The Change in Neutrophil-to-lymphocyte Ratio after Initiation of Nivolumab Monotherapy May be a Strong Marker of Response and Predictor of Prognosis in Advanced Non-small Cell Lung Carcinoma
Download
Abstract
Background: The neutrophil-to-lymphocyte ratio (NLR) is recognized as a predictive and prognostic biomarker in various malignancies. We investigated the utility of the NLR in patients with advanced non-small cell lung cancer (NSCLC) in the early phase of nivolumab monotherapy.
Methods: Thirty-one patients with advanced NSCLC were treated with nivolumab monotherapy from January 2016 to August 2017. They underwent the first response evaluation 8.3±3.3 weeks (mean±SD) after 3.8±1.8 times of administration. The NLR values at baseline (NLR/base) and at the first response evaluation (NLR/1st) were analyzed to evaluate for the association between NLR and the following parameters: treatment response, progression-free survival (PFS) and overall survival (OS).
Results: The median follow-up period was 467 days (range: 38-1903 days). NLR/1st in the disease control (DC) group (n=21, median: 4.36, range: 0.82-11.3) was significantly lower than that in the progression disease (PD) group (n=10, median: 11.91, range 2.04-31.00) (p<0.01). The median PFS and OS for all patients were 184 and 540 days, respectively. A higher NLR/1st resulted in a worse DC rate (OR 0.78, p<0.05), and was associated with shorter PFS (HR 1.11, p<0.005) and OS (HR 1.12, p<0.0005). A greater increase in NLR, from NLR/base to NLR/1st was associated with shorter PFS (HR 2.04, p<0.01) and OS (HR 1.66, p<0.05).
Conclusions: In NSCLC patients receiving nivolumab monotherapy, elevated NLR at the first response evaluation and its inclined change from baseline could be significantly stronger markers of poor response and predictors of worse prognosis than NLR at baseline.
Introduction
Immunotherapy with immune checkpoint inhibitors (ICIs) has become a promising treatment strategy for patients with advanced non-small cell lung cancer (NSCLC). Nivolumab is an anti-programmed cell death protein-1 (PD-1) antibody that has received regulatory approval for the treatment of advanced NSCLC patients, since it has been shown that treatment with nivolumab during or after platinum-based chemotherapy improves overall survival (OS) compared to treatment with docetaxel in phase Ⅲ trials [1-2]. However, these treatments are costly and might have various associated toxicities, and some patients treated with ICIs have only little benefit. Therefore, it is necessary to identify predictive biomarkers for treatment response. Recent studies have shown that the mediators and cellular effectors of inflammation are important constituents of the local environment of tumors, and immune cells are most commonly associated with tumor progression and prognosis [3-4]. The neutrophil- to-lymphocyte ratio (NLR) is an indicator of systemic inflammation and cell-mediated immunity [5]. Previous research has found that a pretreatment NLR ≥ 5 was significantly associated with poor prognosis compared to NLR < 5 [6-7]. Although the baseline NLR is useful, few studies have focused on the changes in NLR during a series of treatments and their prediction of treatment response and prognosis. In this study, we investigated the utility of NLR and its relative change during the early phase of nivolumab monotherapy in patients with advanced NSCLC.
Materials and Methods
Patients and data collection
We performed a retrospective analysis of the electronic records of patients with non-resectable NSCLC who were treated with nivolumab as monotherapy at the Tokyo Saiseikai Central Hospital from January 2016 to August 2017. The data included the following: age, sex, smoking history, Eastern Cooperative Oncology Group Performance Status (ECOG PS), pathological type, lines of prior therapy, programmed cell death protein ligand-1 (PD-L1) status, clinical or pathological stage, baseline complete blood count, molecular profiling for epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK), and history of radiation therapy. The patients received nivolumab at a standard dose of 3 mg/kg per cycle every two weeks. NLR was calculated by dividing the absolute neutrophil count with the lymphocyte count. We recorded NLR at the baseline (NLR/base) and at the first response evaluation (NLR/1st). The former was determined using the complete blood count (CBC) measured before the first cycle of treatment while the latter was determined using the CBC measured 8.3±3.3 weeks (mean ±SD) after 3.8±1.8 times of nivolumab administration. We analyzed the effect of NLR/base, NLR/1st, and NLR ratio (NLR/1st to NLR/base) on the initial treatment effect. Response to therapy was assessed by the treating physician and was classified as progressive disease (PD) or disease control (DC) including complete response (CR), partial response (PR), stable disease (SD) according to the Response Evaluation Criteria in Solid Tumors (version 1.1) (RECIST 1.1). Moreover, we evaluated for the association between each of these three factors and the PFS and OS.
Statistical analysis
We first investigated the effects of NLR/base, NLR/1st, and NLR ratio on disease control (DC) of initial treatment using the Wilcoxon signed-rank sum test, Mann-Whitney U test, and univariate logistic regression analysis.
Progression-free survival (PFS) was defined as the time between the first nivolumab treatment and radiographic or clinical progression according to RECIST 1.1. Overall survival (OS) was defined as the time between the first nivolumab treatment and death due to any cause.
To examine whether each factor contributes to OS and PFS, univariate Cox regression analysis and Kaplan- Meier method were employed. Statistical analyses were performed using BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan). Statistical significance was set at p <0.05.
Ethical approval
The Tokyo Saiseikai Central Hospital Institutional Review Board has approved this retrospective analysis (#RIN 28-73).
Results
Patient characteristics at baseline and outcomes
Thirty-one patients were included, and their baseline characteristics are as follows. The median age was 68 years (range:44-84 years). Altogether, 24 patients (77.4%) were men, 27 patients (87.1%) had a smoking history, and 4 patients (12.9%) had an ECOG PS of 2 or higher. Twenty- three patients (74.2%) had non-squamous histology, and 16 patients (51.6%) were diagnosed with stage IV cancer. Two patients received nivolumab treatment as initial chemotherapy, 16 patients (51.6%) received one prior systemic chemotherapy, and 13 patients (41.9%) had two or more prior systemic chemotherapies. Fourteen patients (45.1%) received radiation therapy. In terms of the tumor PD-L1 expression, 8 patients (25.8%) had more than 50% expression, 4 patients (12.9%) had between 1-49% expression, 9 patients (29.0%) had no expression, and the other patients (32.3%) did not measure PD-L1 expression. Oncogenic alterations were detected in 3 patients who had EGFR mutations (Table 1).
| Age (years) | Median (Range) | 68 |
| Number | ||
| Gender | Male | 24 |
| Female | 7 | |
| Smokig history | Smoker | 27 |
| Never smoker | 4 | |
| ECOG PS | 0 or 1 | 27 |
| 2 | 2 | |
| 3 | 2 | |
| Histology | Adenocarcinoma | 18 |
| Squamous cell carcinoma | 8 | |
| Others | 5 | |
| Tumor Stage | ⅢA | 3 |
| ⅢB | 9 | |
| Ⅳ | 16 | |
| Recurrent | 3 | |
| Lines of therapy | 1 | 2 |
| 2 | 16 | |
| 3 | 4 | |
| 4 | 3 | |
| 5 | 5 | |
| 6 | 1 | |
| Radiation therapy | Undergone | 14 |
| None | 17 | |
| PD-L1 expression, % | 0 | 9 |
| 1-4 | 0 | |
| 5-9 | 1 | |
| 10-49 | 3 | |
| 50≤ | 8 | |
| Not examined | 10 | |
| Targetable driver mutations | EGFR | 3 |
| ALK | 0 | |
| None | 24 | |
| Not examined | 4 | |
| Response | CR | 0 |
| PR | 6 | |
| SD | 15 | |
| PD | 10 |
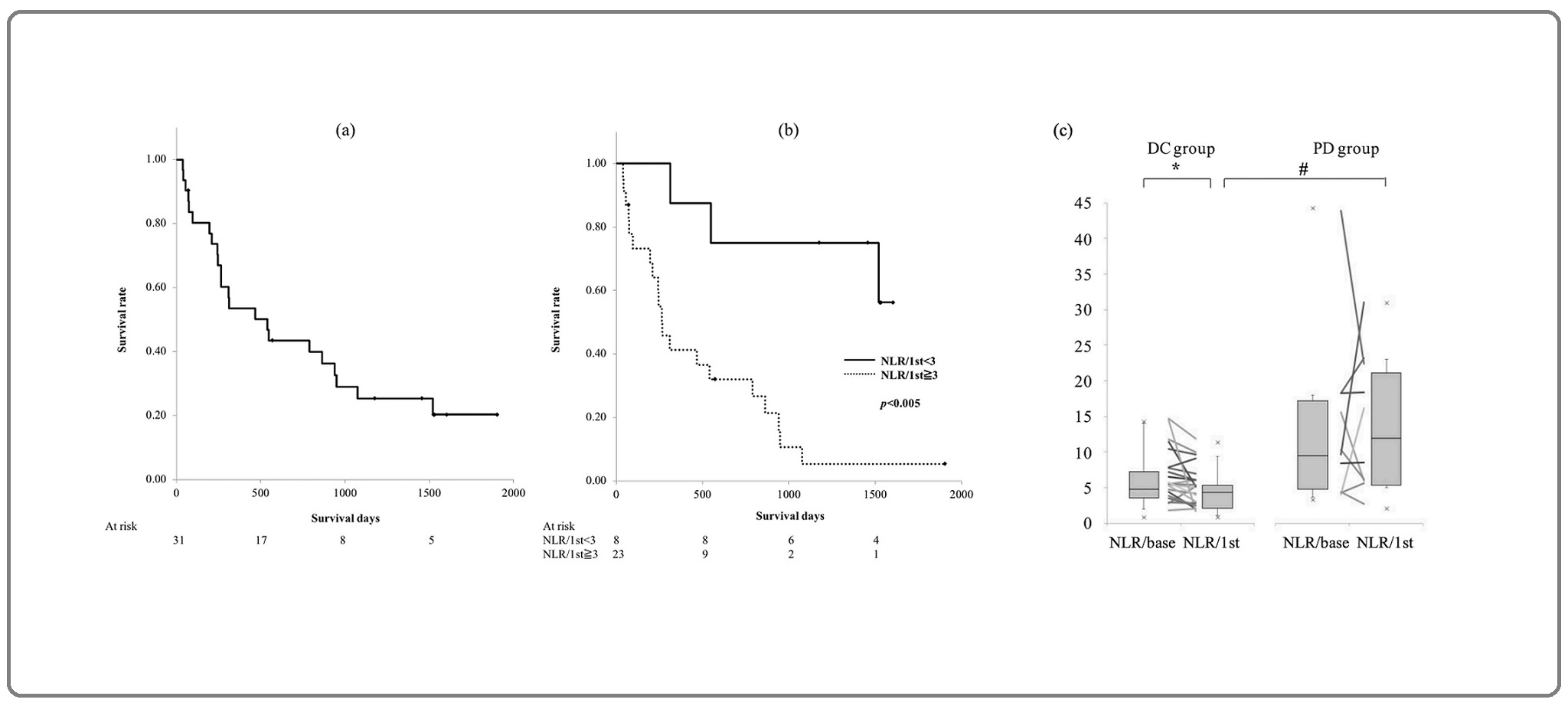
The patients underwent the first evaluation of treatment response 8.3±3.3 weeks (mean±SD) after 3.8±1.8 times of nivolumab administration. Complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) rates were 0%, 19.4% (6 patients), 48.4% (15 patients), and 32.2% (10 patients), respectively (Table 1). The rate of disease control (DC), including PR and SD, was 67.8% (21 patients). For the total population, median PFS and OS were 184 days (interquartile range: 63-325 days) and 540 days (interquartile range: 215-1521 days), respectively. The five-year survival rate was 20.2% (Figure 1a).
Figure 1. Kaplan-Meier Survival Curves for Overall Survival (OS). (a) All patients, (b) solid line, patients with neutrophil-to-lymphocyte ratio assessed at the first response evaluation (NLR/1st) <3; dashed line, patients with NLR/1st ≥ 3, p < 0.005 by log-rank test. (c) Neutrophil-to-lymphocyte ratio (NLR) in disease control (DC) and progressive disease (PD) groups. Median with interquartile range. NLR/base: NLR assessed at the baseline, *p<0.01 by Wilcox signed-rank test, #p<0.01 by Mann-Whitney U test.
NLR at baseline
There was no significant difference between the NLR/ base of the DC and the PD groups (median, range: 4.77, 0.86-14.3 vs 9.43, 3.33-44.3) (Figure 1c). A higher NLR/ base was associated with shorter OS (hazard ratio [HR] 1.04, 95% confidence interval [CI] 1.00-1.09, p<0.05) (Table 2).
| DCR | PFS | OS | |||||||
| OR | (95% CI) | p-value | HR | (95% CI) | p-value | HR | (95% CI) | p-value | |
| NLR/base | 0.85 | (0.71-1.00) | 0.055 | 1.03 | (0.98-1.08) | 0.258 | 1.04 | (1.00-1.09) | <0.05 |
| NLR/1st | 0.78 | (0.63-0.96) | <0.05 | 1.11 | (1.04-1.18) | <0.005 | 1.12 | (1.06-1.19) | <0.0005 |
| NLR ratio (NLR/1st to NLR/base) | |||||||||
| NLR/1st>5 | 0.44 | (0.15-1.30) | 0.435 | 2.04 | (1.23-3.36) | <0.01 | 1.66 | (1.04-2.65) | <0.05 |
| NLR/1st>3 | 0.15 | (0.03-0.91) | <0.05 | 2.59 | (0.84-8.06) | 0.099 | 6.12 | (1.75-21.5) | <0.005 |
| Change in NLR/base to NLR/1st | |||||||||
| Increase | 0.16 | (0.03-0.83) | <0.05 | 2.08 | (0.82-5.31) | 0.125 | 1.75 | (0.74-4.16) | 0.203 |
ECOG PS, Eastern Cooperative Oncology Group Performance Status; PD-L1, programmed cell death protein ligand-1; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NLR, neutrophil-to-lymphocyte ratio; NLR/base, NLR at the baseline; NLR/1st, NLR at the first response evaluation; DCR, disease control rate; OR, odds ratio; 95% CI, 95% confidence interval; PFS, progression-free survival; HR, hazard ratio; OS, overall survival.
NLR at the first evaluation
NLR/1st in the DC group was significantly lower than that in the PD group (median, range: 4.36, 0.82-11.3 vs 11.9, 2.04-31.0; p<0.01). In the DC group, NLR/1st was significantly lower than NLR/base (median, range: 4.36, 0.82-11.3 vs 4.77, 0.86-14.3; p<0.01) (Figure 1c). A higher NLR/1st resulted in a lower DC rate (odds ratio [OR] 0.78, 95%CI 0.63-0.96, p<0.05) and was associated with shorter PFS (HR 1.11, 95% CI 1.04-1.18, p<0.005) and OS (HR 1.12, 95% CI 1.06-1.19, p<0.0005). An increase in NLR/base to NLR/1st and an NLR/1st>5 were both associated with a worse DC rates (OR 0.16, 95% CI 0.03- 0.83, p<0.05, and OR 0.15, 95% CI 0.03-0.91, p<0.05, respectively). A higher NLR ratio (NLR/1st to NLR/base) was associated with a shorter PFS (HR 2.04, 95% CI 1.23-3.36, p<0.01) and OS (HR 1.66, 95% CI 1.04-2.65, p<0.05). NLR/1st>3 was associated with a shorter OS (HR 6.12, 95% CI 1.75-21.5, p<0.005) (Table 2, Figure 1b).
Discussion
In this study, we investigated the predictive and prognostic value of NLR at the initiation of nivolumab monotherapy in patients with advanced NSCLC. Interestingly, we discovered that NLR at the first response evaluation and the change in NLR during treatment from baseline may be more useful markers to assess early response and predict both progression and prognosis than NLR at baseline.
PD-L1 expression is the widely approved and most studied biomarker for immunotherapy with ICIs in advanced NSCLC; however, it is limited by many biological and technical problems in practice. In addition, some patients with high PD-L1 expression have poor therapeutic benefit from this treatment while low or negative PD-L1 has been shown to respond to immunotherapy. Therefore, its predictive role remains unclear [8]. Predictors of the prognosis and therapeutic effect of treatment with ICIs are currently being explored. Among these predictors is the NLR, which can be easily measured and calculated from existing routine laboratories in most medical institutions. Moreover, it can be obtained with a simple white blood cell count and routine peripheral blood draw, which are relatively inexpensive. Owing to these characteristics of NLR, it can be conveniently monitored in a timely manner.
It has been reported that in the treatment with ICIs, NLR can be a predictor of prognosis and therapeutic effect not only at the start of treatment but also thereafter [9-10]. The baseline NLR value would stratify patients who will respond to ICIs, but the change in NLR could be a more useful biomarker. However, further study would be needed to validate the latter. In the past, tumor characteristics have been the focus of the response to ICIs, but host immunity also plays an important role in immunotherapy. The NLR is a biological marker of systemic inflammation and potentially reflects the balance of the immune system. It is also thought to reflect this balance in the context of a malignancy; a high NLR reflects the depletion of lymphocytes and impairment of the host immune response to cancer [9]. Inflammation induces cancer, and the increase in neutrophils could be a reflection of an inflammatory microenvironment that promotes the survival and proliferation of cancer cells [3]. Lymphocytes, on the other hand, have immunosuppressive activity against tumors, and their presence, especially in the tumor microenvironment, is thought to reflect host immunity [11]. This pathway consists of the secretion and release of various chemokines and cytokines, such as helper T-cells producing interleukin-17, which promotes neutrophil migration and the differentiation of tissue macrophages in the peritumor region into tumor-associated macrophages (TAM) [12]. Moreover, this was suggested to contribute to the mechanism of resistance to PD-1 blockade [13]. The decline in NLR before and after treatment may reflect a microenvironment that favors the activation of cellular immunity or the manifestation of ICIs. During treatment with ICIs, a therapeutic response may appear after tumor growth or the appearance of new lesions (called pseudo-progression), and it is important to distinguish between pseudo and true progression. The frequency of pseudoprogression was reported at 3% of all 542 NSCLC patients treated with nivolumab monotherapy and 5% of patients with advanced disease [14]. Chemotherapy can regulate antitumor T cell response and induce tumor cell death eliciting systemic and intratumoral immune responses, contributing to the antitumor immunity. Considering this synergistic effect of chemotherapy after ICIs, it is important to identify true progressive disease and move to an alternative regimen early. In a case-control study, it has been reported that patients with high post-treatment NLR have a true progressive disease [15]. This suggests that the changes in the NLR overtime may be useful in distinguishing between pseudo and true progression.
There are several limitations to our study, including its retrospective nature and the heterogeneous patient status. Most patients received nivolumab as second-line treatment while some received first-line treatment and third-line or later treatment, so potential selection bias may be latent. Survival analysis was performed after the start of nivolumab administration, but the follow-up period may be inadequate. The correlation between NLR and PD-L1 expression is unclear, as three-quarters of patients have not undergone PD-L1 testing. This was an observational study conducted in a single facility with a small number of patients, and the results cannot be regarded as definitive. In practice, various causes, including bone marrow activity, infection, and malnutrition, drive the decline of NLR by the decrease in neutrophils, which could impact prognosis on their own. Further studies regarding the relationship between NLR change and tumor microenvironment, medication interactions, infection, nutrition status, microbiota, line of therapy, and immune-related adverse events need to be done. Prospective studies with a larger patient cohort are also needed for validation. However, the correlation between the changes in NLR after initiation of nivolumab monotherapy and the treatment response or prognosis is statistically significant and may be worth exploring.
In conclusions, in NSCLC patients receiving nivolumab, elevated NLR at the first response evaluation and an inclined change in NLR during treatment from baseline may be useful markers to assess early response and predict both progression and prognosis. This study is retrospective and the correlation between NLR and PD-L1 expression is unclear. However, this NLR can be easily measured and may be useful in distinguishing between pseudo and true progression. Moreover, these might have the potential to aid clinical decision-making for continuing immunotherapy.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors. The authors declare no conflict of interest. We would like to thank Editage (www.editage.com) for English language editing.
References
- Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer Borghaei Hossein, Paz-Ares Luis, Horn Leora, Spigel David R., Steins Martin, Ready Neal E., Chow Laura Q., Vokes Everett E., Felip Enriqueta, Holgado Esther, Barlesi Fabrice, Kohlhäufl Martin, Arrieta Oscar, Burgio Marco Angelo, Fayette Jérôme, Lena Hervé, Poddubskaya Elena, Gerber David E., Gettinger Scott N., Rudin Charles M., Rizvi Naiyer, Crinò Lucio, Blumenschein George R., Antonia Scott J., Dorange Cécile, Harbison Christopher T., Graf Finckenstein Friedrich, Brahmer Julie R.. New England Journal of Medicine.2015;373(17). CrossRef
- Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer Brahmer Julie, Reckamp Karen L., Baas Paul, Crinò Lucio, Eberhardt Wilfried E.E., Poddubskaya Elena, Antonia Scott, Pluzanski Adam, Vokes Everett E., Holgado Esther, Waterhouse David, Ready Neal, Gainor Justin, Arén Frontera Osvaldo, Havel Libor, Steins Martin, Garassino Marina C., Aerts Joachim G., Domine Manuel, Paz-Ares Luis, Reck Martin, Baudelet Christine, Harbison Christopher T., Lestini Brian, Spigel David R.. New England Journal of Medicine.2015;373(2). CrossRef
- Cancer-related inflammation Mantovani Alberto, Allavena Paola, Sica Antonio, Balkwill Frances. Nature.2008;454(7203). CrossRef
- Cancer-related inflammation and treatment effectiveness Diakos Connie I., Charles Kellie A., McMillan Donald C., Clarke Stephen J.. The Lancet. Oncology.2014;15(11). CrossRef
- Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab Diem Stefan, Schmid Sabine, Krapf Mirjam, Flatz Lukas, Born Diana, Jochum Wolfram, Templeton Arnoud J., Früh Martin. Lung Cancer (Amsterdam, Netherlands).2017;111. CrossRef
- Activity of Nivolumab and Utility of Neutrophil-to-Lymphocyte Ratio as a Predictive Biomarker for Advanced Non-Small-Cell Lung Cancer: A Prospective Observational Study Fukui Tomoya, Okuma Yuriko, Nakahara Yoshiro, Otani Sakiko, Igawa Satoshi, Katagiri Masato, Mitsufuji Hisashi, Kubota Masaru, Hiyoshi Yasuhiro, Ishihara Mikiko, Kasajima Masashi, Sasaki Jiichiro, Naoki Katsuhiko. Clinical Lung Cancer.2019;20(3). CrossRef
- Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer Bagley Stephen J., Kothari Shawn, Aggarwal Charu, Bauml Joshua M., Alley Evan W., Evans Tracey L., Kosteva John A., Ciunci Christine A., Gabriel Peter E., Thompson Jeffrey C., Stonehouse-Lee Susan, Sherry Victoria E., Gilbert Elizabeth, Eaby-Sandy Beth, Mutale Faith, DiLullo Gloria, Cohen Roger B., Vachani Anil, Langer Corey J.. Lung Cancer (Amsterdam, Netherlands).2017;106. CrossRef
- Predictive biomarkers of response for immune checkpoint inhibitors in non-small-cell lung cancer Prelaj Arsela, Tay Rebecca, Ferrara Roberto, Chaput Nathalie, Besse Benjamin, Califano Raffaele. European Journal of Cancer (Oxford, England: 1990).2019;106. CrossRef
- Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma Lalani Aly-Khan A., Xie Wanling, Martini Dylan J., Steinharter John A., Norton Craig K., Krajewski Katherine M., Duquette Audrey, Bossé Dominick, Bellmunt Joaquim, Van Allen Eliezer M., McGregor Bradley A., Creighton Chad J., Harshman Lauren C., Choueiri Toni K.. Journal for Immunotherapy of Cancer.2018;6(1). CrossRef
- The time-series behavior of neutrophil-to-lymphocyte ratio is useful as a predictive marker in non-small cell lung cancer Kiriu Tatsunori, Yamamoto Masatsugu, Nagano Tatsuya, Hazama Daisuke, Sekiya Reina, Katsurada Masahiro, Tamura Daisuke, Tachihara Motoko, Kobayashi Kazuyuki, Nishimura Yoshihiro. PloS One.2018;13(2). CrossRef
- The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis Gooden M. J. M., Bock G. H., Leffers N., Daemen T., Nijman H. W.. British Journal of Cancer.2011;105(1). CrossRef
- Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment Motomura Takashi, Shirabe Ken, Mano Yohei, Muto Jun, Toshima Takeo, Umemoto Yuichiro, Fukuhara Takasuke, Uchiyama Hideaki, Ikegami Toru, Yoshizumi Tomoharu, Soejima Yuji, Maehara Yoshihiko. Journal of Hepatology.2013;58(1). CrossRef
- Interleukin-17A Promotes Lung Tumor Progression through Neutrophil Attraction to Tumor Sites and Mediating Resistance to PD-1 Blockade Akbay Esra A., Koyama Shohei, Liu Yan, Dries Ruben, Bufe Lauren E., Silkes Michael, Alam Md Maksudul, Magee Dillon M., Jones Robert, Jinushi Masahisa, Kulkarni Meghana, Carretero Julian, Wang Xiaoen, Warner-Hatten Tiquella, Cavanaugh Jillian D., Osa Akio, Kumanogoh Atsushi, Freeman Gordon J., Awad Mark M., Christiani David C., Bueno Raphael, Hammerman Peter S., Dranoff Glenn, Wong Kwok-Kin. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer.2017;12(8). CrossRef
- Pseudoprogression in Previously Treated Patients with Non-Small Cell Lung Cancer Who Received Nivolumab Monotherapy Fujimoto Daichi, Yoshioka Hiroshige, Kataoka Yuki, Morimoto Takeshi, Hata Tae, Kim Young Hak, Tomii Keisuke, Ishida Tadashi, Hirabayashi Masataka, Hara Satoshi, Ishitoko Manabu, Fukuda Yasushi, Hwang Moon Hee, Sakai Naoki, Fukui Motonari, Nakaji Hitoshi, Morita Mitsunori, Mio Tadashi, Yasuda Takehiro, Sugita Takakazu, Hirai Toyohiro. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer.2019;14(3). CrossRef
- Pseudo-Progression and the Neutrophil-to-Lymphocyte Ratio in Non-Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors: A Case-Control Study Kiriu Tatsunori, Yamamoto Masatsugu, Nagano Tatsuya, Hazama Daisuke, Sekiya Reina, Katsurada Masahiro, Katsurada Naoko, Tachihara Motoko, Kobayashi Kazuyuki, Nishimura Yoshihiro. OncoTargets and Therapy.2019;12. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2022
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times