Demography and Outcome of Cancer Patients with COVID-19: A Retrospective Cohort Study in Dharmais National Cancer Center Indonesia
Abstract
Introduction: Cancer patients have an increased risk of morbidity and mortality due COVID-19 partly due to their immunocompromised status. We aimed to investigate the associations of clinicopathological factors and survival outcomes in cancer patients with COVID-19.
Methods: This was a retrospective cohort study comprised of cancer patients treated in Dharmais National Cancer Center, Indonesia. Main inclusion criteria were pathologically confirmed malignancy with positive results of RTPCR COVID-19 tests.
Results: A total of 16,511 visitors had visited and registered for RTPCR test in Dharmais National Cancer Center from May 2020 to January 2021. Logistic regression showed that male gender (p-value = 0.019; OR = 1.732), haematological type of malignancy (p-value <0.001; OR = 3.073), patients not underwent cancer therapy (p-value = 0.008; OR = 0.485), low RTPCR Ct values (p-value = <0.001; OR = 3.340), poor performance status (p-value = <0.001; OR = 8,194), and disease severity (p-value = <0.001; OR = 5.448) were associated with mortality.
Conclusion: Overall mortality rate in Dharmais cancer patients (25%) was higher than other cancer patients treated in other hospitals in Asia. Moreover, the mortality rate was similar across all age groups. Poor survival in young age might be explained by the fact that median age of cancer patients was 46 years old. In addition to male gender, cancer patients with low Ct values and having delayed cancer treatment were vulnerable groups of having poor outcomes when diagnosed with COVID-19. Long-term follow-up is required to examine the survival rate in cancer patients with COVID-19.
Introduction
Currently, the world is struggling with a global pandemic of coronavirus disease 2019 (COVID-19). World Health Organization (WHO) stated it as a pandemic on March 11, 2020 [1]. Globally, there were 190,671,330 confirmed cases of COVID-19, including 4,098,758 deaths, reported to WHO on July 21, 2021 [2]. During this period, Indonesia had recorded more than 1 million positive cases and 76,200 death cases [3].
While worldwide preventive measures have been ongoing, number of new cases continue to rise and pose persisting threat to all populations, including cancer patients. Patients with cancer had a high risk of contracting severe COVID-19 and with a poorer prognosis than those without cancer [4].
During the pandemic, cancer patients are caught as a unique group with an increased risk of contracting COVID-19 partly due to their immunocompromised condition [4]. Their weakened immunity is resulting from many factors including the malignancy burden and ongoing treatment such as surgery, chemotherapy, radiotherapy, or even due to patients undergoing transplant and use of immunosuppressants [5]. This pandemic has created dilemmas and unpredictable impacts on cancer management which include diagnostic work-ups, treatment schedules, and follow-up visits. The isolation of patients infected with COVID-19 has caused losing valuable clinic visits. New patients could miss an early diagnosis which may lead to worse prognosis. In addition, patients with advanced cancer may have to postpone treatments risking disease progression [6].
Low-and middle-income countries (LMIC) with ailing healthcare systems are more vulnerable to contain the pandemic, especially in efforts to manage cancer patients. Dharmais National Cancer Center in Indonesia has deployed strict mitigation system in order to minimize exposure of viral transmission to healthcare providers and patients to and to optimize cancer management [7]. Our current study analyzed associations of clinical characteristics and outcomes of cancer patients with COVID-19 in Dharmais.
Materials and Methods
Study design and participants
This was a retrospective cohort study to estimate demographic and clinical factors associated with outcome of cancer patients with COVID-19 in National Cancer Center, Indonesia. COVID-19 incidents were obtained from SARS-CoV-2 reverse transcribed polymerase chain reaction (RTPCR) test records of patients registered in medical database of Dharmais Cancer Hospital between May 01, 2020 to January 31, 2021. Based on Indonesia’s national COVID-19 management guidelines [8], Dharmais conducted confirmatory SARS-CoV-2 RTPCR test on nasopharyngeal and/or oropharyngeal swabs specimens in the laboratory. Cancer patients were discharged or proceeded to cancer treatment after demonstrating negative test results of two consecutive RTPCR tests, or being declared free of COVID-19 by pulmonologist-in- charge.
The population in this study were all patients who were treated in and registered at the Dharmais Cancer Hospital. The eligibility criteria were (1) patients with confirmed cancer diagnosis by pathologists or other supporting results in medical records and (2) had positive SARS-CoV-2 RTPCR-positive tests. Patients with suspected or absent of malignancy were excluded.
Data collection and variables
Data were extracted from hospital medical record of RTPCR test results. All patients with RTPCR-positive test results were taken and then evaluated by pathologists to confirm cancer diagnosis. Subsequently, patient demographics (age and gender), clinical characteristics, cancer management, clinical history of COVID-19 treatment, and outcomes were collected. The clinical characteristics included type of malignancy, symptoms, comorbidity, performance status, RTPCR Cycle Threshold Value (Ct value), and disease severity. We categorized cancer treatment status into treatment naïve and previously treated patients and type of cancer management (diagnostic biopsy, evaluation, stabilized clinical condition, as well as having targeted therapy, chemotherapy, surgery, radiotherapy, chemoradiation, and others). Lastly, clinical outcome indicated the number of patients who survived or died. Death cases were patients who died of any cause within 30 days of confirmed COVID-19 diagnosis.
Symptoms and comorbidities were collected from patient self-questionnaires. We used The Eastern Cooperative Oncology Group (ECOG) score to measure performance status cancer patients with COVID-19. The ECOG score was ran from 0 to 5, with 0 denoting perfect health and 5 death [9].
This study distinguished in-patients and out-patients to determine the differences in demographic and clinical characteristics of patients between these groups. In-patients indicated that the patient was infected with COVID-19 while undergoing treatment in the hospital wards. On the other hand, outpatients were all patients who had any symptoms of COVID-19, confirmed by RTPCR who were about to undergo any cancer management modalities. Cancer therapy was divided into two categories i.e. having chemotherapy or no chemotherapy. Surgery, radiotherapy, diagnostics, and treatment for stabilized clinical conditions were included in the non-chemotherapy category.
We also measured COVID-19 disease severity. The Ministry of Health Indonesia classified the disease severity of COVID-19 into four groups, namely asymptomatic or mild, moderate, severe, and critical conditions. Mild symptom is a condition without evidence of viral pneumonia or without hypoxia. Symptoms include fever, cough, fatigue, anorexia, shortness of breath, myalgia. Other non-specific symptoms such as sore throat, nasal congestion, headache, diarrhea, nausea and vomiting, smell (anosmia), or loss of taste (ageusia) [10].
Moderate symptoms in adolescent or adult patients included pneumonia (fever, cough, shortness of breath, rapid breathing) but no signs of severe pneumonia including SpO2> 93% with room air. In children, it showed symptoms of non-severe pneumonia (coughing or difficulty breathing, rapid breathing, and/or chest wall traction) and no signs of severe pneumonia [10].
In adolescent or adult patients, severe symptoms showed the symptoms of pneumonia (fever, cough, shortness of breath, rapid breathing) and/or respiratory rate > 30 bpm, severe respiratory distress, or SpO2 <93% in room air. Lastly, critical symptoms comprised of Acute Respiratory Distress Syndrome (ARDS), sepsis, and septic shock [10].
There were two types of PCR assay available at our hospital, namely open system and closed system PCR. Open system PCR in our hospital is using QIAsymphony DSP Virus/Pathogen mini kit (Qiagen, Germany) for extraction reagent kit, LightMix Modular SARS-CoV-2 (COVID-19) (Roche, Germany) for amplification reagent kit, and Rotor Gene Q (Qiagen, Germany) for real time PCR cycler. Target genes for this open system PCR were E and RdRp gene as target genes with CT cut off point 36 and 41 respectively. We used two type of closed system PCR, that are Abbott Real Time SARS-CoV-2 assay (Abbott, USA) and GeneXpert (Cepheid, USA). Abbott Real Time SARS-CoV-2 assay was real time assay performed on Abbott m2000 system for extraction (m2000sp) and amplification (m2000rt). The Abbott assay used RdRP and N genes as target genes with CT cut off point 31.6. GeneXpert used the E and N2 gene targets with CT cut off point 45 each. We used Abbott PCR assay as the main PCR assay. GeneXpert is used for cases requiring fast result (within 2 hours) such as deteriorating patients who require ICU, patients who need immediate medical intervention, or post mortem COVID-19 status determination.
Statistical analysis
We used descriptive statistics and bivariate analysis. Median and range (min-max) was evaluated for the continuous variables, while the proportion for the categorical variables used to describe the data. We differentiated the proportion of demographics, clinical characters patients in inpatients and outpatients, and death from any cause within 3 months of observation using crosstabulation and chi-square test. Besides, for continous variables using independent samples t-test. The association independent variables with outcome patient were assessed by logistic regression. All analyses were two-sided and the significance level was set at p < 0.05.
Ethical Statement
This study was approved by the Ethical Committee of the Dharmais National Cancer Center (approval No.: 0149/KEPK/X/2020).
Results
A total of 16,511 visitors had visited and registered for RTPCR test in Dharmais National Cancer Center from May 2020 to January 2021 with the results 878 visitors came positive and 15,633 were negative. There were 403 cancer patients with COVID-19 whereby 170 (42.2%) were inpatients and 233 (57.8%) were outpatients. Patient`s demography and clinical characteristics were described, see Table 1.
| Variables | Total (%) (N = 403) | Inpatient (%) (N = 170) | Outpatient (%) (N = 233) |
| Median Age, (min-max) years | 46 (5 - 73) | 46 (5 - 73) | 48 (7 - 77) |
| Gender | |||
| Male | 148 (36.7) | 78 (52.7) | 70 (47.3) |
| Female | 225 (63.3) | 92 (36.1) | 163 (63.9) |
| Type of malignancy | |||
| Solid tumors | 339 (84.1) | 124 (36.6) | 215 (63.4) |
| Breast cancer | 110 (27.3) | 30 (27.3) | 80 (72.7) |
| Lung cancer | 28 (6.9) | 16 (57.1) | 12 (42.9) |
| Nasopharyngeal cancer | 22 (5.5) | 9 (40.9) | 13 (59.1) |
| Cervical cancer | 28 (6.9) | 9 (32.1) | 19 (67.9) |
| Colorectal cancer | 36 (8.9) | 11 (30.6) | 25 (69.4) |
| Others | 112 (27.8) | 48 (42.9) | 64 (57.1) |
| Hematology | 64 (15.9) | 46 (71.9) | 18 (28.1) |
| ALL | 13 (3.2) | 10 (76.9) | 3 (23.1) |
| Lymphoma | 22 (5.5) | 10 (45.5) | 12 (54.5) |
| AML | 18 (4.5) | 15 (83.3) | 3 (16.7) |
| Others | 14 (3.5) | 12 (85.7) | 2 (14.3) |
| Symptoms | |||
| Asymptomatic | 180 (44.7) | 15 (8.3) | 165 (91.7) |
| Symptomatic | 223 (55.3) | 155 (69.5) | 68 (30.5) |
| Respiratory | 167 (41.4) | 115 (68.9) | 52 (31.1) |
| Gastrointestinal (GI) | 12 (3.0) | 6 (50.0) | 6 (50.0) |
| Respiratory and GI | 36 (8.9) | 32 (88.9) | 4 (11.1) |
| Others | 8 (2.0) | 2 (25.0) | 6 (75.0) |
| Comorbidity | |||
| Malignancy alone | 319 (79.2) | 136 (42.6) | 183 (57.4) |
| Additional comorbidity | |||
| Hypertension | 44 (10.9) | 18 (40.9) | 26 (59.1) |
| Heart failure | 7 (1.7) | 4 (57.1) | 3 (42.9) |
| Diabetes | 10 (2.5) | 4 (40.0) | 6 (60.0) |
| Multiple comorbidity | 23 (5.7) | 8 (34.8) | 15 (65.2) |
| Ct value (median, min-max)* | 17.17 (2.81 – 31.33) | 14.38 (2.81-30.95) | 18.00 (3.42-31.33) |
| Therapy status | |||
| Treatment naïve | 168 (41.7) | 93 (55.4) | 75 (44.6) |
| Previously treated | 74 (18.4) | 41 (55.4) | 33 (44.6) |
| Ongoing therapy | 161 (40.0) | 99 (61.5) | 62 (38.5) |
| Cancer management | |||
| Diagnostic biopsy | 58 (14.4) | 18 (31.0) | 40 (69.0) |
| Routine Evaluation | 36 (8.9) | 5 (13.9) | 31 (86.1) |
| Stabilized clinical condition | 48 (11.9) | 45 (93.8) | 3 (6.3) |
| Targeted therapy | 1 (0.2) | 1 (100.0) | 0 (0.0) |
| Chemotherapy | 122 (30.3) | 41 (33.6) | 81 (66.4) |
| Surgery | 31 (7.7) | 21 (67.7) | 10 (32.3) |
| Radiotherapy | 35 (8.7) | 12 (34.3) | 23 (65.7) |
| Chemoradiation | 6 (1.5) | 4 (66.7) | 2 (33.3) |
| Others | 66 (16.4) | 23 (34.8) | 43 (65.2) |
| Performance status (ECOG) | |||
| 0 | 103 (25.6) | 10 (9.7) | 93 (90.3) |
| 1 | 138 (34.2) | 42 (30.4) | 96 (69.6) |
| 2 | 92 (22.8) | 57 (62.0) | 35 (38.0) |
| 3 | 48 (11.9) | 41 (85.4) | 7 (14.6) |
| 4 | 22 (5.5) | 20 (90.9) | 2 (9.1) |
| COVID-19 disease grade | |||
| Asymptomatic | 180 (44.7) | 15 (8.3) | 165 (91.7) |
| Mild | 69 (17.1) | 6 (8.7) | 63 (91.3) |
| Moderate | 54 (13.4) | 52 (96.3) | 2 (3.7) |
| Severe | 96 (23.8) | 93 (96.9) | 3 (3.1) |
| Critical | 4 (1.0) | 4 (100.0) | 0 (0.0) |
| Outcomes | |||
| Survive | 302 (74.9) | 78 (25.8) | 224 (74.2) |
| Deceased | 101 (25.1) | 92 (91.1) | 9 (8.9) |
*based on Abbott Ct value
The median age was 46 years; 84.1% of the patients had a solid tumor and breast cancer was the most frequent malignancy. Approximately, half of patients had respiratory symptoms of COVID-19 and near one-fifth of patients had other comorbidities besides malignancy.
The median Ct values of RTPCR positive results were 16.98, Table 2. Most patients were not under chemotherapy program (68.5%). 61% of patients had mild to asymptomatic COVID-19, 59% had ECOG performance score 0-1, and 25% of patients died of any cause.
| Ct value | n | Ct value Cut off | Mean | Median | Min - Max |
| Abbott* | 365 | 31.6 | 16.52 | 17.17 | 2.81 – 31.33 |
| GeneXpert | 8 | ||||
| E | 45 | 27.4 | 26.85 | 16.20 – 42.80 | |
| N2 | 45 | 30.1 | 29.4 | 18.70 – 38.80 | |
| Qiagen | 20 | ||||
| E | 36 | 25.48 | 27.78 | 10.90 – 33.47 | |
| RdRP | 41 | 28.75 | 31.37 | 11.49 – 38.63 |
*frequently-used
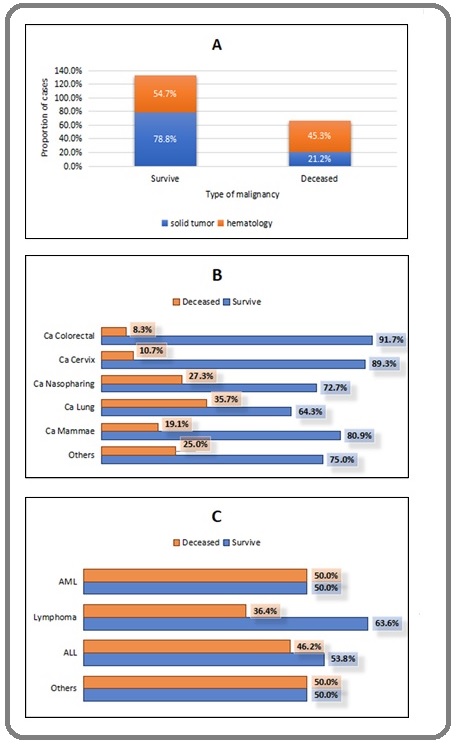
Male patients were mostly in-patients while female patients were in outpatients. Haematological cancer especially Acute myeloid leukemia (AML) had higher proportions of in-patients care than solid tumor. Most common symptoms of in-patients COVID-19 cancer patients were respiratory (cough, shortness of breath, fever) and gastrointestinal (nausea, vomiting, and diarrhea). The inpatients disease severity and mortality were also higher than outpatients. Mortality rate in haematological cancer was also higher than solid tumors. Based on type of malignancy, hematological mortality was higher than solid tumors, lung cancer and nasopharyngeal cancer were the highest deaths in solid tumors, AML was the highest mortality in hematology (Figure 1).
Figure 1. Outcome of Cancer Patients with COVID-19. Based on Type of Malignancy, Hematological Mortality was Higher than Solid Tumors (A), Lung Cancer and Nasopharyngeal Cancer were the Highest Deaths in Solid Tumors (B), AML was the Highest Mortality in Hematology (C).
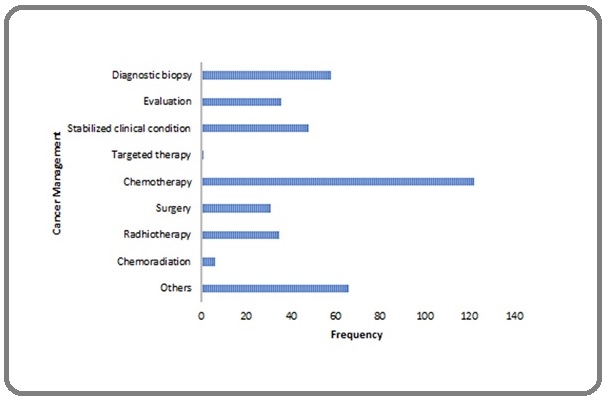
Chemotherapy was the most frequent modality of cancer management (Figure 2).
Figure 2. Cancer Management in COVID-19 Patients. Chemotherapy is the most common cancer management when patients diagnosed with COVID-19.
Logistic regression showed that gender (p-value = 0.019; OR = 1.732, 95% CI OR = 1.095-2.739), type malignancy (p-value = <0.001; OR = 3.073, 95% CI OR = 1.761-5.362), Ct value (p-value = <0.001; OR = 3.340, 95% CI OR = 0.192-0.547), performance status (p-value = <0.001; OR = 8,194, 95% CI OR = 4,669-14,381), and disease severity (p-value = <0.001; OR = 5.448, 95% CI OR = 3,916-7,578) were associated with mortality. We did adjustments in therapy cancer and status performance to confirmed the number of deaths in patients with ECOG score ≤ 2. The results showed that mortality in patients who did not undergo chemotherapy was higher than those having chemotherapy, see Table 3.
| Variables | Survive (%) | Deceased (%) | Sig | OR | 95% CI OR |
| Age | |||||
| < 50 | 165 (74.7) | 56 (25.3) | 0.649 | 0.94 | 0.721-1.226 |
| 50 – 59 | 79 (74.5) | 27 (25.5) | |||
| 60 – 69 | 45 (73.8) | 16 (26.2) | |||
| > 70 | 13 (86.7) | 2 (13.3) | |||
| Gender | |||||
| Female | 201 (78.8) | 54 (21.2) | 0.019 * | 1.732 | 1.095-2.739 |
| Male | 101 (68.2) | 47 (31.8) | |||
| Types of malignancy | <0.001 * | 3.073 | 1.761-5.362 | ||
| Solid tumor | 267 (78.8) | 72 (21.2) | |||
| Haematology | 35 (54.7) | 29 (45.3) | |||
| Comorbidity | 0.672 | 1.046 | 0.850-1.287 | ||
| No comorbidity | 238 (74.6) | 81 (25.4) | |||
| Additional comorbidity besides malignancy | |||||
| Hypertension | 37 (84.1) | 7 (15.9) | |||
| Heart failure | 3 (42.9) | 4 (57.1) | |||
| Diabetes | 8 (80.0) | 2 (20.0) | |||
| Double comorbidity | 16 (69.6) | 7 (30.4) | |||
| Therapy status | |||||
| Treatment naive | 121 (72.0) | 47 (28.0) | 0.193 | 0.846 | 0.659-1.088 |
| Previously treated | 55 (74.3) | 19 (25.7) | |||
| Ongoing therapy | 126 (78.3) | 35 (21.7) | |||
| Ct value# | |||||
| Abbott (median = 17.17) | |||||
| ≥ median | 159 (86.9) | 24 (13.1) | <0.001 * | 3.34 | 1.970-5.664 |
| < median | 121 (66.5) | 61 (33.5) | |||
| GeneXpert (median = 26.85) | |||||
| < median | 2 (50.0) | 2 (50.0) | 0.429 | 0.5 | 0.188-1.332 |
| ≥ median | 0 (0.0) | 4 (100.0) | |||
| Qiagen (median = 31.37) | |||||
| < median | 9 (60.0) | 6 (40.0) | 0.613 | 0.375 | 0.033-4.228 |
| ≥ median | 4 (80.0) | 1 (20.0) | |||
| Performance status | |||||
| ECOG ≤ 2 | 276 (82.9) | 57 (17.1) | <0.001 * | 8.194 | 4.669-14.381 |
| ECOG >2 | 26 (37.1) | 44 (62.9) | |||
| ECOG ≤ 2 | |||||
| not chemotherapy | 179 (80.3) | 44 (19.7) | 0.074 | 0.545 | 0.280-1.061 |
| chemotherapy | 97 (88.2) | 13 (11.8) | |||
| Disease severity | |||||
| Asymptomatic | 174 (96.7) | 6 (3.3) | <0.001 * | 5.448 | 3.916-7.578 |
| Mild | 66 (95.7) | 3 (4.3) | |||
| Moderate | 39 (72.2) | 15 (27.8) | |||
| Severe | 23 (24.0) | 73 (76.0) | |||
| Critical | 0 (0.0) | 4 (100.0) |
#based on Abbott; * Significance < 0.05; OR, Odds Ratio
Discussion
Given the wide range in disease course for COVID-19, the ability to predict which cancer patients are at particularly high risk of deterioration and negative outcomes would be of particular value in the clinical setting. Our study found five risk factors that were significantly associated with mortality in cancer patients with COVID-19, i.e. gender, type malignancy, RTPCR Ct values, performance status, and COVID-19 disease severity. Demographic factors and clinical characteristics are important in predicting mortality.
This study investigated the association of age and gender to mortality in cancer patients with COVID-19. The results showed that age was not significant risk factor of mortality and had been showed by previous studies [11,12]. The median age in cancer patients was lower (46 years old) than the general population (50 years old) in Indonesia [13] and over 50 years old in globally [14,15]. In Asia Pacific (China, Singapura, Korea, and Australia) [16-18], Europe (Italy, Spain, France, and United Kingdom) [19-22], and America (USA and Brazil) [23,24] has a median aged more than 60 years [25]. Our data suggest that vulnerability of cancer patients to high risk of mortality was independent of age.
In our cohort, male cancer patients had higher risk of COVID-19 mortality than female patients. Females and males have differences in their susceptibility and response to viral infections, leading to gender differences in the incidence and severity of the disease [26]. However, and likely in association with differences in the incidence of poor condition, risky and preventive behaviours, or immune systems, male gender was overrepresented among COVID-19 fatalities. This observation was consistent with recent data from China, Spain, and Italy regarding the COVID-19 outbreak showed that the percentage of males who have died due to the infection was much higher than females [27]. In China, for example, found that the fatality rate among men with the virus was about 65% higher than among women. A study in 177 countries and territories suggested that more consideration should be paid to male patients, particularly those over 65 years old for enhanced clinical management [25].
Cancer patients are particularly susceptible to respiratory pathogens and severe pneumonia, because they are at an immunosuppressive state due to malignancy and anticancer therapy. Nearly half of haematology malignancy had high death rates in this study. One of the reasons why outcomes may be worse in patients with haematologic malignancies is that in many cases, immune responses to the virus are less pronounced and highly variable compared with people with other types of cancer, with delayed or negligible seroconversion, prolonged viral shedding, and sustained immune dysregulation [28]. Lower respiratory tract diseases caused by human coronaviruses in patients with haematological malignancies have been associated with high rates of oxygen use and mortality [29]. Solid tumor on the other hand had good prognoses. More than half solid tumor patients survived from COVID-19.
Cancer management such as chemotherapy, surgery, and radiotherapy protocols may suppress immune system, which may lead to worsening of COVID-19 associated morbidity and/or mortality [30]. Furthermore, cancer patients who were being diagnosed with COVID-19 may also risk cancer progression which may jeopardize a chance of survival [31]. However, there was no strong association between status of cancer therapy and mortality within this study cohort.
Cancer therapy can cause a weakened immune system, including chemotherapy. The first objective of using chemotherapy is to kill cancer cells. Chemotherapy can also affect immune cells and thus contribute not only to effective tumor response but also to tumor resistance [32]. Chemotherapy can cause neutropenia (decreased number of neutrophils, a type of white blood cell). This means the body may not be able to fight infection properly. While chemotherapy may affect survival of blood cancer patients with COVID, others studies show that chemotherapy may not imposed additional survival risk [33,34].
Cancer patients with COVID-19 had delayed treatment because they have to be quarantined for approximately 14 days or more. Delayed treatment of cancer can make adverse consequences on outcome. A four-week delay in treatment was associated with an increased risk of death. For surgery, 6-8% increased risk of death for every four-week delay. This effect was even more marked in some radiotherapy and systemic indications, with an increased risk of death of 9% and 13% for definitive head and neck radiotherapy and adjuvant systemic treatment for colorectal cancer, respectively [35]. Therefore, our data may indicate that the increased rate of mortality in patients who did not undergo chemotherapy may die due to malignancy instead of COVID-19. At this stage we could not distinguish the death of COVID-19 or of malignancy. The cycle threshold (Ct) value is the number of cycles of amplification to detect the virus genetic materials. It is an estimate of how much virus was likely in the sample to start with – instead of the actual number of viable virus particles. If the virus is found in a low number of cycles (Ct value under 30), it means that the virus was easier to find in sample and that the sample started out with a large amount of the virus [36]. Clinical knowledge of COVID-19 is constantly evolving, with studies being published at a high rate; however, there is currently only limited data relating to the correlation of viral loads with patient prognoses, such as mortality or disease progression. A study reported on the association between mortality and SARS-CoV-2 Ct value and showed that lower Ct values correlated with increased risk of death, [37] which is consistent with data for previous epidemic-causing coronaviruses [38,39].
Many studies reported on the correlation of Ct values with symptom severity that indicated that lower Ct values were associated with more severe disease. This is consistent with some previous studies of Ct values in other respiratory infections, although other studies do not show correlation [40]. The limitation of our study, we did not perform quantitative measurement of viral particles in laboratory culture and correlate with Ct values.
Lastly, patients with ECOG > 2 had 8 times risk for death. We did an adjustment and the data showed in this category the patients were doing ‘not chemotherapy’ category and stabilized the clinical condition. In Dharmais Hospital as a tertiary hospital. So, the patients that came to us more likely had poor prognostic. For the patient that came for diagnostic, the moment they got COVID-19, we had to delayed any action or treatment for the cancer. In conclusion, when exposed to COVID-19, cancer patients had higher mortality rate than general population. Gender as demographics factors has association with mortality significantly. Clinical characteristics such as type malignancy, Ct value, performance status, and disease severity were associated with mortality. COVID-19 had a huge impact on cancer treatment and management. Cancer patients with COVID-19 would postpone doctor visits, diagnostic work-up, chemotherapy, and others which can increase the risk of mortality. Further research is required to investigate the others factor of infection COVID-19 such as body mass index (BMI), blood type, smoking history and examine the survival rate in cancer patients with COVID-19.
Acknowledgments
We would like to thank all the COVID-19 mitigation team in Dharmais National Cancer Center, Jakarta, Indonesia.
Conflict Of Interest
The authors report no conflict of interest
References
- World Health Organization. Coronavirus disease (COVID-19) pandemic [Internet]. Coronavirus disease. 2020 [cited 2021 July 21] Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019..
- World Health Organization. WHO Coronavirus (COVID-19) [Internet]. Coronavirus disease. 2021 [cited 2021 July 21] Available from: https://covid19.who.int/..
- Satuan Tugas COVID-19. Data Sebaran Situasi virus COVID-19 [Internet]. 2020 [cited 2021 July 21] Available from: https://www.covid19.go.id/.;:1-5.
- Risk of COVID-19 for patients with cancer Y Xia, R Jin, J Zhao, W Li, H Shen. The Lancet. Oncology.2020;21(4). CrossRef
- Outcome of Oncology Patients Infected With Coronavirus Jazieh Abdul-Rahman, Alenazi Thamer H., Alhejazi Ayman, Al Safi Faisal, Al Olayan Ashwaq. JCO global oncology.2020;6. CrossRef
- Active and Effective Measures for the Care of Patients With Cancer During the COVID-19 Spread in China Wang Zhijie, Wang Jie, He Jie. JAMA oncology.2020;6(5). CrossRef
- COVID-19 and Cancer Care in Indonesia: What we have done in Dharmais Cancer Center Hospital Dwijayanti Fifi, Setiadi Hendi, Makful Martya. Indonesian Journal of Cancer.2020;14. CrossRef
- Ministry of Health. Guidelines for the prevention and control of coronavirus disease COVID-19. 4th edition. Jakarta: Ministry of Health Indonesia; 2020 ;:1-35.
- Toxicology and response criteria of the Eastern Cooperative Oncology Group Oken MM, Creech RH, Davis TE. American Journal of Clinical Oncology: Cancer Clinical Trials.1982;5:649-655.
- Guidelines of COVID-19 Management. Third edit. Jakarta: Ministry of Health Indonesia Burhan E, Susanto AD, Nasution SA, Ginanjar E, Pitoyo CW, Susilo A, et al. . 2020;3–6:88-89.
- Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies Parohan Mohammad, Yaghoubi Sajad, Seraji Asal, Javanbakht Mohammad Hassan, Sarraf Payam, Djalali Mahmoud. The Aging Male: The Official Journal of the International Society for the Study of the Aging Male.2020;23(5). CrossRef
- Clinical Characteristics and Outcomes in Patients with COVID-19 and Cancer: a Systematic Review and Meta-analysis Zarifkar P., Kamath A., Robinson C., Morgulchik N., Shah S. F. H., Cheng T. K. M., Dominic C., Fehintola A. O., Bhalla G., Ahillan T., Mourgue d'Algue L., Lee J., Pareek A., Carey M., Hughes D. J., Miller M., Woodcock V. K., Shrotri M.. Clinical Oncology (Royal College of Radiologists (Great Britain)).2021;33(3). CrossRef
- Clinical characteristics and mortality associated with COVID-19 in Jakarta, Indonesia: A hospital-based retrospective cohort study Surendra Henry, Elyazar Iqbal Rf, Djaafara Bimandra A., Ekawati Lenny L., Saraswati Kartika, Adrian Verry, Widyastuti null, Oktavia Dwi, Salama Ngabila, Lina Rosa N., Andrianto Adhi, Lestari Karina D., Burhan Erlina, Shankar Anuraj H., Thwaites Guy, Baird J. Kevin, Hamers Raph L.. The Lancet Regional Health. Western Pacific.2021;9. CrossRef
- The Landscape of COVID-19 in Cancer Patients: Prevalence, Impacts, and Recommendations Abdihamid Omar, Cai Changjing, Kapesa Linda, Zeng Shan. Cancer Management and Research.2020;12. CrossRef
- COVID-19 with Different Severities: A Multicenter Study of Clinical Features Feng Yun, Ling Yun, Bai Tao, Xie Yusang, Huang Jie, Li Jian, Xiong Weining, Yang Dexiang, Chen Rong, Lu Fangying, Lu Yunfei, Liu Xuhui, Chen Yuqing, Li Xin, Li Yong, Summah Hanssa Dwarka, Lin Huihuang, Yan Jiayang, Zhou Min, Lu Hongzhou, Qu Jieming. American Journal of Respiratory and Critical Care Medicine.2020;201(11). CrossRef
- Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak Dai Mengyuan, Liu Dianbo, Liu Miao, Zhou Fuxiang, Li Guiling, Chen Zhen, Zhang Zhian, You Hua, Wu Meng, Zheng Qichao, Xiong Yong, Xiong Huihua, Wang Chun, Chen Changchun, Xiong Fei, Zhang Yan, Peng Yaqin, Ge Siping, Zhen Bo, Yu Tingting, Wang Ling, Wang Hua, Liu Yu, Chen Yeshan, Mei Junhua, Gao Xiaojia, Li Zhuyan, Gan Lijuan, He Can, Li Zhen, Shi Yuying, Qi Yuwen, Yang Jing, Tenen Daniel G., Chai Li, Mucci Lorelei A., Santillana Mauricio, Cai Hongbing. Cancer Discovery.2020;10(6). CrossRef
- Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study Chen Nanshan, Zhou Min, Dong Xuan, Qu Jieming, Gong Fengyun, Han Yang, Qiu Yang, Wang Jingli, Liu Ying, Wei Yuan, Xia Jia'an, Yu Ting, Zhang Xinxin, Zhang Li. Lancet (London, England).2020;395(10223). CrossRef
- Epidemiology and Clinical Features of Emergency Department Patients with Suspected COVID-19: Initial Results from the COVID-19 Emergency Department Quality Improvement Project (COVED-2) [Internet]. Emergency Medicine Australasia. 2020 [cited 2021 May 29] O’Reilly GM, GM, Mitchell RD, RD, Wu J, J, Rajiv P, P, Bannon-Murphy H, H, Amos T, T, et al. . Available from: https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/fr/covidwho-603489?lang=en..
- Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy Grasselli Giacomo, Zangrillo Alberto, Zanella Alberto, Antonelli Massimo, Cabrini Luca, Castelli Antonio, Cereda Danilo, Coluccello Antonio, Foti Giuseppe, Fumagalli Roberto, Iotti Giorgio, Latronico Nicola, Lorini Luca, Merler Stefano, Natalini Giuseppe, Piatti Alessandra, Ranieri Marco Vito, Scandroglio Anna Mara, Storti Enrico, Cecconi Maurizio, Pesenti Antonio. JAMA.2020;323(16). CrossRef
- COVID-19 in solid organ transplant recipients: A single-center case series from Spain Fernández-Ruiz Mario, Andrés Amado, Loinaz Carmelo, Delgado Juan F., López-Medrano Francisco, San Juan Rafael, González Esther, Polanco Natalia, Folgueira María D., Lalueza Antonio, Lumbreras Carlos, Aguado José M.. American Journal of Transplantation: Official Journal of the American Society of Transplantation and the American Society of Transplant Surgeons.2020;20(7). CrossRef
- Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected with Pulmonary CT Angiography Grillet Franck, Behr Julien, Calame Paul, Aubry Sébastien, Delabrousse Eric. Radiology.2020;296(3). CrossRef
- Characteristics, Symptom Management, and Outcomes of 101 Patients With COVID-19 Referred for Hospital Palliative Care Lovell Natasha, Maddocks Matthew, Etkind Simon N., Taylor Katie, Carey Irene, Vora Vandana, Marsh Lynne, Higginson Irene J., Prentice Wendy, Edmonds Polly, Sleeman Katherine E.. Journal of Pain and Symptom Management.2020;60(1). CrossRef
- Characteristics of Emergency Department Patients With COVID-19 at a Single Site in Northern California: Clinical Observations and Public Health Implications Duanmu Youyou, Brown Ian P., Gibb William R., Singh Jessica, Matheson Loretta W., Blomkalns Andra L., Govindarajan Prasanthi. Academic Emergency Medicine: Official Journal of the Society for Academic Emergency Medicine.2020;27(6). CrossRef
- Coronavirus Infections-More Than Just the Common Cold Paules Catharine I., Marston Hilary D., Fauci Anthony S.. JAMA.2020;323(8). CrossRef
- Influence of age and gender on the epidemic of COVID-19 : Evidence from 177 countries and territories-an exploratory, ecological study Hu Dingtao, Lou Xiaoqi, Meng Nana, Li Zhen, Teng Ying, Zou Yanfeng, Wang Fang. Wiener Klinische Wochenschrift.2021;133(7-8). CrossRef
- Impact of sex and gender on COVID-19 outcomes in Europe Gebhard Catherine, Regitz-Zagrosek Vera, Neuhauser Hannelore K., Morgan Rosemary, Klein Sabra L.. Biology of Sex Differences.2020;11. CrossRef
- Gender dimension of the COVID-19 pandemic. Gend Dimens COVID-19 pandemic Paz C de, Mulle M, Boudet AMM, Gaddis I. 18(April).2020;:964-969.
- Acute Immune Signatures and Their Legacies in Severe Acute Respiratory Syndrome Coronavirus-2 Infected Cancer Patients Abdul-Jawad Sultan, Baù Luca, Alaguthurai Thanussuyah, Molino del Barrio Irene, Laing Adam G., Hayday Thomas S., Monin Leticia, Muñoz-Ruiz Miguel, McDonald Louisa, Francos Quijorna Isaac, McKenzie Duncan, Davis Richard, Lorenc Anna, Chan Julie Nuo En, Ryan Sarah, Bugallo-Blanco Eva, Yorke Rozalyn, Kamdar Shraddha, Fish Matthew, Zlatareva Iva, Vantourout Pierre, Jennings Aislinn, Gee Sarah, Doores Katie, Bailey Katharine, Hazell Sophie, De Naurois Julien, Moss Charlotte, Russell Beth, Khan Aadil A., Rowley Mark, Benjamin Reuben, Enting Deborah, Alrifai Doraid, Wu Yin, Zhou You, Barber Paul, Ng Tony, Spicer James, Van Hemelrijck Mieke, Kumar Mayur, Vidler Jennifer, Lwin Yadanar, Fields Paul, Karagiannis Sophia N., Coolen Anthony C.C., Rigg Anne, Papa Sophie, Hayday Adrian C., Patten Piers E.M., Irshad Sheeba. Cancer Cell.2021;39(2). CrossRef
- Clinical Significance of Human Coronavirus in Bronchoalveolar Lavage Samples From Hematopoietic Cell Transplant Recipients and Patients With Hematologic Malignancies Ogimi Chikara, Waghmare Alpana A., Kuypers Jane M., Xie Hu, Yeung Cecilia C., Leisenring Wendy M., Seo Sachiko, Choi Su-Mi, Jerome Keith R., Englund Janet A., Boeckh Michael. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America.2017;64(11). CrossRef
- Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study Kuderer Nicole M., Choueiri Toni K., Shah Dimpy P., Shyr Yu, Rubinstein Samuel M., Rivera Donna R., Shete Sanjay, Hsu Chih-Yuan, Desai Aakash, Lima Lopes Gilberto, Grivas Petros, Painter Corrie A., Peters Solange, Thompson Michael A., Bakouny Ziad, Batist Gerald, Bekaii-Saab Tanios, Bilen Mehmet A., Bouganim Nathaniel, Larroya Mateo Bover, Castellano Daniel, Del Prete Salvatore A., Doroshow Deborah B., Egan Pamela C., Elkrief Arielle, Farmakiotis Dimitrios, Flora Daniel, Galsky Matthew D., Glover Michael J., Griffiths Elizabeth A., Gulati Anthony P., Gupta Shilpa, Hafez Navid, Halfdanarson Thorvardur R., Hawley Jessica E., Hsu Emily, Kasi Anup, Khaki Ali R., Lemmon Christopher A., Lewis Colleen, Logan Barbara, Masters Tyler, McKay Rana R., Mesa Ruben A., Morgans Alicia K., Mulcahy Mary F., Panagiotou Orestis A., Peddi Prakash, Pennell Nathan A., Reynolds Kerry, Rosen Lane R., Rosovsky Rachel, Salazar Mary, Schmidt Andrew, Shah Sumit A., Shaya Justin A., Steinharter John, Stockerl-Goldstein Keith E., Subbiah Suki, Vinh Donald C., Wehbe Firas H., Weissmann Lisa B., Wu Julie Tsu-Yu, Wulff-Burchfield Elizabeth, Xie Zhuoer, Yeh Albert, Yu Peter P., Zhou Alice Y., Zubiri Leyre, Mishra Sanjay, Lyman Gary H., Rini Brian I., Warner Jeremy L.. Lancet (London, England).2020;395(10241). CrossRef
- SARS-CoV-2 Infection in Cancer Patients: Effects on Disease Outcomes and Patient Prognosis Seth Gaurav, Sethi Saira, Bhattarai Shristi, Saini Geetanjali, Singh Chandra Bhushan, Aneja Ritu. Cancers.2020;12(11). CrossRef
- Cytotoxic effects of chemotherapy on cancer and immune cells: how can it be modulated to generate novel therapeutic strategies? Rébé Cédric, Ghiringhelli François. Future Oncology (London, England).2015;11(19). CrossRef
- Clinical outcomes and risk factors for severe COVID-19 in patients with haematological disorders receiving chemo- or immunotherapy Fox Thomas A., Troy-Barnes Ethan, Kirkwood Amy A., Chan Wei Yee, Day James W., Chavda Selina J., Kumar Emil A., David Kate, Tomkins Oliver, Sanchez Emilie, Scully Marie, Khwaja Asim, Lambert Jonathan, Singer Mervyn, Roddie Claire, Morris Emma C., Yong Kwee L., Thomson Kirsty J., Ardeshna Kirit M.. British Journal of Haematology.2020;191(2). CrossRef
- COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study Lee Lennard Yw, Cazier Jean-Baptiste, Angelis Vasileios, Arnold Roland, Bisht Vartika, Campton Naomi A., Chackathayil Julia, Cheng Vinton Wt, Curley Helen M., Fittall Matthew W., Freeman-Mills Luke, Gennatas Spyridon, Goel Anshita, Hartley Simon, Hughes Daniel J., Kerr David, Lee Alvin Jx, Lee Rebecca J., McGrath Sophie E., Middleton Christopher P., Murugaesu Nirupa, Newsom-Davis Thomas, Okines Alicia Fc, Olsson-Brown Anna C., Palles Claire, Pan Yi, Pettengell Ruth, Powles Thomas, Protheroe Emily A., Purshouse Karin, Sharma-Oates Archana, Sivakumar Shivan, Smith Ashley J., Starkey Thomas, Turnbull Chris D., Várnai Csilla, Yousaf Nadia, Kerr Rachel, Middleton Gary. Lancet (London, England).2020;395(10241). CrossRef
- Mortality due to cancer treatment delay: systematic review and meta-analysis Hanna Timothy P., King Will D., Thibodeau Stephane, Jalink Matthew, Paulin Gregory A., Harvey-Jones Elizabeth, O'Sullivan Dylan E., Booth Christopher M., Sullivan Richard, Aggarwal Ajay. BMJ (Clinical research ed.).2020;371. CrossRef
- Diagnostic SARS-CoV-2 Cycle Threshold Value Predicts Disease Severity, Survival, and Six-Month Sequelae in COVID-19 Symptomatic Patients Trunfio Mattia, Venuti Francesco, Alladio Francesca, Longo Bianca Maria, Burdino Elisa, Cerutti Francesco, Ghisetti Valeria, Bertucci Roberto, Picco Carlo, Bonora Stefano, Di Perri Giovanni, Calcagno Andrea. Viruses.2021;13(2). CrossRef
- Chronological Changes of Viral Shedding in Adult Inpatients with COVID- 19 in Wuhan, China Jing-Tao Huang J-T, Ran R-X, Lv Z-H, Feng L-N, Ran C-Y, Tong Y-Q, et al . .
- Initial viral load and the outcomes of SARS Chu Chung-Ming, Poon Leo L.M., Cheng Vincent C.C., Chan Kin-Sang, Hung Ivan F.N., Wong Maureen M.L., Chan Kwok-Hung, Leung Wah-Shing, Tang Bone S.F., Chan Veronica L., Ng Woon-Leung, Sim Tiong-Chee, Ng Ping-Wing, Law Kin-Ip, Tse Doris M.W., Peiris Joseph S.M., Yuen Kwok-Yung. CMAJ : Canadian Medical Association Journal.2004;171(11). CrossRef
- Association of Higher MERS-CoV Virus Load with Severe Disease and Death, Saudi Arabia, 2014 Feikin Daniel R., Alraddadi Basem, Qutub Mohammed, Shabouni Omaima, Curns Aaron, Oboho Ikwo K., Tomczyk Sara M., Wolff Bernard, Watson John T., Madani Tariq A.. Emerging Infectious Diseases.2015;21(11). CrossRef
- Is Higher Viral Load in the Upper Respiratory Tract Associated With Severe Pneumonia? Findings From the PERCH Study Feikin Daniel R., Fu Wei, Park Daniel E., Shi Qiyuan, Higdon Melissa M., Baggett Henry C., Brooks W. Abdullah, Deloria Knoll Maria, Hammitt Laura L., Howie Stephen R. C., Kotloff Karen L., Levine Orin S., Madhi Shabir A., Scott J. Anthony G., Thea Donald M., Adrian Peter V., Antonio Martin, Awori Juliet O., Baillie Vicky L., DeLuca Andrea N., Driscoll Amanda J., Ebruke Bernard E., Goswami Doli, Karron Ruth A., Li Mengying, Morpeth Susan C., Mwaba John, Mwansa James, Prosperi Christine, Sawatwong Pongpun, Sow Samba O., Tapia Milagritos D., Whistler Toni, Zaman Khalequ, Zeger Scott L., O' Brien Katherine L., Murdoch David R.. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America.2017;64(suppl_3). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2021
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times