Persistent Mullerian Duct Syndrome with Malignant Conversion of Mullerian Remnant
Download
Abstract
A case report of a very rare entity of persistent Müllerian duct syndrome with malignant transformation of Mullerian remnant is presented. A twenty-two-year-old phenotypically normal male presented with complain of hematuria and lower abdominal pain. On examination patient had bilateral cryptorchidism and on imaging bilateral duplication of pelvicalyceal system and ureters and left sided ureterocoele. There was a malignant mass lesion with metastatic lymphadenopathy in pelvis. The diagnosis of persistent Mullerian duct syndrome was suspected on MRI pelvis and confirmed by genetic and pathological testings. Risk of malignant transformation of undescended testis and Mullerian remnants increases with age and have impact on psychological health of patient, so early diagnosis becomes crucial.
Introduction
Persistent Müllerian Duct Syndrome is a very rare type of pseudo hermaphroditism with less than 250 cases reported till date in medical literature. It is type of pseudo hermaphroditism with male phenotype and genotype. There is unilateral or bilateral undescended testis and presence of Müllerian structures like Fallopian tube, uterus, cervix or vagina. There is associated increased risk of germ cell malignancy as consistent with cryptorchidism [1]. Some cases of malignant transformation of Müllerian remnants have been reported. Age at presentation is highly variable from infant to old age. Clinical presentation is most commonly as unilateral or bilateral undescended testis associated with or without inguinal hernia, as abdominal mass or hematuria. Sometimes, it is an incidental diagnosis at time of surgery for above conditions when Müllerian remnants like Fallopian tubes, uterus are found. Early recognition and diagnosis are essential to prevent development of malignancy as well as associated psychosocial impact on patient. For diagnosis and treatment, holistic multidisciplinary approach is needed [2].
Case Presentation
A 22-year-old phenotypically male patient presented to surgical emergency at tertiary hospital with chief complaints of lower abdominal pain and one episode of Hematuria. On examination patient was tall (193 cm) with supernumerary nipple along right milk line. Physical examination revealed bilateral empty scrotal sac with coronal type of hypospadias (Figure 1).
Figure 1. (A) Image Showing Tall Stature of the Patient with Supernumerary Nipple and (B) Male External Genitalia with Hypospadias on Examination.

Breast development corresponds to Tanner stage I (only the papilla is elevated above the level of the chest wall), pubic hairs development corresponds to Tanner stage V (adult type hair with inverse triangle shaped distribution). Axillary hairs were present, however no facial hair and chest wall hair were seen. Patient had coarse voice. Clubbing was present in both hands. Birth history revealed full term birth of male child from a non- consanguineous marriage. There was episode of cyanosis at birth and was diagnoses with VSD for which was operated at three years of age. Developmental history was normal in terms of achievement of milestones with normal intelligent quotient. Family history suggested two younger male siblings with similar tall height but otherwise normal.
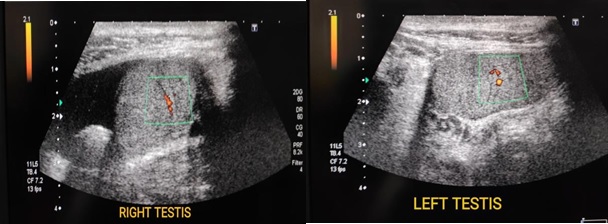
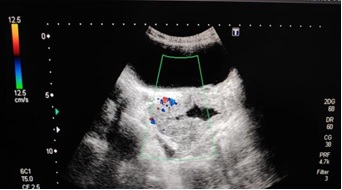
Patient was referred for abdominal and inguinoscrotal ultrasound to radiology department. The USG was performed on TOSHIBA-DIAGNOSTIC ULTRASOUND SYSYTEM-SSA-660A. Inguinoscrotal ultrasound examination, revealed bilateral empty scrotal sac with both normal appearing testis in deep inguinal rings on either side suggesting -bilateral undescended testis. Both testis showed preserved vascularity on colour Doppler study. Mild hydrocele was noted on right side (Figure 2).
Figure 2. Images Showing Ultrasound and Doppler Images of Bilateral Testes in Deep Inguinal Ring with Minimal Fluid Surrounding Right Testis.
Abdominal ultrasound examination revealed a uterus like structure with central fluid filled cavity posterior to bladder and anterior to rectum. There is no definite visualization of prostate. A small ureterocele projecting from left posterolateral wall of urinary bladder with periodical collapse after ureteric jet was visualized (Figure 3).
Figure 3. Image Showing Uterus Like Structure Posterior to Bladder and Left Sided Ureterocele.
Mild hydroureter of both ureters seen on left side. Both kidneys were enlarged with double collecting system and ureters. MRI pelvis was done for further delineation of the pelvic structures. High resolution MR imaging was performed using 1.5 Tesla Achieva system (Philips Medical systems). MR imaging protocol included the following: T1 and T2 weighted spin echo sequence in axial, coronal, and Sagittal planes, T1 noncontrast FAT SAT, contrast FAT SAT images in axial, coronal and sagittal plane were obtained after giving intravenous Gadolinium contrast (Figure 4).
Figure 4. (A) T2 Weighted Axial Imagine Shows Left Sided Uretrocoele and Retrobladder Central Fluid Filled Mullerian remnant. (B) T2 weighted axial image showing bilateral undescended testis (C) T2 weight axial image shows left pelvic metastatic lymph node (D) T2 weighted Sagitta images shows common opening of the Mullerian remnant into urethra (E) post Gadolinium T1 weighted axial FATSAT images showing internal genital organs(testes) and infiltrating mass of mullerian structure.
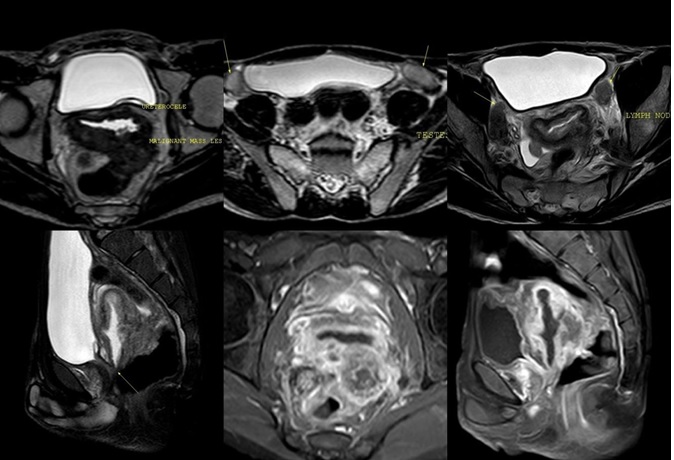
MRI of pelvis showed infiltrating mass lesion with central fluid filled cavity of the Mullerian structures with metastatic pelvic lymphadenopathy. The mass was infiltrating left pyriformis muscle. Central fluid filled cavity merges with urethra and opens externally via common external urethral opening (Figure 5).
Figure 5. (A) Delayed Phase in Axial Plane of CT IVP Shows Bilateral Double Ureters (B) Delayed Phase in Axial Plane at Bladder Level Showing Normal Excretion on Right Side and both Ureter Combining Together as Single unit to Open into Bladder (C) Nephrogram Phase in Coronal Reformatted Section Shows Enlarged Bilateral Kidneys (D) 3D Reconstruction Shows Bilateral Duplex System.
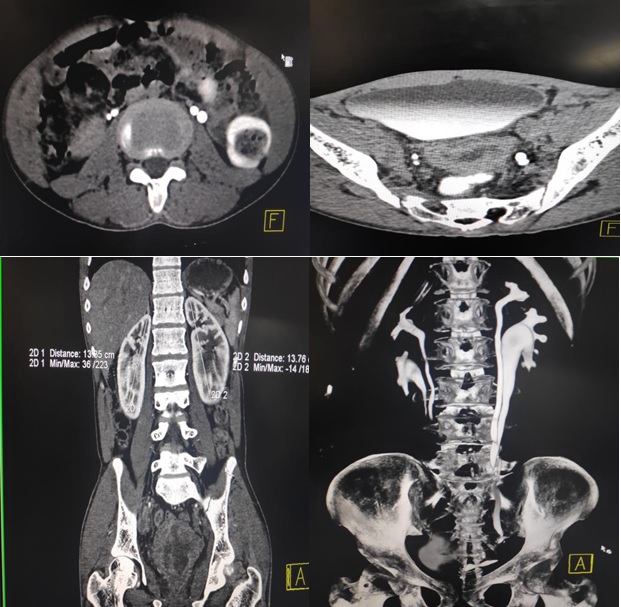
Ovaries were not visualised. Double ureters and left uretrocoele was noted. Lower part of ureter on left side was encased by infiltrating mass lesion. There was mild left sided hydroureter. CT Intravenous pyelography was performed which revealed duplex pelvicalyceal system with bifid ureter on both sides. Upper and lower moiety of right kidney opening at normal position into bladder without any obstruction or hydroureter, while on left side upper and lower moiety unite few centimeter before its opening at junction of bladder neck and posterior urethra with obstructing ureterocele. Hormonal assay revealed increase level of Follicle stimulating hormone-47.50 mIU/ml (Normal range:0.95 to 11.95 mIU/ml) with normal levels of Luteinizing hormone, Prolactin, Testosterone and Thyroid stimulating hormone.
Discussion
Persistent Mullerian Duct syndrome shows autosomal recessive inheritance in most cases with some cases showing familial X-linked inheritance as well as sporadic occurrence [3]. Both the Müllerian (paramesonephric) as well as Wolffian (mesonephric) ducts are present in a fetus at 7th week of gestation. In male fetus (XY), SRY gene on Y chromosome determines development of testis as gonads. Leydig cells secrete testosterone, acts locally on Wolffian duct and helps in differentiation and development of male internal genital organs epididymis, vas deferens, and seminal vesicle, whereas dihydrotestosterone helps in the formation of urogenital sinus and external genitalia-penis and scrotum. Immature Sertoli cells in testis secrete Müllerian-inhibiting factor (MIF) or Anti Mullerian hormone (AMH), which is a glycoprotein that helps in regression of Müllerian ducts in male fetuses [4]. Mutations of the Mullerian inhibiting substance (MIS) gene or the MIS type II receptor (MISRII) gene have been identified in PMDS patients with autosomal recessive transmission [5]. Most common point mutation being 27bp deletion in kinase domain [6].
Out of the reported cases in literature, these are broadly classified into following variants [7]: (i) In the most common male type, there is bilateral undescended testis; (ii) Second variant in which one testis is found within the scrotum; the uterus and ipsilateral fallopian tube are either in the inguinal canal or can be brought into it by gentle traction on the presenting testis; (iii) In some cases, the contralateral testis and tube are also in the hernial sac; transverse testicular ectopia can also occur; (iv). The least common form, or female type, is characterized by bilateral cryptorchidism with testes embedded in the broad ligaments in an ovarian position with regard to the uterus, which is fixed in the pelvis.
As development of testis is normal as determined by SRY gene on Y chromosome, testosterone production is normal which leads to normal male external genitalia development with near normal development of Wolffian derivatives and secondary sexual characteristics. Males with persistent Mullerian development syndrome may be fertile if Mullerian derived structures have not compromised the integrity of Wolffian structures and correction of cryptorchidism is timely done, preferably before the age of one year without significant germ cell hypoplasia [6]. For such timely diagnosis and management, MRI is investigation of choice as it provides great tissue contrast and offers a unique capability to delineate the pelvic organs and the complex structures in PMDS, which are difficult to be determined on either US or CT. In this case also application MRI has been very useful in proper and apt diagnosis.
In conclusion, in patient presenting with cryptorchidism, a scrutinize examination should be made for persistent Müllerian duct syndrome. Patient of cryptorchidism if presents with hematuria, further investigation should be performed to rule out malignancy of Mullerian structures in persistent Müllerian duct syndrome. MRI pelvis is investigation of choice in early diagnosis of persistent Müllerian duct syndrome. Early suspicion and diagnosis should be made as there is risk of malignancy in Mullerian structures in persistent Müllerian duct syndrome.
References
- Persistent Müllerian duct syndrome: lessons learned from managing a series of eight patients over a 10-year period and review of literature regarding malignant risk from the Müllerian remnants Farikullah Jasmin, Ehtisham Sarah, Nappo Simona, Patel Leena, Hennayake Supul. BJU international.2012;110(11 Pt C). CrossRef
- Persistent Mullerian Duct Syndrome: a rare entity with a rare presentation in need of multidisciplinary management Da Aw Lin, Zain Murizah M., Esteves Sandro C., Humaidan Peter. International Braz J Urol: Official Journal of the Brazilian Society of Urology.2016;42(6). CrossRef
- Persistent Müllerian duct syndrome Hm Dekker, Ij de Jong, J Sanders, Rf Wolf. Radiographics : a review publication of the Radiological Society of North America, Inc.2003;23(2). CrossRef
- Anti-müllerian hormone in early human development Josso N., Lamarre I., Picard J. Y., Berta P., Davies N., Morichon N., Peschanski M., Jeny R.. Early Human Development.1993;33(2). CrossRef
- Persistent Mullerian duct syndrome caused by both a 27-bp deletion and a novel splice mutation in the MIS type II receptor gene Hoshiya Makiko, Christian Benjamin P., Cromie William J., Kim Hyung, Zhan Yong, MacLaughlin David T., Donahoe Patricia K.. Birth Defects Research. Part A, Clinical and Molecular Teratology.2003;67(10). CrossRef
- The Persistent Müllerian Duct Syndrome: An Update Based Upon a Personal Experience of 157 Cases Jy Picard, Rl Cate, C Racine, N Josso. Sexual development : genetics, molecular biology, evolution, endocrinology, embryology, and pathology of sex determination and differentiation.2017;11(3). CrossRef
- Persistent müllerian duct syndrome: a case report Patil Vijaya, Muktinaini Sunilkrishna, Patil Rashmi, Verma Ashish. The Indian Journal of Surgery.2013;75(Suppl 1). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2022
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times