Prognostic Significance of Pre-treatment Serum Inflammatory Biomarkers on Survival in Patients with Carcinoma Cervix Treated by Radical Radiotherapy or Chemo-radiation
Download
Abstract
Background: Inflammation has an important role in the initiation and progression of carcinoma cervix. The measurement of inflammatory biomarkers is a cost-effective method of identifying patients at high risk of recurrence after treatment. This study was done to identify the influence of pre-treatment inflammatory biomarkers on survival in patients treated with radical chemo-radiation or radiation.
Methods: Patients with biopsy proven carcinoma cervix treated with Radiotherapy or Chemo-radiation from January 1st, 2016 to September 30th, 2017 were included. Pre-treatment complete blood counts, differential counts, serum albumin and C-reactive protein (CRP) were obtained. Neutrophil lymphocyte ratio (NLR), platelet lymphocyte ratio (PLR) and CRP Albumin ratio (CAR) were calculated. The best cut off values for NLR, PLR, serum albumin, CRP and CAR were found out from receiver operating characteristic (ROC) curves. OS (Overall Survival) and DFS (Disease Free Survival) were estimated using Kaplan -Meier method. The prognostic value of inflammatory biomarkers on survival was assessed by cox regression model.
Results: Sixty-three patients were included. The median follow up was 42.5 months. The best cut off values for NLR, PLR, albumin, CRP and CAR from the ROC curve were 2.36, 122.725, 3.95, 0.65 and 0.8 respectively. The three-year OS and DFS probability were 68.3% and 63.5% respectively. Patients with CAR >0.8 had 5.7 times more risk of death and 6.01 times risk of relapse or progression compared to patients with CAR ≤0.8.
Conclusion: Patients with a CRP-Albumin ratio of more than 0.8 are at significant risk of relapse and death after chemoradiation or radiation.
Introduction
Cervical cancer is the second most common cancer in women in India and it constitutes the most common cause of death due to cancer [1]. Almost one third of the patients develops relapse or disease progression and eventually dies due to the disease. The treatment options are limited for relapsed disease and hence identifying the poor prognostic group is crucial. The well- established prognostic factors for recurrence include clinical stage, depth of invasion, lymph node involvement and lympho- vascular invasion. But in advanced cases, patients are treated by radical chemo-radiation and hence these adverse pathological features cannot be determined. Hence it is relevant to find out other prognostic biomarkers.
Inflammation has an important role in the initiation and progression of many cancers [2,3].Various inflammatory mediators promote tumour growth, invasion, metastasis and angiogenesis [4]. Inflammatory blood parameters like neutrophil- lymphocyte ratio and platelet lymphocyte ratio have been extensively studied in various cancers [5,6]. C-Reactive Protein (CRP) is an acute phase reactant and the prognostic significance of pre-treatment CRP has been evaluated in many cancers [7-9]. Another inflammatory biomarker that has been extensively evaluated is serum albumin. Decreased levels of serum albumin was associated with poorer outcome in many cancers [10-12]. CRP to albumin ratio is considered to be a more reliable prognostic marker in some malignancies [13,14].
The inflammation based prognostic markers namely platelet lymphocyte ratio (PLR), neutrophil lymphocyte ratio (NLR), serum albumin, CRP, CRP albumin ratio (CAR) have been studied in cervical cancer patients also [4,15-17]. Some complex nutrition and inflammation based scores like Cervical Cancer Systemic Inflammation Score, Prognostic Nutritional Index, modified Glasgow Prognostic Score, Systemic Immune Inflammation Index have been evaluated in cervical cancer patients [4,18–20]. The identification of these inflammatory biomarkers is a cost-effective method of identifying patients at high risk of recurrence after radical treatment for carcinoma cervix, especially in a developing country like India. Hence, we conducted this prospective observational study to identify the effect of pre-treatment inflammatory biomarkers on survival in cervical cancer patients treated by radical radiotherapy or chemo-radiation.
Materials and Methods
Patients and Methods
This prospective observational study was conducted after getting approval from the Institutional Review Board. Patients with a histopathological diagnosis of carcinoma cervix who were planned to be treated with radical Radiotherapy or Chemo-radiation from January1st, 2016 to September 30th, 2017 were included after getting written informed consent. Patients aged more than 75 years were excluded from the study. Patient demographics and tumour characteristics including FIGO stage, histological type, lymph node status was documented. Magnetic Resonance Imaging of the pelvis and Computed Tomography scan of thorax and abdomen were used for staging.
Measurement of inflammatory parameters
Complete blood counts including platelet count, differential counts, serum albumin and CRP were obtained before starting radical treatment. Neutrophil lymphocyte ratio was calculated as the ratio of absolute neutrophil count to absolute lymphocyte count. The ratio of platelet count to absolute lymphocyte count was taken as platelet lymphocyte ratio. CRP Albumin ratio was calculated as the ratio of CRP to serum albumin values. The best cut off values for NLR, PLR, serum albumin, CRP and CRP albumin ratios were found out from ROC curves.
Treatment and follow up
Patients with stage IB1 disease were treated with pelvic radiotherapy, 46Gy in 23 fractions using four field technique followed by brachytherapy, 7Gy prescribed to point A for 3 sittings. Patients from stage IB2 onwards received external beam radiotherapy to pelvis 46Gy in 23 fractions with 15MV photons using four field technique.
A boost dose of 10Gy in 5 fractions was given to grossly enlarged nodes using opposed anterior-posterior fields with midline shielding. Patients with enlarged Para-aortic nodes were treated with IMRT. This was followed by intra-cavitary brachytherapy, 7Gy prescribed to point A for three sittings using Iridium- 192 after-loading technique. Chemotherapy consisted of concurrent cisplatin 40mg/m2 weekly for 4-5 cycles. Patients were followed up three-monthly for first two years and six monthly thereafter. Loco-regional control was assessed at six months of completion of treatment using clinical examination. MRI or PET-CT was done in patients with clinical residual disease. Follow up information till August 31st 2020 was updated.
Statistical analysis
Statistical analysis was done using SPSS software version 11.0. Categorical variables were summarized using counts and percentages. OS was taken from the date of registration to date of death or last follow up. DFS was calculated from date of registration to date of recurrence or death. Most of the patients with carcinoma cervix will have residual disease at the end of brachytherapy. The time point at which these patients become disease free is highly variable, usually ranges from 3 to 6 months. Hence DFS was calculated from the date of registration similar to OS. OS and DFS were estimated using Kaplan -Meier method and were compared using log rank test. The best cut off points of NLR, PLR, CRP, albumin and CAR were determined using receiver operating characteristic (ROC) curve analysis. Cox proportional hazard regression models were done to assess the prognostic value of inflammatory biomarkers on survival outcomes. A two-sided p value <0.05 was considered statistically significant.
Results
Sixty-three patients with carcinoma cervix who underwent radical radiotherapy or chemo-radiotherapy were included. Median age at diagnosis was 56 years (43-79). Median follow up was 42.5 months. The best cut off values for NLR, PLR, albumin, CRP and CAR from ROC curve were 2.36, 122.725, 3.95, 0.65 and 0.8 respectively corresponding to maximum joint sensitivity and specificity. Table 1 show the baseline patient and tumour characteristics.
| Patient characteristic | Number (%) |
| Age | |
| <50 years | 11 (17.46) |
| ≥50 years | 52 (82.53) |
| Histological type | |
| SCC | 49 (77.77) |
| Adenocarcinoma | 9 (14.28) |
| Others | 5 (7.93) |
| Stage | |
| Stage I | 2 (3.17) |
| Stage II | 24 (38.09) |
| Stage III | 33 (52.38) |
| Stage IV | 4 (6.34) |
| Pelvic nodes | 16 (25.39) |
| Paraaortic nodes± pelvic nodes | 6 (9.52) |
| NLR | |
| ≤2.36 | 37 (58.73) |
| >2.36 | 26 (41.26) |
| PLR | |
| ≤122.72 | 32 (50.79) |
| >122.72 | 31 (49.20) |
| CRP | |
| ≤0.65 | 24 (38.09) |
| >0.65 | 39 (61.90) |
| Albumin | |
| ≤3.95 | 14 (22.22) |
| >3.95 | 49 (77.77) |
| CAR | |
| ≤0.8 | 59 (93.65) |
| >0.8 | 4 (6.34) |
Two patients with stage IBI disease received radical radiotherapy. All other patients received radical chemo- radiation with concurrent weekly cisplatin. No significant association was noticed between the inflammatory markers (NLR, PLR, CRP, Albumin, CAR) and FIGO stage or presence of lymph node metastasis. The three- year overall survival probability was 68.3% with a standard error of 5.9% (Figure 1).
Figure 1. Kaplan-Meier Curve Showing Overall Survival Probability.
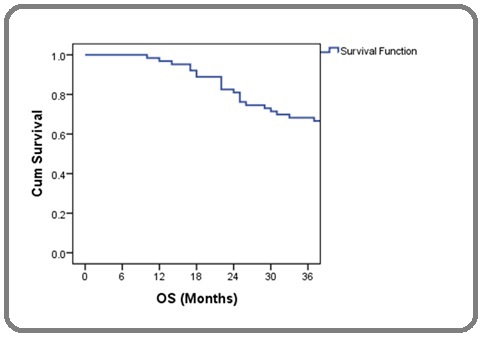
Overall survival was 80.8% for early stages (I and II) and 59.5% for locally advanced cases (stage III and IV). There was no difference in OS according to age, NLR, PLR, albumin and CRP. But there was significant survival difference with respect to CAR. The difference in survival according to various groups is summarised in Table 2.
| Groups | Survival probability (%) | Standard error (%) | P value |
| Age in years | |||
| ≤50 | 71.4 | 12.1 | 0.988 |
| >50 | 67.3 | 6.7 | |
| Histology | |||
| SCC | 71.4 | 6.5 | 0.248 |
| Non squamous | 57.1 | 13.2 | |
| Stage | |||
| I+II | 80.8 | 7.7 | 0.16 |
| III+IV | 59.5 | 8.1 | |
| NLR | |||
| ≤2.36 | 73 | 7.3 | 0.387 |
| >2.36 | 61.5 | 9.5 | |
| PLR | |||
| ≤122.72 | 68.8 | 8.2 | 0.899 |
| >122.72 | 67.7 | 8.4 | |
| CRP | |||
| ≤0.65 | 75 | 8.8 | 0.177 |
| >0.65 | 64.1 | 7.7 | |
| Albumin | |||
| ≤3.95 | 71.4 | 12.1 | 0.595 |
| >3.95 | 67.3 | 6.7 | |
| CAR | |||
| ≤0.8 | 72.9 | 5.8 | 0.001 |
| >0.8 | 0 | 0 |
The DFS probability at 3 years was 63.5% (Figure 2).
Figure 2. Kaplan-Meier Curve Showing Disease Free Survival Probability.
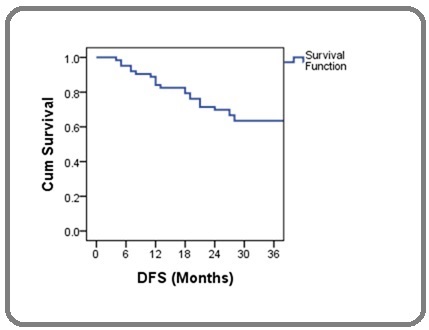
The 3 year DFS probability was 69.2% for early stages and 59.5% for locally advanced stages. Table 3 show the differences in DFS probability according to various groups.
| Groups | Disease Free Survival probability (%) | Standard error (%) | P value |
| Age in years | |||
| ≤50 | 57.1 | 13.2 | 0.725 |
| >50 | 65.3 | 6.8 | |
| Histology | |||
| SCC | 69.4 | 6.6 | 0.12 |
| Non squamous | 42.9 | 13.2 | |
| Stage | |||
| I+II | 69.2 | 9.1 | 0.243 |
| III+IV | 59.5 | 8.1 | |
| NLR | |||
| ≤2.36 | 67.6 | 7.7 | 0.655 |
| >2.36 | 57.7 | 9.7 | |
| PLR | |||
| ≤122.72 | 65.6 | 8.4 | 0.988 |
| >122.72 | 61.3 | 8.7 | |
| CRP | |||
| ≤0.65 | 70.8 | 9.3 | 0.291 |
| >0.65 | 59 | 7.9 | |
| Albumin | |||
| ≤3.95 | 71.4 | 12.1 | 0.526 |
| >3.95 | 61.2 | 7 | |
| CAR | |||
| ≤0.8 | 67.8 | 6.1 | 0.001 |
| >0.8 | 0 | 0 |
There was no significant difference in DFS according to age, histological type, stage, NLR, PLR, CRP and albumin. A significant difference in DFS was noted in patients with CAR ≤0.8 compared to patients with CAR >0.8. Out of the 63 patients, 6 patients had died before two years due to distant metastasis and hence their locoregional control could not be assessed. Of the remaining 57 patients, 14 patients had developed loco-regional failure at the time of assessment. Hence only the 43 patients who were loco-regionally controlled were assessed for association between pre-treatment factors and loco-regional control. There was no significant difference in loco-regional control at 2 years with respect to age, stage, histology, NLR, PLR, CRP, albumin or CAR. Table 4 shows the relationship between pre-treatment factors and locoregional control at 2 years.
| Parameters | Loco-regional control at 2years (%) | P value |
| Age | ||
| ≤50 | 10 (90.9) | 1 |
| >50 | 33 (63.46) | |
| FIGO stage | ||
| I+II | 22 (84.61) | 0.067 |
| III+IV | 21 (56.75) | |
| Histology | ||
| SCC | 35 (71.42) | |
| non squamous | 8 (57.14) | 1 |
| NLR | ||
| ≤2.36 | 26 (70.27) | 1 |
| >2.36 | 17 (65.38) | |
| PLR | ||
| ≤122.72 | 21 (65.6) | 1 |
| >122.72 | 22 (70.96) | |
| CRP | ||
| ≤0.65 | 18 (75) | 0.714 |
| >0.65 | 25 (64.10) | |
| Albumin | ||
| ≤3.95 | 9 (64.28) | 0.057 |
| >3.95 | 34 (69.38) | |
| CAR | ||
| ≤0.8 | 43 (72.88) | 0.056 |
| >0.8 | 0 |
Pre-treatment NLR, PLR, CRP and albumin were not significant predictors of OS or DFS in univariate cox regression model. Patients with CAR >0.8 had 5.7 times more risk of death and 6.01 times more risk of recurrence or progression compared to patients with CAR≤0.8. Table 5 shows the univariate cox regression analysis for OS and DFS.
| OS | DFS | |||||||
| Factors | Hazard ratio (HR) | 95% confidence interval | P value | HR | 95% CI | P value | ||
| Lower | Upper | Lower | Upper | |||||
| Age(≤50 vs >50) | 0.993 | 0.368 | 2.674 | 0.988 | 0.849 | 0.337 | 2.139 | 0.728 |
| Squamous vs non squamous | 0.598 | 0.245 | 1.456 | 0.257 | 0.519 | 0.222 | 1.215 | 0.131 |
| Stage III+IV vs I+II | 1.864 | 0.765 | 4.542 | 0.171 | 1.643 | 0.702 | 3.847 | 0.252 |
| NLR >2.36 vs ≤2.36 | 1.428 | 0.629 | 3.242 | 0.394 | 1.199 | 0.537 | 2.676 | 0.658 |
| PLR >122.25 vs ≤122.25 | 0.949 | 0.418 | 2.152 | 0.9 | 1.006 | 0.452 | 2.24 | 0.988 |
| CRP >0.65 vs ≤0.65 | 1.869 | 0.736 | 4.742 | 0.188 | 1.592 | 0.66 | 3.841 | 0.301 |
| Albumin ≤3.95 vs >3.95 | 1.336 | 0.454 | 3.932 | 0.599 | 1.409 | 0.481 | 4.125 | 0.532 |
| CAR>0.8 vs ≤0.8 | 5.703 | 1.852 | 17.562 | 0.002 | 6.01 | 1.969 | 18.343 | 0.002 |
Discussion
The established prognostic factors in carcinoma cervix include tumour size, FIGO stage, depth of invasion, lympho-vascular space involvement and lymph node involvement. But these factors are insufficient to explain all the patterns of failure or progression. Inflammation plays an important role in the pathogenesis of carcinoma cervix and the role of serum biomarkers of inflammation in the prognosis of carcinoma cervix are under investigation. Here we evaluated the prognostic significance of biomarkers of inflammation namely PLR, NLR, CRP, albumin and CAR.
Raised inflammatory markers were associated with advanced stage and presence of lymph node metastasis in some studies [4,21]. But we could not find any significant association between raised inflammatory markers and stage or lymph node metastasis. The non-significant association may be due to the small patient population. Advanced stage was reported to be associated with inferior OS and DFS in many studies [22,23]. There was an absolute difference of 21% in 3year OS between early and advanced stages in this study also, but the difference did not reach statistical significance due to the small patient population. Similarly, the absolute difference in 3year DFS between early and advanced stages was 9.7%, but not statistically significant.
High NLR was reported to be associated with decreased survival after surgical and non-surgical treatment for carcinoma cervix in many studies [16, 24].This study also favours the same finding. But the difference did not reach statistical significance. The mechanism by which increased neutrophil count and reduced lymphocyte count produces tumour progression is not clear. The proposed explanation is that neutrophils release angiogenic factors like vascular endothelial growth factor and matrix metalloproteinase-9 [25]. Lymphocytes secrete interleukin-2 which stimulate the proliferation of cytotoxic lymphocytes and inhibits tumour cell proliferation [26]. Low NLR was associated with complete response after chemo-radiation in a study [27]. But this study did not show any difference in loco- regional control with regard to NLR.
Many studies have reported elevated platelet lymphocyte ratio as a poor prognostic factor for survival [15,24,28]. Platelet activation causes release of angiogenic and anti-angiogenic factors, the overall effect of platelet endothelial interaction is stimulation of tumour angiogenesis [29]. PLR was not related to survival or loco-regional control in the present study
CRP is an acute phase protein secreted by hepatocytes in response to inflammatory mediators such as IL-1, IL-6 and TNF [30]. CRP is known to promote metastatic potential by stimulation of angiogenesis and increasing vascular permeability [31]. It is a cheap and widely available laboratory test which can be used in prognostication of cervical cancer patients. Higher CRP levels were associated with disease recurrence and decreased survival [31,32]. But we could not find any significant association between serum CRP levels and survival or loco-regional control.
Serum albumin is used as an indicator of nutritional status. The reasons for decreased serum albumin in malignancy are inflammation, cachexia and malnutrition [11]. Serum albumin was an independent prognostic factor in operable cancer of cervix in the study by Zheng et al [4]. Survival was not related to serum albumin levels in the present study.
Another inflammation- nutrition score, CRP to albumin ratio (CAR) was evaluated in some recent studies and was found to be a negative prognostic factor in carcinoma cervix [17,33]. This was confirmed in our study and appeared the most significant factor. Patients with CAR >0.8 were found to have 5.7 times increased risk of death and 6.01 times increased risk of recurrence and this was statistically significant. But only four patients had CAR>0.8 which accounts for only 6.34% of the total population. Hence this data should be interpreted with caution. All the four patients developed disease recurrence (three patients developed distant metastasis and one patient had locoregional recurrence) and eventually died due to disease. This data could not be extrapolated to real-world scenario due to the small number of cases. More-over all the four patients had stage III disease at presentation, two patients had pelvic or paraaortic nodes. The histopathological subtype was adenocarcinoma in two patients. All these factors could have contributed to the decreased DFS and OS in this group of patients.
There are ongoing trials examining value of adjuvant systemic therapy in locally advanced carcinoma cervix [34]. Patients with these raised inflammatory markers may be evaluated in these adjuvant trials.
The study is limited by small sample size and hence the results of the study should be interpreted with caution. The role of inflammatory markers was not separately analysed for early and advanced stages due to the small patient population in this study.
In conclusion, cervical cancer patients treated by radiotherapy or chemo-radiation with raised CRP-Albumin ratio are at significant risk of relapse and death. These patients are potential candidates for trials of adjuvant systemic therapy.
Acknowledgements
Nil
Funding
None
Conflicts of Interest
nil
Author contributions
All authors contributed to study concept and study design. Data collection was done by Dr Lekha M Nair and Dr Aneesha Babu. Dr JagathnathKrishna K M and Dr Lekha M Nair contributed to the data analysis and interpretation. All authors contributed to the drafting of the manuscript. All authors contributed to reviewing and approval of the final version of the manuscript.
Ethics approval
The study was approved by the institutional review board.
Consent to participate
Written informed consent was obtained from all individual participant
References
- Epidemiology of cervical cancer with special focus on India Aswathy Sreedevi, Reshma Javed, Avani Dinesh. International Journal of Women's Health.2015. CrossRef
- Cancer-Related Inflammation Candido Juliana, Hagemann Thorsten. Journal of Clinical Immunology.2012;33(S1). CrossRef
- The fundamental role of mechanical properties in the progression of cancer disease and inflammation Mierke Claudia Tanja. Reports on Progress in Physics.2014;77(7). CrossRef
- Cervical cancer systemic inflammation score: a novel predictor of prognosis Zheng Ru-ru, Huang Min, Jin Chu, Wang Han-chu, Yu Jiang-tao, Zeng Lin-chai, Zheng Fei-yun, Lin Feng. Oncotarget.2016;7(12). CrossRef
- The systemic inflammation-based neutrophil–lymphocyte ratio: Experience in patients with cancer Guthrie Graeme J.K., Charles Kellie A., Roxburgh Campbell S.D., Horgan Paul G., McMillan Donald C., Clarke Stephen J.. Critical Reviews in Oncology/Hematology.2013;88(1). CrossRef
- Establishing the prognostic value of platelet-to-lymphocyte ratio in cervical cancer: a systematic review and meta-analysis Yang Lixiao, Chen Huixiao. International Journal of Gynecologic Cancer.2019;29(4). CrossRef
- C-reactive protein and ovarian cancer: a prospective study nested in three cohorts (Sweden, USA, Italy) Lundin Eva, Dossus Laure, Clendenen Tess, Krogh Vittorio, Grankvist Kjell, Wulff Marianne, Sieri Sabina, Arslan Alan A., Lenner Per, Berrino Franco, Hallmans Goran, Zeleniuch-Jacquotte Anne, Toniolo Paolo, Lukanova Annekatrin. Cancer Causes & Control.2009;20(7). CrossRef
- The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma Hashimoto Koji, Ikeda Yasuharu, Korenaga Daisuke, Tanoue Kazuo, Hamatake Motoharu, Kawasaki Katsumi, Yamaoka Terutoshi, Iwatani Yasue, Akazawa Kohei, Takenaka Kenji. Cancer.2005;103(9). CrossRef
- Significance of preoperative C-reactive protein as a parameter of the perioperative course and long-term prognosis in squamous cell carcinoma and adenocarcinoma of the oesophagus Gockel Ines. World Journal of Gastroenterology.2006;12(23). CrossRef
- Baseline serum albumin and other common clinical markers are prognostic factors in colorectal carcinoma González-Trejo S, Carrillo JF, Carmona-Herrera DD, Baz-Gutiérrez P, Herrera-Goepfert R, Núñez G, et al. . Medicine (United States).2017;96(15):1-7.
- Pretreatment Serum Albumin Level is an Independent Prognostic Factor in Patients with Stage IIIB Non-Small Cell Lung Cancer: A Study of the Turkish Descriptive Oncological Researches Group Tanriverdi Ozgur, Avci Nilufer, Oktay Esin, Kalemci Serdar, Pilanci Kezban Nur, Cokmert Suna, Menekse Serkan, Kocar Muharrem, Sen Cenk Ahmet, Akman Tulay, Ordu Cetin, Goksel Gamze, Meydan Nezih, Barutca Sabri. Asian Pacific Journal of Cancer Prevention.2015;16(14). CrossRef
- Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutrition Journal. 2010;9(1):69 .
- The C-Reactive Protein/Albumin Ratio, a Novel Inflammation-Based Prognostic Score, Predicts Outcomes in Patients with Hepatocellular Carcinoma Kinoshita Akiyoshi, Onoda Hiroshi, Imai Nami, Iwaku Akira, Oishi Mutumi, Tanaka Ken, Fushiya Nao, Koike Kazuhiko, Nishino Hirokazu, Matsushima Masato. Annals of Surgical Oncology.2014;22(3). CrossRef
- C-Reactive Protein to Albumin Ratio in Colorectal Cancer: A Meta-Analysis of Prognostic Value Zhou Qiang-ping, Li Xiu-jiang. Dose-Response.2019;17(4). CrossRef
- Pretreatment neutrophil-lymphocyte and platelet-lymphocyte ratio predict clinical outcome and prognosis for cervical Cancer Zhu MeiLin, Feng Min, He Fei, Han BangCai, Ma Ke, Zeng XinYu, Liu ZhiRong, Liu XinLian, Li Juan, Cao Hui, Liang YunDan, Jia Cui, Zhang LuShun. Clinica Chimica Acta.2018;483. CrossRef
- Lee YY, Choi CH, Kim HJ, Kim TJ, Lee JW, Lee JH, et al. Pretreatment neutrophil:lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Research. 2012;32(4):1555–61. .
- Pretreatment C-reactive protein/albumin ratio is associated with poor survival in patients with stage IB-IIA cervical cancer Zhang Weiwei, Liu Kejun, Ye Bin, Liang Weijiang, Ren Yazhou. Cancer Medicine.2017;7(1). CrossRef
- Pretreatment prognostic nutritional index is a significant predictor of prognosis in patients with cervical cancer treated with concurrent chemoradiotherapy Haraga Junko, Nakamura Keiichiro, Omichi Chiaki, Nishida Takeshi, Haruma Tomoko, Kusumoto Tomoyuki, Seki Noriko, Masuyama Hisashi, Katayama Norihisa, Kanazawa Susumu, Hiramatsu Yuji. Molecular and Clinical Oncology.2016;5(5). CrossRef
- Prognostic Value of Preoperative Systemic Immune-Inflammation Index in Patients with Cervical Cancer Huang Huaping, Liu Qin, Zhu Lixia, Zhang Yan, Lu Xiaojuan, Wu Yawei, Liu Li. Scientific Reports.2019;9(1). CrossRef
- Xiao Y, Ren YK, Cheng HJ, Wang L, Luo SX. Modified Glasgow prognostic score is an independent prognostic factor in patients with cervical cancer undergoing chemoradiotherapy. International Journal of Clinical and Experimental Pathology. 2015;8(5):5273–81. .
- Predictive value of hematological markers of systemic inflammation for managing cervical cancer Wang Lin, Jia Jing, Lin Lu, Guo Junying, Ye Xingming, Zheng Xiongwei, Chen Ying. Oncotarget.2017;8(27). CrossRef
- Locally advanced cervical cancer: A study of 5-year outcomes Chopra Supriya, Gupta Meetakshi, Mathew Ashwathy, Mahantshetty Umesh, Engineer Reena, Lavanya G, Gupta Sudeep, Ghosh Jaya, Thakur Meenakshi, Deodhar Kedar, Menon Santosh, Rekhi Bharat, Bajpai Jyoti, Gulia Seema, Maheshwari Amita, Kerkar Rajendra, Shylasree TS, Shrivastava SK. Indian Journal of Cancer.2018;55(1). CrossRef
- Balasubramaniam G, Gaidhani RH, Khan A, Saoba S, Mahantshetty U, Maheshwari A. Survival rate of cervical cancer from a study conducted in India. Indian Journal of Medical Sciences [Internet]. 2020 Dec 22 [cited 2021 Jun 29];0(0):1–10. Available from: https://ijmsweb.com/survival-rate-of-cervical-cancer-from-a-study-conducted-in-india/ .
- Pretreatment neutrophil to lymphocyte and platelet to lymphocyte ratios as predictive factors for the survival of cervical adenocarcinoma patients Jonska-Gmyrek Joanna, Gmyrek Leszek, Zolciak-Siwinska Agnieszka, Kowalska Maria, Fuksiewicz Malgorzata, Kotowicz Beata. Cancer Management and Research.2018;Volume 10. CrossRef
- Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis Ardi Veronica C., Kupriyanova Tatyana A., Deryugina Elena I., Quigley James P.. Proceedings of the National Academy of Sciences.2007;104(51). CrossRef
- Pleiotropic Effects of IL-2 on Cancer: Its Role in Cervical Cancer Valle-Mendiola Arturo, Gutiérrez-Hoya Adriana, Lagunas-Cruz María del Carmen, Weiss-Steider Benny, Soto-Cruz Isabel. Mediators of Inflammation.2016;2016. CrossRef
- Neutrophil lymphocyte ratio is significantly associated with complete response to chemoradiation in locally advanced cervical cancer Gangopadhyay Aparna. Acta Oncologica.2019;58(3). CrossRef
- The pretreatment platelet-to-lymphocyte ratio predicts clinical outcomes in patients with cervical cancer Ma Jian-ying, Ke Li-chi, Liu Qin. Medicine.2018;97(43). CrossRef
- The role of blood platelets in tumor angiogenesis Sabrkhany Siamack, Griffioen Arjan W., oude Egbrink Mirjam G.A.. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer.2011;1815(2). CrossRef
- C-Reactive Protein and Cancer—Diagnostic and Therapeutic Insights Hart Peter C., Rajab Ibraheem M., Alebraheem May, Potempa Lawrence A.. Frontiers in Immunology.2020;11. CrossRef
- Bodner-Adler B, Kimberger O, Schneidinger C, Kölbl H, Bodner K. Prognostic significance of pre-treatment serum c-reactive protein level in patients with adenocarcinoma of the uterine cervix. Anticancer Research. 2016;36(9):4691–6. .
- C-reactive protein is a prognostic parameter in patients with cervical cancer Polterauer S, Grimm C, Tempfer C, Sliutz G, Speiser P, Reinthaller A, et al . Gynecologic Oncology.2007;107(1). CrossRef
- Prognostic value of C-reactive protein/albumin ratio in predicting overall survival of Chinese cervical cancer patients overall survival: comparison among various inflammation based factors He Xia, Li Jian-Pei, Liu Xiao-Hua, Zhang Jing-Ping, Zeng Qiu-Yao, Chen Hao, Chen Shu-Lin. Journal of Cancer.2018;9(10). CrossRef
- Mileshkin LR, Narayan K, Moore KN, Rischin D, King M, Kolodziej I, et al. A phase III trial of adjuvant chemotherapy following chemoradiation as primary treatment for locally advanced cervical cancer compared to chemoradiation alone: Outback (ANZGOG0902/GOG0274/RTOG1174). Journal of Clinical Oncology. 2014 May 20;32(15_suppl):TPS5632–TPS5632. .
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2021
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times