Role of DOSE Escalation in whole Brain Radiotherapy for Brain Metastasis in Patients with a Favorable Survival Prognosis
Download
Abstract
Background: Brain metastases is a major health care problem and is the most common intracranial cancer in adults. These patients may benefit from intensive treatments including neurosurgery and radiosurgery but many patients cannot receive such treatment, and whole brain radiotherapy (WBRT) alone is the only option. The most common WBRT schedule is 30 Grays (Gy) in 10 fractions (fr). However there is need to develop fractionation schedules, but total dose still remain dilemma.The Purpose of this study to assess the potential benefit of dose escalation beyond 30 Gy.
Patients and methods: Total 120 patients with multiple brain metastases, previously untreated with WBRT were randomly assigned into two arms. All patients have favorable survival prognosis. Seventy patients received a total 30Gy in 10fr, 3Gy/fr administered daily 5days/week in arm A and 50 patients received 39Gy in 13fr, with same schedule in arm B. Both arms were compared for local control (LC) and overall survival (OS).
Results: The LC rate at 18 months was 11% after 30 Gy and 24% after 39 Gy (p value=0.068). The survival rate at18 months was 21% after 30 Gy and 38% after 39Gy (OS 21% vs. 38%, hazard ratio 0.593; 95% CI: .395-.891; p value=0.047). On subgroup analysis of primary tumor, the 18 months LC rate was 16% vs 53% in Breast (p value=0.020) and10% vs 11% in Lung cancer (p value=0.809) in arm A and B respectively; the 18 months OS rate was 14% vs26% in Lung (P value=0.160) and 37% vs 73% in Brest cancer (p value=0.034) in arm A and B respectively.
Conclusion: Escalation of the WBRT dose beyond 30 Gy resulted in better outcomes, particularly for patients with primary breast cancer.
Introduction
Brain metastases is most common intra-cranial malignancy in adults [1]. Around 10% to 30% of all cancer patients, develop brain metastases during course of their disease [2]. Brain metastases can be diagnosed as a synchronous or metachronous malignancy [3]. The most common primary site is lung followed by breast. Most of patients presents with neurologic sign and symptoms. Whole-brain radiotherapy (WBRT) alone is the most common treatment for these patients, particularly in those who have multiple lesions. Majority of patients with multiple brain metastases have a poor survival prognosis of only a few months [4]. It has been seen that patients who have up to 3 brain lesions may have a considerably more favorable survival prognosis. It has been reported that these patients benefit from more intensive treatment, including neurosurgery or radiosurgery, in terms of better intracerebral control and overall survival [5-7]. If more intensive treatment cannot be used, and although, neurosurgery and radiosurgery are not available in many institutions worldwide, even in developed countries then WBRT alone is the only reasonable treatment option. Dexamethasone is frequently used to control brain edema. At present supportive care along with WBRT remains the standard of care for all symptomatic patients with multiple brain lesions that are not amenable for surgery.
as the overall survival for patients with brain metastases remains poor, the use of prognostic scales help to guide therapies. One of the useful prognostic scales was based on 1200 patient’s consecutive Radiation Therapy Oncology Group (RTOG) phase 3 brain metastases trials [8] from 1979 to 1993. Using recursive partitioning analysis (RPA) three well defined prognostic groups (RPA class I, II and III) were identified based on age (< 65 or = 65 and older), KPS of>70, = 70 or < 70, absence or presence of extracranial metastases and primary tumor status.
In this study, only patients who had the most favorable survival score were included. This study compared 30 Gy in 10 fractions with 39 Gy in 13 fractions to evaluate local control and overall survival.
Materials and Methods
This was a randomized prospective comparative study conducted at Regional Cancer Treatment and Research Institute.
Eligibility Criteria
The study protocol included total 120 patients of brain metastases with a known primary of breast and lung only; who were enrolled from March 2016 to November 2017. The majority of these patients had favorable prognosis. Further inclusion criteria were as follows: no prior radiotherapy to the brain, confirmation of metastases by CECT or MRI imaging and administration of dexamethasone (12-32 mg daily) during WBRT. The data were obtained from the patients and their files.
The protocol was approved by hospital’s institutional ethical committee, and all patients were properly informed and consented for the treatment study. Study design was intent to treat.
Study design
Patients were randomly assigned to two arms, A and B; 70 patients in armAand 50 patients in arm B. randomization was done by using the web site randomization.com prior to start WBRT. Patients characterstics is described in Table 1.
| Patients characteristics | Number of patients | P value | ||
| Arm A, n=70 (30 Gy/10 fr) | Arm B, n=50 (39 Gy/13fr) | |||
| Age | < 65 years | 59 | 40 | 0.542 |
| >65 years | 11 | 10 | ||
| Sex | Male | 45 | 31 | 0.798 |
| Female | 25 | 19 | ||
| KPS score | ≥70 | 46 | 34 | 0.793 |
| < 70 | 24 | 16 | ||
| Socioeconomic status | Urban | 13 | 8 | 0.715 |
| Rural | 57 | 42 | ||
| Number of lesion | Single | 8 | 5 | 0.803 |
| Multiple | 62 | 45 | ||
| Extra cranial metastasis | Yes | 17 | 14 | 0.647 |
| No | 53 | 36 | ||
| Primary disease | Lung | 51 | 35 | 0.732 |
| Breast | 19 | 15 | ||
| Interval from tumor diagnosis to WBRT | ≤ 6 months | 55 | 39 | 0.94 |
| ≥ 6 months | 15 | 11 |
Total 70 patients in arm A treated with WBRT dose 30 Gy; 3 Gy/fraction in total 10 fractions and 50 patients in arm B patients treated 39 Gy; 3 Gy/fr (1fr per day and 5fr per week in both arms) in total 13 fractions on telecobalt units Theratron 780C/780E/ Bhabhatron with photon energy of 1.25 Mev. WBRT was given by parallel opposed right-left lateral portals. supportive care specially mannitol, dexamethasone was started at the beginning of treatment and also continued during radiotherapy. All statistical analysis were performed by using SPSS for windows, version 20.0.
Alongwith the WBRT regimen, some potential prognostic factors were evaluated like Age (<65 years vs >65 yrs), Sex, Karnofsky performance score (KPS<70 vs >70), Primary tumor type ( breast vs lung cancer), the no. of brain metastases (single vs multiple) and the presence of extracranial metastases (yes vs no). both groups were also comparable for these factors (Table 1).
Assessment of overall survival were done by using the kaplan-meier-method [10].
Results
The baseline patients and tumor characteristics are described in Table 1. All characteristics were balanced and comparable. The local control and overall survival on different follow-up visits are shown in Table 2-3. The median follow up was 18 months (6 months, 12 months, and 18 months).
Patients were followed up until death or on 3 months, 6 months, 12 months and 18 months. The median survival after WBRT was 10.4 months for whole cohort. The median survival was 13.4 months for 39 Gy arm and 9.2 months for 30 Gy arm.
On subgroup analysis improved local control was significantly associated with KPS>70 (p value =0 .015) and primary breast cancer (p=0.004). A strong trend toward better local control was seen for dose escalated 39 Gy arm (p=0.068). Significantly increased overall survival was associated with 39 Gy arm (p=.047), KPS> 70 (p=.0003), female sex (p=.008) and breast primary (p=0.0002).
The rate of grade 2 acute toxicity, including nausea, headache, and oral mucositis according to version 2.0 of the Common Toxicity Criteria [11], was 20% after 30 Gy in 10 fractions and 18% after 39 Gy in 13 fractions (P value=0.783). Relevant late toxicity rates, including neurocognitive deficits, visual disturbances, and hearing problems, were 29% after 30 Gy in 10 fractions and 24 % after 39 Gyin13 fractions (P value=0.576)).
Discussion
With gradual improvements in the care of cancer patients, longer survival is expected even in patients having multiple brain metastases. WBRT is most frequently used treatment for patients with brain metastases. For patients who have 1 to 3 brain metastases and favorable survival prognosis, more intensive treatment approaches, including neurosurgery or radiosurgery, are used.
Although, because of inappropriate size or location of tumor, such intensive treatment may not be possible. If WBRT alone is administered, then 30 Gy in 10 fractions is considered the “standard” regimen. However, the results of WBRT alone with 30 Gy is associated with relatively poor treatment outcomes and need to be improved with respect to overall survival and local intracerebral control specially for the patients of relatively favorable survival prognosis, because these patients are likely to live long enough to develop such a recurrence.
There are so many approaches have been tried to improve the results of WBRT alone. Many randomized control studies compared WBRT alone with WBRT plus various radio-sensitizing agents like misonidazole, metronidazole, ionidazole, and motexafin gadolinium [12-15]. However, the administration of such radiosensitizers did not improve treatment outcomes. The addition of chemotherapy to WBRT were also not be beneficial [16-20]. Another approach to improve treatment outcomes is the escalation of WBRT dose beyond the standard (30 Gy in 10 fraction). In this current study, we compared 30 Gy in 10 fractions with 39 Gy in 13 fractions. The biologic effectiveness of irradiation depends on both total dose and dose per fraction. Different radiation schedule scan be compared with the equivalent dose in 2-Gyfractions (EQD2), which takes into account the total dose and the dose per fraction [9]. The EQD2 is calculated with the equation EQD2= D x ([d + a/b]/(2Gy + a/b), as derived from the linear-quadratic model; where D is the total dose, d is the dose per fraction, a is the linear (first-order, dose- dependent) component of cell killing, b is the quadratic (second order, dose-dependent) component of cell killing, and the a/b ratio is the dose at which both components of cell killing are equal. Assuming an a/b ratio of 10 Gy for tumor cell kill, the EQD2 of the radiation schedules are 32.5Gy (30 Gy in 10 fractions) and 42.4 Gy (39 Gy in 13 fractions), respectively. Thus, the regimen investigated in the current study represented a dose escalation by almost 30% compared with 30 Gy in 10 fractions. Previous studies, including Kurtz et al [21] and Chatani et al [22] compared 30 Gy in 10 fractions with 50 Gy in [20] fractions reported median survival of 4.4 months vs 3.9 months and 5.4 months vs 4.8 months in both arms of above studies respectively (p value= 0.84). This current study included only patients who had a favourable survival prognosis and results suggest that patients who have a relatively favourable survival prognosis do benefit from WBRT regimen favoring 39 Gy in 13 fractions (P ¼ .064; Figure 1).
Figure 1. This Figure Compares whole-brain Radiotherapy at 30 grays (Gy) in 10 fractions with 39 Gy in 13 fractions with respect to overall survival. (OS 21% vs. 38%, hazard ratio 0.593; 95% CI: .395-.891; p value=0.047).
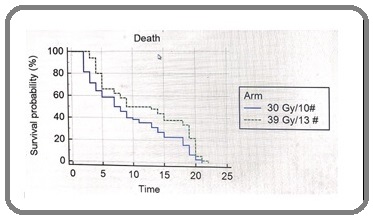
Both local control and overall survival were significantly better after 39 Gy in 13 fractions than after 30 Gy in 10 fractions (Table 2 and 3).
| Variable | At 6 months | At 12 month | At 18 months | P value |
| WBRT schedule | 0.068 | |||
| 30 Gy/10 fr (N=70) | 50 % (35) | 26% (18) | 11% (08) | |
| 39Gy/13 fr (N=50) | 56% (28) | 36% (18) | 24% (12) | |
| Age | 0.334 | |||
| <65 yr (N= 99) | 60% (59) | 30% (30) | 17% (18) | |
| >65 yr (N=21) | 19% (04) | 09% (02) | 09% (02) | |
| Sex | 0.734 | |||
| Female (N=44) | 61% (27) | 43% (19) | 18% (08) | |
| Male (N=76) | 47% (36) | 22% (17) | 16% (12) | |
| KPS | 0.015 | |||
| >70 (N=80) | 68% (54) | 41% (33) | 23% (18) | |
| <70 (N= 40) | 23% (09) | 08% (03) | 05% (02) | |
| Primary tumor | 0.004 | |||
| Lung (N=86) | 47% (41) | 21% (18) | 10% (09) | |
| Breast (N=34) | 65% (22) | 53% (18) | 32% (11) | |
| Extracranial mets | 0.513 | |||
| Yes (N=31) | 42% (13) | 19% (06) | 13% (04) | |
| No (N=89) | 56% (50) | 34% (30) | 18% (16) | |
| Number of mets | 0.148 | |||
| Single (N=13) | 46% (6) | 46% (6) | 31% (4) | |
| Multiple (N=107) | 53% (57) | 28% (30) | 15% (16) |
| Variables | At 6 months | At 12 months | At 18 months | P value |
| WBRT schedule | 0.047 | |||
| 30 Gy/10 fr (N=70) | 57% (40) | 39% (27) | 21% (15) | |
| 39Gy/13 fr (N=50) | 66% (33) | 50% (25) | 38% (19) | |
| Age | 0.298 | |||
| <65 yr (N= 99) | 67% (66) | 47% (47) | 30% (30) | |
| >65 yr (N=21) | 33% (07) | 24% (05) | 19% (04) | |
| Sex | 0.008 | |||
| Male (N=76) | 48% (37) | 35% (27) | 20% (15) | |
| Female(N=45) | 80% (36) | 56% (25) | 42% (19) | |
| KPS | 0.0003 | |||
| >70 (N=80) | 77% (62) | 59% (47) | 39% (31) | |
| <70 (N= 40) | 28% (11) | 13% (05) | 08% (03) | |
| Primary tumor | 0.0002 | |||
| Lung (N=86) | 52% (45) | 34% (29) | 19% (16) | |
| Breast (N=34) | 82% (28) | 68% (23) | 53% (18) | |
| Extracranial mets | 0.198 | |||
| Yes (N=31) | 48% (15) | 23% (07) | 19% (06) | |
| No (N=89) | 65% (58) | 51% (45) | 31% (28) | |
| Number of mets | 0.131 | |||
| Single (N=13) | 77% (10) | 61% (08) | 46% (6) | |
| Multiple (N=107) | 59% (63) | 41% (44) | 26% (28) |
In conclusion, this study results suggest that WBRT at 39 Gy in 13 fractions have better intracerebral control and overall survival than 30 Gy in 10 fractions for patients who have relatively good survival prognosis.
Ethical Approval
The study was approved by the institutional Review Board.
References
- Recent trends in Epidemiology of brain metastases: An Overview Tabouret B, Chinot O, Metellus P, Tallet A, Viens P, Goncalves A. Anticancer Res.2012;32(11):4655-4662.
- Diagnosis of cerebral metastases: doubledose delayed CT vs. contrast-enhanced MR imaging Davis PC, Hudgins PA, Peterman SB, et al . AJNR Am J Neuroradiol.1991;12:293-300.
- Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma Schouten Leo J., Rutten Joost, Huveneers Hans A. M., Twijnstra Albert. Cancer.2002;94(10). CrossRef
- Prognosis of patients treated for intracranial metastases with whole-brain irradiation Sundström Jari T, Minn Heikki, Lertola Kaarlo K, Nordman Eeva. Annals of Medicine.1998;30(3). CrossRef
- Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial Andrews David W, Scott Charles B, Sperduto Paul W, Flanders Adam E, Gaspar Laurie E, Schell Michael C, Werner-Wasik Maria, Demas William, Ryu Janice, Bahary Jean-Paul, Souhami Luis, Rotman Marvin, Mehta Minesh P, Curran Walter J. The Lancet.2004;363(9422). CrossRef
- Surgical Resection Followed by Whole Brain Radiotherapy Versus Whole Brain Radiotherapy Alone for Single Brain Metastasis Rades Dirk, Kieckebusch Susanne, Haatanen Tiina, Lohynska Radka, Dunst Juergen, Schild Steven E.. International Journal of Radiation Oncology*Biology*Physics.2008;70(5). CrossRef
- Whole-brain radiotherapy versus stereotactic radiosurgery for patients in recursive partitioning analysis classes 1 and 2 with 1 to 3 brain metastases Rades Dirk, Pluemer Andre, Veninga Theo, Hanssens Patrick, Dunst Juergen, Schild Steven E.. Cancer.2007;110(10). CrossRef
- Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials Gaspar Laurie, Scott Charles, Rotman Marvin, Asbell Sucha, Phillips Theodore, Wasserman Todd, McKenna W.Gillies, Byhardt Roger. International Journal of Radiation Oncology*Biology*Physics.1997;37(4). CrossRef
- The linear-quadratic approach to fractionation and calculation of isoeffect relationships. In: Steel GG, ed. Basic Clinical Radiobiology Joiner MC, Van der Kogel AJ. New York: Oxford University Press.1997;:106-112.
- Nonparametric Estimation from Incomplete Observations Kaplan E. L., Meier Paul. Journal of the American Statistical Association.1958;53(282). CrossRef
- Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy Trotti Andy, Byhardt Roger, Stetz Joanne, Gwede Clement, Corn Benjamin, Fu Karen, Gunderson Leonard, McCormick Beryl, Morris∫ Mitchell, Rich Tyvin, Shipley William, Curran Walter. International Journal of Radiation Oncology*Biology*Physics.2000;47(1). CrossRef
- Randomized trial of radiotherapy versus radiotherapy plus metronidazole for the treatment metastatic cancer to brain Eyre HarmonJ, Ohlsen JoelD, Frank Jess, LoBuglio AlbertF, McCracken JosephD, Weatherall TJ, Mansfield CM. Journal of Neuro-Oncology.1984;2(4). CrossRef
- The combined use of radiation therapy and lonidamine in the treatment of brain metastases DeAngelis LisaM., Currie ViolanteE., Kim Jae-Ho, Krol George, O'Hehir MaureenA., Farag FouadM., Young CharlesW., Posner JeromeB.. Journal of Neuro-Oncology.1989;7(3). CrossRef
- A randomized phase iii protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (RTOG-7916) T.Komarnicky Lydia, Phillips Theodore L, Martz Karen, Asbell Sucha, Isaacson Steven, Urtasun Raul. International Journal of Radiation Oncology*Biology*Physics.1991;20(1). CrossRef
- Survival and Neurologic Outcomes in a Randomized Trial of Motexafin Gadolinium and Whole-Brain Radiation Therapy in Brain Metastases Mehta Minesh P., Rodrigus Patrick, Terhaard C.H.J., Rao Aroor, Suh John, Roa Wilson, Souhami Luis, Bezjak Andrea, Leibenhaut Mark, Komaki Ritsuko, Schultz Christopher, Timmerman Robert, Curran Walter, Smith Jennifer, Phan See-Chun, Miller Richard A., Renschler Markus F.. Journal of Clinical Oncology.2003;21(13). CrossRef
- Chemotherapy of brain metastases from lung carcinoma Ushio Y, Arita N, Hayakawa T, Mogami H, Hasegawa H, Bitoh S, Oku Y, Ikeda H, Kanai N, Kanoh M. Neurosurgery.1991. CrossRef
- Treatment of Brain Metastases of Small-Cell Lung Cancer: Comparing Teniposide and Teniposide With Whole-Brain Radiotherapy—A Phase III Study of the European Organization for the Research and Treatment of Cancer Lung Cancer Cooperative Group Postmus Pieter E., Haaxma-Reiche Hanny, Smit Egbert F., Groen Harry J. M., Karnicka Hanna, Lewinski Tadeusz, van Meerbeeck Jan, Clerico Mario, Gregor Anna, Curran Desmond, Sahmoud Tarek, Kirkpatrick Anne, Giaccone Giuseppe. Journal of Clinical Oncology.2000;18(19). CrossRef
- Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Français de Pneumo-Cancérologie (GFPC) Protocol 95-1 Robinet G., Thomas P., Breton J.L., Léna H., Gouva S., Dabouis G., Bennouna J., Souquet P.J., Balmes P., Thiberville L., Fournel P., Quoix E., Riou R., Rebattu P., Pérol M., Paillotin D., Mornex F.. Annals of Oncology.2001;12(1). CrossRef
- Phase II Randomized Trial of Temozolomide and Concurrent Radiotherapy in Patients With Brain Metastases Antonadou D., Paraskevaidis M., Sarris G., Coliarakis N., Economou I., Karageorgis P., Throuvalas N.. Journal of Clinical Oncology.2002;20(17). CrossRef
- Randomized phase III trial of fotemustine plus whole brain irradiation in cerebral metastases of melanoma Mornex F, Thomas L, Mohr P, et al . Cancer Radiother.2003;7:1-8.
- The palliation of brain metastases in a favorable patient population: A randomized clinical trial by the radiation therapy oncology group Kurtz John M., Gelber Richard, Brady Luther W., Carella Richard J., Cooper Jay S.. International Journal of Radiation Oncology*Biology*Physics.1981;7(7). CrossRef
- Radiation therapy for brain metastases from lung carcinoma. Prospective randomized trial according to the level of lactate dehydrogenase Chatani M, Matayoshi Y, Masaki N, Inoue T. StrahlentherOnkol.1994;170:155-161.
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2022
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times