Cell of Origin Based on Hans’ Algorithm as Prognostic Factor in Diffuse Large B-Cell Lymphoma: A Clinicopathologic and Survival Study
Download
Abstract
Background: Diffuse Large B-Cell Lymphoma (DLBCL) has been classified based on Cell of Origin (COO) into Germinal Center B-cell (GCB) and Non-Germinal Center B-cell (Non-GCB). The practical application of this concept by immunohistochemistry (IHC)-based subtyping with Hans’ algorithm and its role as a prognostic factor has been widely investigated with conflicting results. Moreover, the recent deeper molecular characterizations of DLBCL have been another challenges for this approach to remain as a prognostic factor.
Objective: To determine the prognostic value of COO by IHC-based subtyping with Hans’ algorithm for DLBCL.
Materials and Methods: This was a single-center retrospective analysis including DLBCL cases diagnosed in 2014-2019 from Dr. Sardjito Hospital, Yogyakarta, Indonesia. Hans’ algorithm with CD10, BCL6, and MUM1 were used. The two subtypes were compared in terms of clinicopathological features and 3 years overall survival.
Results: Seventy patients were included in this study. Non-GCB was predominant than GCB 74.3% vs 25.7%, with the ratio of 2.8:1. No statistical difference in the clinicopathological features from both subtypes in terms of age, gender, Ann Arbor stadium, ECOG performance status, extra-nodal involvement, and LDH level. Ann Arbor stadium and ECOG showed significant prognostic value for patients outcome with multivariate analysis (OR 3.64; 95%CI 0.18-0.61; p=0.001) and (OR 2.68; 95%CI 0.11-0.77; p=0.009). Survival analysis based on COO did not show significant difference between GCB and non-GCB subtypes (log rank=0.46). Further analysis based on COO and the use of rituximab showed better OS in the two subtypes than without rituximab although not significant (log rank=0.67).
Conclusion: The role of COO with Hans’ algorithm as prognostic factor in DLBCL is not confirmed in this study. Some related factors may be the addition of rituximab and the recent molecular characterization of DLBCL which covered a more subtle classification of the disease beyond GCB and non-GCB subtypes.
Introduction
Diffuse Large B-cell Lymphoma (DLBCL) is an aggressive lymphoma that accounts for about 40% of Non-Hodgkin Lymphoma. Gene expression profiling (GEP) has set up molecular classification of this entity into two different categories of cell of origin (COO): germinal center B-like (GCB) DLBCL which showed genes characteristic of germinal center B cells and non- GCB or activated B-cell (ABC) which expressed genes normally induced during in vitro activation of peripheral blood B cells. This molecular classification has been the basis of prognostication in DLBCL for the last two decades [1]. The sophisticated GEP study has been translated with immunohistochemistry (IHC) application for diagnostics in clinical practice. One of the most popular algorithm to determine COO in lymphoma is by Hans’ algorithm with concordance rate of 80% with GEP. Hans’ panel includes CD10, BCl6 and MUM1, where GCB subtype is CD10 + or BCL6 + /CD10–/MUM1or IRF4– and non-GCB is with CD10–/ MUM1 or IRF4 + (BCL6 positive or negative) [2].
The prognostic impact of COO to the disease outcome of DLBCL in clinical practice has been controversial. Previous study reported that GCB subtype had better progression free survival (PFS) and overall survival (OS) than non-GCB or ABC [3]. A significant relationship between GCB subtype and lower international prognostic index (IPI) score was also reported [4]. However, other study confirmed that the two subtypes of DLBCL did not show any relationship either with OS or IPI [5].
Despite the various conflicting reports, it is still very important to explore the potential of COO by immunohistochemistry approach such as Hans’ algorithm as one prognostic factor in DLBCL, particularly due to its practical and economical concerns, in various populations including Indonesia.
Materials and Methods
This was a retrospective cohort study involving cases of DLBCL diagnosed from January 2014 to December 2019 from Dr. Sardjito Hospital, Yogyakarta, Indonesia which is one referral cancer center in the country. Tissue blocks were collected from the Department of Anatomical Pathology. Clinicopathological and laboratory parameters were extracted from patient’s medical record, including age, Ann-Arbor stadium, extra nodal tumor involvement, Eastern Cooperative Oncology Group (ECOG) performance status, lactate dehydrogenase (LDH) level, chemotherapy regiment, and 3 years overall survival status. This study has been approved by the Ethical Committee of Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada (No. KE/ FK/1371/2020).
Immunohistochemistry Analysis
For immunostaining, 4-μm thick sections were cut from formalin-fixed paraffin-embedded (FFPE) tissue blocks and placed on electrostatic-charged, poly- L-lysine-coated slides (Biogear, Microscope Slide, Biogear Scientific, BioVentures, Inc., Coralville, Iowa, USA). Sections were dehydrated at 45°C overnight. All immunostaining procedures including deparaffinization were performed on Semi-automatic Intellipath FLX (Biocare Medical, Concord, Massachusetts, USA) with open kit. The antigen retrieval process was performed on Deckloaking Chamber from Biocare Medical. The counterstaining process with hematoxylin was performed under a semi-automatic slide stainer. After that, the dehydration process was achieved, followed by clearing with xylene, and finally the mounting process was finished to end the entire immunostaining process. The following antibodies were used in this study: BCl-6 (BCL-6 oncoprotein 7ml ready to use PA0204, clone: LN22), CD10 (CD10 7ml bond ready to use PA0270, clone: 56C6) and MUM1 (dilution: 1/50, clone: MUM1p, Dako SA, Glostrup, Denmark). Reactive lymph nodes tissue samples were used as positive controls. Negative controls were treated with the same immunohistochemical method by omitting the primary antibody. The cut-off level for interpreting BCl6, CD10 and MUM1 as positive was >30% tumor cell staining from 500 cells [6]. There were two pathologists interpreting the staining result with inter-observer agreement using Cohen’s Kappa >75% (substantial agreement). CD10 positivity was shown by brown granules on the cell membranes, while Bcl-6 and MUM1 positivity was shown by brown staining on the cell nucleus.
Statistical analysis
The proportions of nominal parameters were compared between groups using Chi-square or Fisher’s exact text, as appropriate, while the median of numerical parameters was compared using the Mann-whitney test. Using logistic regression, the odds ratio (OR) with 95 percent confidence interval (95 percent CI) was obtained for each variable. To assess independent associations, univariate logistic regression analysis was performed initially, followed by multivariate logistic regression analysis.
The period from the day of initial diagnosis until death or the last follow-up was referred to as overall survival (OS). The duration of the follow-up was set at three years. The Kaplan-Meier survival analysis was used to assess the OS distributions in relation to clinical parameters and immunohistochemistry subtypes, and the log-rank test was used to assess the differences between survival curves. SPSS Statistics 17.0 software (SPSS Inc., Chicago, Illinois) and R studio Version 1.4.1717 were used to conduct the above statistical analyses (RStudio Team, Boston, MA, USA). The differences were considered statistically significant if the p-value was less than 0.05.
Results
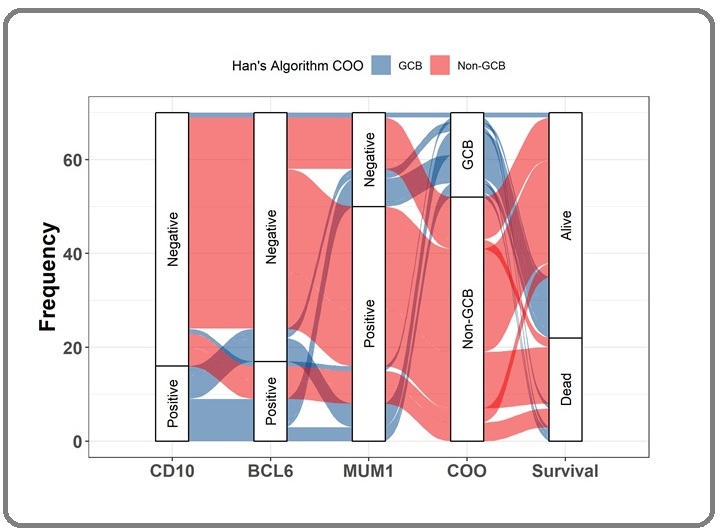
There were seventy (70) cases of DLBCL in this study. Median age of the patient was 59 years old, 57.1% with age <60 years. There was almost equal proportion between male and female (55.7% vs 44.3%). More cases presented with early stage of Ann-Arbor stadium I-II 71.4% than stage III-IV of 28.6%. Most cases had good ECOG performance status 0-2 (91.4%) and limited stage with IPI score 0-2 (90%). Only few patients had multiple extranodal site (10%). Many patients did not perform LDH level examination in their initial visit, with only 15 patients among the available LDH group (n=21) had increased level of LDH (71.4%). Thirty two (45.7%) patients received rituximab based regimen while 38 (54.3%) did not. Median follow up of the cohort was 28.5 months, with 48 patients (68.6%) were still alive and 22 patients (31.2%) deceased. There were more non-GCB than GCB subtypes DLBCL in our series, 52 (74.3%) vs 18 (25.7%). Baseline characteristics of the subjects are presented in Table 1, while distribution and frequency of the COO with survival status are showed in Figure 1.
| Parameter | n = (70) | |
| Age | Median [IQR] | 59 [53-65] years |
| Gender | Male | 39 (55.7%) |
| Female | 31 (44.3%) | |
| International Prognostic Index | ||
| Age | <60 | 40 (57.1%) |
| ≥60 | 30 (42.9%) | |
| Ann-Arbor Stadium | I-II | 50 (71.4%) |
| III-IV | 20 (28.6%) | |
| ECOG status | 0-2 | 64 (91.4%) |
| >2 | 6 (8.6%) | |
| LDH level | Normal | 6 (28.6%) |
| Increased | 15 (71.4%) | |
| Extranodal involvement | Single | 64 (91.4%) |
| Multiple | 6 (8.6%) | |
| Total IPI score | 0-2 | 63 (90%) |
| >2 | 7 (10%) | |
| Therapy | With Rituximab | 32 (45.7%) |
| No Rituximab | 38 (54.3%) | |
| B-cell Lymphoma-6 (BCL-6) | Negative | 53 (75.7%) |
| Positive | 17 (24.3%) | |
| Multiple Myeloma Oncogene-1 (MUM-1) | Negative | 20 (28.6%) |
| Positive | 50 (71.4%) | |
| CD 10 | Negative | 54 (77.1%) |
| Positive | 16 (22.9%) | |
| Origin of cells | GCB | 18 (25.7%) |
| Non-GCB | 52 (74.3%) | |
| Median follow-up | 28.5 months | |
| Patients status | Alive | 48 (68.6%) |
| Dead | 22 (31.4%) |
Figure 1. Distribution and Frequency of the Cell of Origin with Survival Status. The Sankey plot depicted all DLBCL patients (n=70) for whom IHC staining was performed based on the Hans Algorithm, proceeding with CD10, BCl6, and MUM1. The results of these three staining panels showed that DLBCL cells were either GCB (blue color) or non-GCB (red color). The majority of the DLBCL patients were non-GCB subtypes and most were CD10-/Bcl6-/ Mum1+. The survival of patient was then evaluated for three years to assess dead or alive status.
Clinicopathological comparison between GCB and non-GCB DLBCL is presented in Table 2. There was no statistical difference in terms of age, sex, extranodal involvement, ECOG performance status, LDH level, nodal involvement and total IPI score between the two groups. Non-GCB group consisted more cases with advanced stage indicated by Ann Arbor stage III-IV (p 0.057).
| Parameter | GCB (n=18) | Non-GCB (n=52) | p-value | |
| N (%) | N (%) | |||
| Age (years) | Mean (±SD) | 55.56 (±12.41) | 59.60 (±9.74) | 0.163 |
| Gender | Male | 12 (30.8) | 27 (69.2) | 0.278 |
| Female | 6 (19.4) | 25 (80.6) | ||
| International Prognostic Index | ||||
| Age | <60 | 8 (20) | 32 (80) | 0.207 |
| ≥60 | 10 (33.3) | 20 (66.7) | ||
| ECOG status | 0-2 | 17 (26.6) | 47 (73.4) | 0.596 |
| >2 | 1 (16.7) | 5 (83.3) | ||
| LDH | Normal | 1 (16.7) | 5 (83.3) | 0.861 |
| Increased | 12 (80) | 3 (20) | ||
| Ann-Arbor Stadium | I-II | 16 (32) | 34 (68) | 0.057 |
| III-IV | 2 (10) | 18 (90) | ||
| Extranodal involvement | Single | 17 (26.6) | 47 (73.4) | 0.596 |
| Multiple | 1 (16.7) | 5 (83.35) | ||
| Total IPI | 0-2 | 18 (28.6) | 45 (71.4) | 0.101 (Fisher Exact) |
| >2 | 0 (0%) | 7 (100%) |
Univariate analysis in Table 3 showed no significant impact of patient characteristics such as age, sex, LDH, and nodal involvement to the patient outcome. Patient with more advanced stage of the disease had an increased risk than with lower stage (OR 6; 95% CI 1.93 – 18.6; p=0.002).
| Parameter | Alive (n=48) | Dead (n=22) | p-value | OR (CI 95%) | |
| N (%) | N (%) | ||||
| Age | <60 | 27 (67.5) | 13 (32.5) | 0.824 | 0.89 (0.32-2.47) |
| ≥60 | 21 (70) | 9 (30) | |||
| Gender | Male | 24 (61.5) | 15 (38.5) | 0.159 | 2.14 (0.74-6.18) |
| Female | 24 (77.4) | 7 (22.6) | |||
| Ann Arbor stadium | I-II | 40 (80) | 10 (20) | 0.002 | 6 (1.93 – 18.6) |
| III-IV | 8 (40) | 12 (60) | |||
| ECOG | 0-2 | 46 (73) | 17 (27) | 0.03 | 6.76 (1.19- 38.21) |
| >2 | 2 (28.6) | 5 (71.4) | |||
| LDH (n=21) | Normal | 3 (50) | 3 (50) | 0.677 | 0.66 (0.09 – 4.47) |
| Increase | 9 (60) | 6 (40) | |||
| Extranodal Involvement | Single | 45 (70.3) | 19 (29.7) | 0.317 | 2.36 (0.43 – 12.8) |
| Multiple | 3 (50) | 3 (50) | |||
| Cell of Origins | GCB | 14 (77.8) | 4 (22.2) | 0.333 | 1.85 (0.53 – 6.46) |
| Non-GCB | 34 (65.4) | 18 (34.6) | |||
| Therapy | With Rituximab | 25 (65.8) | 13 (34.2) | 0.585 | 0.75 (0.27-2.09) |
| No Rituximab | 23 (71.9) | 9 (28.1) |
A worse ECOG performance status also related with an increased risk of worse outcome (OR 6.76; 95% CI 1.19-38.21; p=0.03). Cell of origin (COO) and rituximab did not show significant impact to the patient outcome.
Multivariate analysis in Table 4 consistently showed Ann Arbor stadium and ECOG performance status as worse prognostic outcome for the patient (OR 3.64; 95% CI 0.18-0.61; p=0.001) and (OR 2.68; 95% CI 0.11-0.77; p=0.009).
| Parameter | Univariate Analysis | Multivariate Analysis | ||
| OR (95% CI) p | p-value | OR (95% CI) | p-value | |
| Ann Arbor stadium | 6 (1.93 – 18.6) | 0.002 | 3.64 (0.18-0.61) | 0.001 |
| ECOG | 6.76 (1.19- 38.21) | 0.03 | 2.68 (0.11-0.77) | 0.009 |
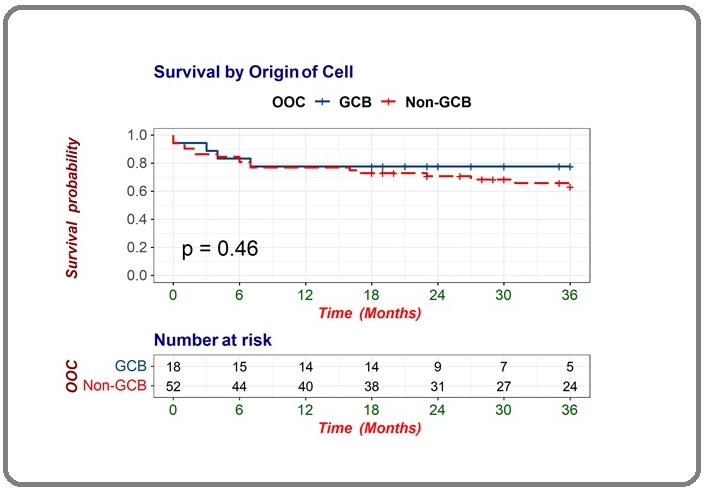
Survival analysis based on COO in Figure 2 showed that DLCBL with GCB subtype had median OS of 28.7 months (95% CI 22.51 – 35.04), while in non-GCB the median OS was 27 months (95% CI 23.1 – 30.9). The difference was not significant (log rank=0.46). Based on this insignificant result of COO in affecting the OS of DLBCL patients, we further analyzed the effect of treatment based on the use of rituximab in the treatment regimen.
Figure 2. Survival Analysis of DLBCL Patients between Germinal Center and non-Germinal Center Subtypes.
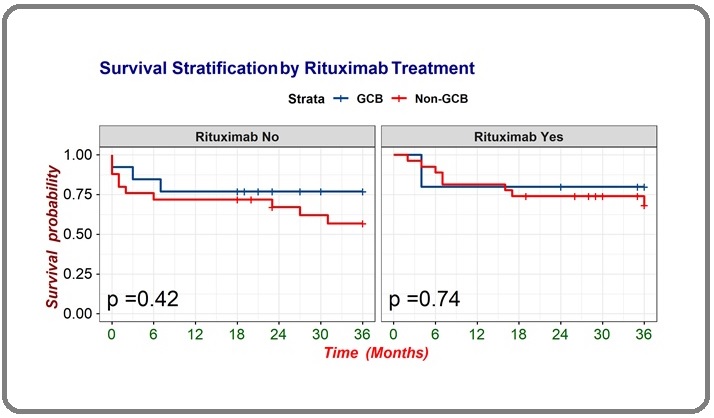
Analysis of survival based on the COO and the use of rituximab in Figure 3 showed that DLBCL patients with GCB subtype treated with rituximab showed median OS 29.6 months (95% CI 18.38-40.82), compared without rituximab showing median OS 28.4 months (95% CI 20.94-35.98). Meanwhile, DLBCL with non-GCB subtype treated with rituximab showed median OS 28.8 months (95% CI 23.85 – 33.84), compared without rituximab 24.9 months (95% CI 19.02 – 30.92). Overall, patients with GCB and non-GCB subtype who got rituximab showed better OS than without rituximab median OS 29.6 months (95% CI 18.38-40.82) vs median OS 28.8 months (95% CI 23.85 – 33.84), although the difference was not significant (log rank=0.67).
Figure 3. Survival Analysis Based on Combination of Cell of Origin (COO) and Treatment .
Discussion
The mean of age of DLBCL in this cohort was 59 years, which is in coherent with previous reports in eastern countries (60 years) and in western countries (67 years) [7, 8]. There were more male than female in this study with ratio of 1,2:1. This is accordance with previous studies in western, eastern countries and Indonesia [7, 9-11]. Environmental factors such as occupational risks with higher exposure to carcinogens such as pesticides and other chemicals, smoking and alcohol consumption may be related with male predominance [12]. Estrogen was also reported to have protective effect for lymphoma. Exposure of any chemicals that were related with estrogen metabolism increased the risk of occurrence of mature B cell lymphoma in male but not in female [13].
DLBCL subtype of non-GCB (n=52;74.3%) was predominant than GCB (n=18; 25.7%) with ratio of 2.8:1 in this cohort, which is in accordance with other studies in Southeast Asia which reported 60% of non-GCB in Malaysia and Singapore, and 63.8% in Thailand. In the Asian region, such as in Japan and Taiwan the non-GCB subtype was 71% and 78.2%, subsequently. Other study showed that 31% of DLBCL patients (102/330) had the GCB subtype, similar results were also found in Asian countries such as Korea, China, and Japan [14-18]. Some possible factors contributing to the difference in the proportions between GCB and non-GCB in Asian and Western countries is the lower rate of translocation (14;18) in Asian DLBCL which is in accordance with the low proportion of GCB molecular subtypes [14, 15, 19]. Second, environmental factors such as the interaction with the Epstein - Barr virus (EBV) which is dominant in the Asian region can influence the pattern of COO in DLBCL [15]. It is well known that NF-kB activation is the main underlying mechanism in activated B-cell (ABC) DLBCL, which is part of the non-GCB DLBCL subtype, and EBV has been known to be capable of activating canonical NF-kB in tumor cells [20].
This study showed that 66.7% patients above 60 years were non-GCB subtype. A previous study found the similar result where older age >50 years were more prevalent in non-GCB subtype [10]. This may be related with pathological character and natural aging process of B-cells [10, 21].
Extranodal involvement in this cohort occurred more in non-GCB subtype. This is in accordance with a previous study showing higher percentage of extranodal involvement in non-GCB subtype [22]. One study reported that patient with nodal DLBCL had better survival than extranodal DLBCL, although there were no significant relationship between molecular subtype, age and gender with nodal location [23].
Ann-Arbor stadium and ECOG performance status were significant as prognostic factors for patient’s outcome in this study. Such findings are generally acceptable considering the relevance between disease burden and clinical status of the patient [24].
This study did not confirm the role of COO as prognostic factor to the patient outcome among DLBCL patients. The controversy over the significance of the prognostic value of the DLBCL molecular subtype may be related with several explanations. These include standardization of methods and evaluation of immunohistochemical examinations, as well as the heterogeneity of clinical characteristics of patients in various studies. Germinal Center B cell Expressed Transcript 1 (GCET 1) and metastasis associated gene 3 (MTA 3) had been proposed as complementary markers for the algorithm panel to differ GCB from non-GCB [25, 26].
There was a tendency to improved survival in GCB and non-GCB subtypes receiving chemotherapy with Rituximab, although the difference was not statistically significant (p=0.67). A study suggested that in the CHOP therapy group, the molecular subtype of GCB had a significantly better prognosis than non-GCB. However, in the R-CHOP therapy group, there was no significant difference. This study showed that the addition of rituximab has altered the prognostic role of COO [3]. The mechanism of action of antibody therapy on non-GCB molecular subtype DLBCL is thought to be related to the NFκB pathway through increased RKIP expression which reduces NFκB pathway activity and weakens DNA binding thereby increasing chemotherapy sensitivity [27, 28]. Previous studies found the average two years survival rate of DLBCL patients with addition of rituximab was 85.6% and 64% with CHOP only [29]. R-CHOP improved survival in the non-GCB subtype (61.3% versus 40.9%; P = 0.0303), but not in the GCB subtype (76.5% versus 61.3%; P = 0.141). These data also suggested that rituximab diminished the prognostic value of COO [30].
Recent advances in next generation sequencing (NGS) provide a broad and comprehensive diversity of cancer genes involved in the pathogenesis of DLBCL. Whole exome sequencing (WES) of more than two hundred DLBCLs has completely redefined the genetic landscape by identifying single nucleotide variants and providing new therapeutic opportunities for GCB, non-GCB, or primary mediastinal B-cell lymphoma (PMBL) subtypes. This study revealed that Double Hit Lymphoma (DHL) / Triple Hit Lymphoma (THL), an aggressive subtype of B cell lymphoma, have identified multiple mutations in genes involved in apoptosis/cell cycle and epigenetic regulatory pathways. The lymphopanel consisting of 43 genes identified distinct mutational signatures of DHL THL with higher mutation rates of CREBBP, BCL2, KMT2D, MYC, EZH2 and FOXO1 in the GCB DLBCL subtype [31]. The ReMODal study described a new molecular classification of DLBCL by GEP which stated that Molecular High Grade B-Cell Lymphoma (MHG) group is a group that has different characteristics from the GCB subtype. With this classification, MHG is a new subtype of DLBCL, which is part of the molecular subtype of GCB but with a more aggressive behavior and resembles the non-GCB and Burkitt Lymphoma (BL) subtypes [11]. Furthermore, a molecular study exploring the immunoglobulin gene classified the molecular subtype of DLBCL based on the expression of 36 somatic hypermutation (SHM) target genes into four subtypes namely SHM 1, SHM 2, SHM 3 and SHM 4 subtypes. GCB subtypes dominate SHM 1 and SHM 3 subtypes, while non-GCB are mostly SHM 2 and a small proportion in the SHM subtype 4. Overall survival between the four subtype groups differed, where SHM subtype 1 is the majority of GCB subtypes with a poor prognosis after receiving R-CHOP chemotherapy. In contrast, SHM 3 subtype has a better prognosis after R-CHOP chemotherapy. The non-GCB subtypes consisting of SHM 2 and SHM 4 had poor survival among all SHM subtypes [32]. Based on these three latest molecular classifications of DLBCL, practical application of immunohistochemisty to determine the cell of origin (COO) required to be improved with wider panel of staining to enable more detail molecular classification of DLBCL that is beneficial for diagnostic and clinical practice.
In conclusion, the role of COO with Hans’ algorithm as prognostic factor in DLBCL is not confirmed in this study. Several possible explanations include the addition of rituximab and the recent molecular characterization of DLBCL which covered a more subtle classification of the disease beyond GCB and non-GCB subtypes.
Acknowledgments
Funding Statement
This research was supported by Hibah Penelitian Dosen-Mahasiswa PPDS Dana Masyarakat (DAMAS) Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada. 2019, and partially by PDUPT Grant of Indonesian Ministry of Research and Technology/ National Agency for Research and Innovation 2020 (No. 2758/UN1.DITLIT/DIT-LIT/ PT/2020).
References
- Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling Alizadeh A. A., Eisen M. B., Davis R. E., Ma C., Lossos I. S., Rosenwald A., Boldrick J. C., Sabet H., Tran T., Yu X., Powell J. I., Yang L., Marti G. E., Moore T., Hudson J., Lu L., Lewis D. B., Tibshirani R., Sherlock G., Chan W. C., Greiner T. C., Weisenburger D. D., Armitage J. O., Warnke R., Levy R., Wilson W., Grever M. R., Byrd J. C., Botstein D., Brown P. O., Staudt L. M.. Nature.2000;403(6769). CrossRef
- High concordance of gene expression profiling-correlated immunohistochemistry algorithms in diffuse large b-cell lymphoma, not otherwise specified Hwang Hee Sang, Park Chan Sik, Yoon Dok Hyun, Suh Cheolwon, Huh Jooryung. American Journal of Surgical Pathology.2014;38(8). CrossRef
- Reassessment of the prognostic value of the International Prognostic Index and the revised International Prognostic Index in patients with diffuse large B-cell lymphoma: A multicentre study Huang Hong-Hui, Xiao Fei, Chen Fang-Yuan, Wang Ting, Li Jun-Min, Wang Jian-Min, Cao Jun-Ning, Wang Chun, Zou Shan-Hua. Experimental and Therapeutic Medicine.2012;4(3). CrossRef
- Evaluation of BCL-6, CD10, CD138 and MUM-1 expression in diffuse large B-cell lymphoma patients: CD138 is a marker of poor prognosis Bodoor Khaldon, Matalka Ismail, Hayajneh Rami, Haddad Yazan, Gharaibeh Waleed. Asian Pacific journal of cancer prevention: APJCP.2012;13(7). CrossRef
- CELL-OF-ORIGIN FAILS TO PREDICT SURVIVAL IN PATIENTS WITH DIFFUSE LARGE B-CELL LYMPHOMA TREATED WITH AUTOLOGOUS HEMATOPOIETIC STEM CELL TRANSPLANTATION Gu Keni, Weisenburger Dennis D, Fu Kai, Chan Wing C, Greiner Timothy C, Aoun Patricia, Smith Lynette M, Bast Martin, Liu Zhongfen, Bociek R. Gregory, Bierman Philip J, Armitage James O, Vose Julie M. Hematological oncology.2012;30(3). CrossRef
- Poor concordance among nine immunohistochemistry classifiers of cell-of-origin for diffuse large B-cell lymphoma: implications for therapeutic strategies Coutinho Rita, Clear Andrew James, Owen Andrew, Wilson Andrew, Matthews Janet, Lee Abigail, Alvarez Rute, Gomes da Silva Maria, Cabeçadas José, Calaminici Maria, Gribben John G.. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2013;19(24). CrossRef
- Incidence and Mortality Trends and Risk Prediction Nomogram for Extranodal Diffuse Large B-Cell Lymphoma: An Analysis of the Surveillance, Epidemiology, and End Results Database Yin Xuejiao, Xu Aoshuang, Fan Fengjuan, Huang Zhenli, Cheng Qianwen, Zhang Lu, Sun Chunyan, Hu Yu. Frontiers in Oncology.2019;9. CrossRef
- Diffuse large B-cell lymphoma: 10 years' real-world clinical experience with rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisolone Horvat Matej, Zadnik Vesna, Južnič Šetina Tanja, Boltežar Lučka, Pahole Goličnik Jana, Novaković Srdjan, Jezeršek Novaković Barbara. Oncology Letters.2018;15(3). CrossRef
- Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001 Morton Lindsay M., Wang Sophia S., Devesa Susan S., Hartge Patricia, Weisenburger Dennis D., Linet Martha S.. Blood.2006;107(1). CrossRef
- Molecular Subtypes, Apoptosis and Proliferation Status in Indonesian Diffuse Large B-Cell Lymphoma Cases Snak Yosinta, Indrawati null, Widayati Kartika, Arfian Nur, Anggorowati Nungki. Asian Pacific journal of cancer prevention: APJCP.2018;19(1). CrossRef
- Molecular High-Grade B-Cell Lymphoma: Defining a Poor-Risk Group That Requires Different Approaches to Therapy Sha Chulin, Barrans Sharon, Cucco Francesco, Bentley Michael A., Care Matthew A., Cummin Thomas, Kennedy Hannah, Thompson Joe S., Uddin Rahman, Worrillow Lisa, Chalkley Rebecca, Hoppe Moniek, Ahmed Sophia, Maishman Tom, Caddy Josh, Schuh Anna, Mamot Christoph, Burton Catherine, Tooze Reuben, Davies Andrew, Du Ming-Qing, Johnson Peter W. M., Westhead David R.. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2019;37(3). CrossRef
- Sex Disparities in Cancer Incidence by Period and Age Cook Michael B., Dawsey Sanford M., Freedman Neal D., Inskip Peter D., Wichner Sara M., Quraishi Sabah M., Devesa Susan S., McGlynn Katherine A.. Cancer Epidemiology, Biomarkers & Prevention.2009;18(4). CrossRef
- Reproductive factors and lymphoid neoplasms in Europe: findings from the EpiLymph case-control study Costas Laura, Casabonne Delphine, Benavente Yolanda, Becker Nikolaus, Boffetta Paolo, Brennan Paul, Cocco Pierluigi, Foretova Lenka, Maynadié Marc, Staines Anthony, Kane Eleanor, Nieters Alexandra, Sanjosé Silvia. Cancer causes & control: CCC.2012;23(1). CrossRef
- Diffuse large B-cell lymphoma in Chinese patients: immunophenotypic and cytogenetic analyses of 124 cases Chen Yan, Han Tao, Iqbal Javeed, Irons Richard, Chan Wing C., Zhu Xiongzeng, Fu Kai. American Journal of Clinical Pathology.2010;133(2). CrossRef
- Immunophenotypic and Genetic Characteristics of Diffuse Large B-Cell Lymphoma (DLBCL), and Prognostic Significance of Cell of Origin (COO) in a Southeast Asian Cohort Chong Vanessa CL, Yap Eng Soo, Xin Liu, Chin Suk Teng, Jeyasekharan Anand, Chee Yen-Lin, Chan Hian Li Esther, De Mel Sanjay, Lee Joanne Shu Xian, Lee Yee Mei, Yuen Yi Ching, Koh Liang Piu, Poon Michelle. Blood.2018;132(Supplement 1). CrossRef
- Shorter Overall Survival in Non-Germinal Center of Diffuse Large B-Cell Lymphoma Based on Hans ’ Criteria among Thai Patients Noiwattanakul J, Assanasen T, Rojnuckarin P, Intragumtornchai T. J. Hematol. Transfus. Med.2017;27(4).
- Comparison of protein-based cell-of-origin classification to the Lymph2Cx RNA assay in a cohort of diffuse large B-cell lymphomas in Malaysia Phang Kean-Chang, Akhter Ariz, Tizen Nur Maya Sabrina, Rahman Faridah Abd, Zahratul Azma Raja, Elyamany Ghaleb, Shabani-Rad Meer-Taher, Masir Noraidah, Mansoor Adnan. Journal of Clinical Pathology.2018;71(3). CrossRef
- Germinal Center B Cell-Like (GCB) and Activated B Cell-Like (ABC) Type of Diffuse Large B Cell Lymphoma (DLBCL): Analysis of Molecular Predictors, Signatures, Cell Cycle State and Patient Survival Blenk S., Engelmann J., Weniger M., Schultz J., Dittrich M., Rosenwald A., Müller-Hermelink H. K., Müller T., Dandekar T.. Cancer Informatics.2007;3. CrossRef
- Sequential Vinorelbine and Docetaxel in Advanced Non-small Cell Lung Cancer Patients Age 70 and Older and/or with a Performance Status of 2: A Phase II Trial of the Southwest Oncology Group (S0027) Hesketh Paul J., Chansky Kari, Lau Derick H. M., Doroshow James H., Moinpour Carol M., Chapman Robert A., Goodwin J. Wendall, Gross Howard M., Crowley John J., Gandara David R.. Journal of Thoracic Oncology.2006;1(6). CrossRef
- Prevalence and clinical implications of epstein-barr virus infection in de novo diffuse large B-cell lymphoma in Western countries Ok Chi Young, Li Ling, Xu-Monette Zijun Y., Visco Carlo, Tzankov Alexander, Manyam Ganiraju C., Montes-Moreno Santiago, Dybkaer Karen, Dybaer Karen, Chiu April, Orazi Attilio, Zu Youli, Bhagat Govind, Chen Jiayu, Richards Kristy L., Hsi Eric D., Choi William W. L., Krieken J. Han, Huh Jooryung, Ai Weiyun, Ponzoni Maurilio, Ferreri Andrés J. M., Farnen John P., Møller Michael B., Bueso-Ramos Carlo E., Miranda Roberto N., Winter Jane N., Piris Miguel A., Medeiros L. Jeffrey, Young Ken H.. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2014;20(9). CrossRef
- Classification of diffuse large B-cell lymphoma by immunohistochemistry demonstrates that elderly patients are more common in the non-GC subgroup and younger patients in the GC subgroup Thunberg Ulf, Enblad Gunilla, Berglund Mattias. Haematologica.2012;97(2). CrossRef
- Frequent Expression of CD10 and Bcl-6 in High-Grade Primary Extranodal Lymphoma with Diffuse Large B-cell Lymphoma Morphology Tai Yan-Chin, Peh Suat-Cheng. Journal of Clinical and Experimental Hematopathology.2004;44(2). CrossRef
- Clinical features and outcomes of diffuse large B-cell lymphoma based on nodal or extranodal primary sites of origin: Analysis of 1,085 WHO classified cases in a single institution in China Shi Yuankai, Han Ying, Yang Jianliang, Liu Peng, He Xiaohui, Zhang Changgong, Zhou Shengyu, Zhou Liqiang, Qin Yan, Song Yongwen, Liu Yueping, Wang Shulian, Jin Jing, Gui Lin, Sun Yan. Chinese Journal of Cancer Research = Chung-Kuo Yen Cheng Yen Chiu.2019;31(1). CrossRef
- An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era Zhou Zheng, Sehn Laurie H., Rademaker Alfred W., Gordon Leo I., LaCasce Ann S., Crosby-Thompson Allison, Vanderplas Ann, Zelenetz Andrew D., Abel Gregory A., Rodriguez Maria A., Nademanee Auayporn, Kaminski Mark S., Czuczman Myron S., Millenson Michael, Niland Joyce, Gascoyne Randy D., Connors Joseph M., Friedberg Jonathan W., Winter Jane N.. Blood.2014;123(6). CrossRef
- Clinico-biological characterization and outcome of primary nodal and extranodal diffuse large B-cell lymphoma in the rituximab era Gutiérrez-García Gonzalo, Colomo Lluis, Villamor Neus, Arenillas Leonor, Martínez Antonio, Cardesa Teresa, García-Herrera Adriana, Setoain Xavier, Rodríguez Sonia, Ghita Gabriela, Abrisqueta Pau, Giné Eva, Bosch Francesc, Campo Elías, Montserrat Emilio, López-Guillermo Armando. Leukemia & Lymphoma.2010;51(7). CrossRef
- Ki-67 expression as a prognostic factor in diffuse large B-cell lymphoma patients treated with rituximab plus CHOP Yoon Dok Hyun, Choi Dae Ro, Ahn Heui June, Kim Shin, Lee Dae Ho, Kim Sang-We, Park Bong-Hee, Yoon Sun Och, Huh Jooryung, Lee Sang-Wook, Suh Cheolwon. European Journal of Haematology.2010;85(2). CrossRef
- Role of nuclear factor kappa B in neuropathological mechanisms Cechetto DF. Prog. Brain Res.2001;132:391-404. CrossRef
- Cooperative signaling through the signal transducer and activator of transcription 3 and nuclear factor-{kappa}B pathways in subtypes of diffuse large B-cell lymphoma Lam Lloyd T., Wright George, Davis R. Eric, Lenz Georg, Farinha Pedro, Dang Lenny, Chan John W., Rosenwald Andreas, Gascoyne Randy D., Staudt Louis M.. Blood.2008;111(7). CrossRef
- Prognostic factors in patients with diffuse large B cell lymphoma: Before and after the introduction of rituximab Ngo Lynette, Hee Siew-Wan, Lim Lay-Cheng, Tao Miriam, Quek Richard, Yap Swee-Peng, Loong Er-Li, Sng Ivy, Hwan-Cheong Tan Leonard, Ang Mei-Kim, Ngeow Joanne, Tham Chee-Kian, Tan Min-Han, Lim Soon-Thye. Leukemia & Lymphoma.2008;49(3). CrossRef
- Outcome of R-CHOP or CHOP regimen for germinal center and nongerminal center subtypes of diffuse large B-cell lymphoma of Chinese patients Huang Ying, Ye Sheng, Cao Yabing, Li Zhiming, Huang Jiajia, Huang He, Cai Muyan, Luo Rongzhen, Lin Tongyu. TheScientificWorldJournal.2012;2012. CrossRef
- Next-Generation Sequencing in Diffuse Large B-Cell Lymphoma Highlights Molecular Divergence and Therapeutic Opportunities: a LYSA Study Dubois Sydney, Viailly Pierre-Julien, Mareschal Sylvain, Bohers Elodie, Bertrand Philippe, Ruminy Philippe, Maingonnat Catherine, Jais Jean-Philippe, Peyrouze Pauline, Figeac Martin, Molina Thierry J., Desmots Fabienne, Fest Thierry, Haioun Corinne, Lamy Thierry, Copie-Bergman Christiane, Brière Josette, Petrella Tony, Canioni Danielle, Fabiani Bettina, Coiffier Bertrand, Delarue Richard, Peyrade Frédéric, Bosly André, André Marc, Ketterer Nicolas, Salles Gilles, Tilly Hervé, Leroy Karen, Jardin Fabrice. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2016;22(12). CrossRef
- Distinct subtypes of diffuse large B-cell lymphoma defined by hypermutated genes Alkodsi Amjad, Cervera Alejandra, Zhang Kaiyang, Louhimo Riku, Meriranta Leo, Pasanen Annika, Leivonen Suvi-Katri, Holte Harald, Leppä Sirpa, Lehtonen Rainer, Hautaniemi Sampsa. Leukemia.2019;33(11). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2022
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times