research.2020;08(01). CrossRef
- GATA3 Immunohistochemical Expression in Salivary Gland Neoplasms Schwartz Lauren E., Begum Shahnaz, Westra William H., Bishop Justin A.. Head and Neck Pathology.2013;7(4). CrossRef
- Unknown Primary/Undifferentiated Neoplasm. Handbook of Practical Immunohistochemistry Lin F, Liu H. Springer New York.2015;:119-163. CrossRef
- p40 Immunohistochemistry Is an Excellent Marker in Primary Lung Squamous Cell Carcinoma Affandi Khairunisa Ahmad, Tizen Nur Maya Sabrina, Mustangin Muaatamarulain, Zin Reena Rahayu MdReena Rahayu Md. Journal of Pathology and Translational Medicine.2018;52(5). CrossRef
- Role of P63 expression in diagnosis of thymoma subtypes Sun Y, Zhang L, Yu H, Cao X, Jia X, Chen G. Academic Journal of Second Military Medical University.2013;33(11). CrossRef
- Metastatic Treated Malignant Germ Cell Tumors Andeen Nicole K., Tretiakova Maria S.. Applied Immunohistochemistry & Molecular Morphology.2016;24(3). CrossRef
- Best Practices Recommendations in the Application of Immunohistochemistry in Testicular Tumors Ulbright Thomas M., Tickoo Satish K., Berney Daniel M., Srigley John R.. American Journal of Surgical Pathology.2014;38(8). CrossRef
- Evaluation of central nervous system metastases with immunohistochemistry correlation Sharma Pranshuta, Trivedi Priti, Shah ManojJ. Indian Journal of Pathology and Microbiology.2014;57(3). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2021
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times