A Study of Multivariate Modalities of Therapy in Gall Bladder Malignancies with Different Clinical, Radiological and Histo-Pathological Profiles at a Low Resource Indian Cancer Centre
Download
Abstract
Introduction: The effectiveness of adjuvant radiation therapy (RT) in the treatment of GBC has not yet been established. The effectiveness of adjuvant radiation therapy (RT) in the treatment of GBC has not yet been established. Following surgical resection, postoperative external beam RT can diminish local relapse; though effect on global survival has not been confirmed due to lack of good quality clinical trials. Thus the aim of the study was to assess the efficacy of the multivariate modality of therapy in Gallbladder malignancies.
Materials and method: The present prospective comparative study of multivariate modality of therapy in Gallbladder malignancies was carried out in a low resource North Indian cancer center from. Only those patients were selected who were histologically confirmed of adenocarconima of gall bladder, squamous cell carcinoma and oat cell carcinoma. Staging was done according to TNM classification no concurrent medical illness, Karnofsky performances status of patient >70. Statistical analyses were performed using SPSS version 23.0 (SPSS Inc., Chicago, IL, USA). A value of p<0.05 was considered statistically significant.
Result: Majority (29.3%) of the cases were aged 61-70 years just followed by the age group 41-50 years, females were in majority (73.2%). Higher stage had significantly higher risk of partial or no response (p<0.001). An increasing trend of toxicities was observed with increasing dose. Significant association was found between toxicities and treatment response at dose 2000 cGy (p<0.001) and 4500 cGy (p<0.001). The Cox regression analysis showed higher hazard ratio for stage IV (HR=2.33 95% CI : 0.31-17.66) followed by stage II/III (HR=1.67 95% CI : 0.17-16.02).
Conclusion: Healthcare professionals should discuss the indeterminate benefit and possible risks of adjuvant therapy when counseling their patients and reassure enrollment in clinical trials of novel regimens. The curative potential of current adjuvant therapy in gallbladder cancer is questionable, justifying placebo-controlled investigation of novel chemotherapy combinations or alternative approaches. To confirm the efficacy of adjuvant therapy, a major randomized controlled trial is required with large sample size. The improvement in radiation delivery with intensity modulated radiotherapy (IMRT), image guided radiotherapy has further paved the way for exploration of adjuvant radiation for GBC.
Introduction
Gall bladder carcinoma (GBC) is fifth most common and one of the most prevalent malignancies of gastro intestinal tract [1, 2]. It has been found that most of the GBC′s are adenocarcinoma which arises from the epithelium. It has been recounted to be three to five times more common in females than males. The GBC pathogenesis has been elucidated by two hypothesis, gall stone-cholecystitis (relative risk of 4.9) and anomalous biliary ducts [3]. Because of the prevalence, randomized controlled studies to determine the best therapy have been few and far between. As a result, therapy recommendations are based on retrospective research and limited prospective cohort studies, both of which should be read cautiously. Surgical excision has long been thought to be the sole curative therapy for these tumors. In majority of the described studies, however, even after a successful surgical resection, median survival does not surpass 14–15 months. As a result, adjuvant treatment looks to be a really good idea for better tumor control and survival [3].
Surgery is contemplated as the only conclusive treatment for non-metastatic gall bladder cancer. For tumors confined to the lamina propria [T1a], a simple cholecystectomy is sufficient. In all other situations, a prolonged cholecystectomy [T1b onward] is suggested. Matsumoto et al. evaluated the three-year survival of Stage II GBC patients who had simple cholecystectomy vs a radical cholecystectomy [4]. The authors found a three-year survival rate of 29–57 percent vs. 100 percent in favor of radical cholecystectomy, making it the standard surgical technique for Stage II GBC. However, even after extensive surgical resection, three-year survival rates in Stage III and Stage IV ranged from 7% to 80% [5].
Extended cholecystectomy involves cholecystectomy, en bloc hepatic lobe IVB and V resection, as well as nodal resection [6]. Attaining an R0 resection is important to ameliorate survival outcomes of GBC patients [4]. Study conducted by Itoh et al found that overall 5-year survival rates of GBC patients were 73%, 40%, and 0% for R0, R1 and R2, respectively [5]. The prominence of R0 resection in improving the survival rate has also been revealed in other studies [7]. Despite an R0 resection, dismal prognosis in GBC has been confronted with high rates of loco regional failures in addition to systemic metastasis. Hence, adjuvant radiation or chemo-radiation has been espoused in many healthcare centers to augment tumor control and thus improvement in survival rate.
The pattern of local recurrence and nodal distribution in gall bladder tumors will aid us in determining the target volumes for post-operative radiation. The lymph nodes along the cystic and common bile ducts are the principal nodal drainage sites, after which they expand to pancreaticoduodenal nodes and para-aortic nodes. Local– regional relapse affects 45 percent of relapsing patients. The hilum, anastomotic site, resection border of the liver, and retroperitoneal lymph nodes are the most common locations of solitary loco regional recurrence in GBC [8]. Matsumoto et al. studied lymph node metastasis and divided it into three groups: cystic, peridochal, and hilar lymph nodes, peripancreatic, portal, and hepatic lymph nodes, and celiac, periduodenal, and peri-mesenteric lymph nodes. In groups 1, 2, and 3, the authors reported a 17 percent, 18.8 percent, and 14.5 percent probability of lymph node metastasis, respectively [4].
The tumor bed and nearby lymph nodes must be included in the post-operative radiation volumes. The porta hepatis, celiac, para-aortic, and pancreaticoduodenal lymph nodes must all be included in the regional lymph nodes. The surgical clips with 1 cm isotropic extension and the GB fossa, as seen in the pre-operative CECT picture, make up the CTV primary [9, 10].
A dosage of 45 Gy delivered in 25 halves over 5 weeks has been proven to enhance outcomes in GBC patients. In an R1 resection, a 5.4 Gy increase may be considered. In situations with substantial residual lesion, a 15-Gy increase may be administered [11-14]. The liver and the right kidney are the key organs at risk in GBC radiation planning. The spinal cord is another organ at danger whose tolerance must be considered. Three-dimensional conformal radiation based on computed tomography should be employed. The ideal field configuration may be a three-field method, with differential weightage of these beams allowing irradiation of these volumes while retaining the tolerance of the organs at risk. The left lateral beam may be delivered by higher energy photons like 15 MV or 18 MV to further optimize the plan. It must be an aim to keep the average dosage to the liver under 30 Gy and the amount of liver that receives 30 Gy under 60%. The renal mean dosage must be kept below 16–18 Gy volume, with 20 Gy getting less than 66 percent of the total dose. We could also strive to keep the total volume of both kidneys getting 20 Gy to less than 75%. The maximal dosage to the spinal cord should be maintained below 45 Gy if possible [8, 15-17].
The effectiveness of adjuvant radiation therapy (RT) in the treatment of GBC has not yet been established. Following surgical resection, postoperative external beam RT can diminish local relapse; though effect on global survival has not been confirmed due to lack of good quality clinical trials. Retrospective analyses with a small sample size in which either RT alone or CRT (generally with a concomitant fluoropyrimidine) was administered have proposed improved survival [17-21]. In most circumstances, the inference was that the patients who underwent RT as a part of therapy (particularly at doses ≥40 Gy) had enhanced survival rate than those who did not. The aim of the present study was to assess the efficacy of multivariate modality of therapy in Gallbladder malignancies.
Materials and Methods
The present prospective comparative study of multivariate modality of therapy in Gallbladder malignancies was carried out in a low resource North Indian cancer center. Only those patients were selected who were histologically confirmed of adenocarconima of gall bladder, squamous cell carcinoma and oat cell carcinoma. In addition the only those patients were included who did not receive any prior treatment for their present illness surgery/radiotherapy / chemotherapy. Staging was done according to TNM classification no concurrent medical illness, Karnofsky performances status of patient >70.
Study group selection and treatment protocol
41 patients were treated with surgery/ cholecystectomy /extended cholecystectomy,as indicated and adjuvant radiotherapy and combination chemotherapy.
Patients at stage 0/1 underwent surgery while patients with stage II/III were treated with extended cholecystectomy followed by radiotherapy, maximum in the form of 45Gy/4 weeks/200 cGy/fraction to the large field size i.e.; gall bladder and its associated area (Right hypochondrium / epigastrium). Patients at stage IV were treated with chemotherapy in the form of 400mg/m 5FU IV 14 day, 200 mg/ml IV leucovorin, Mitomycin C 8 mg/ml IV one day and repeat after 28 days into 6 cycles / other chemotherapy like cisplatin and 5-fluorouracil in poor patients.
Among 41 patients who were treated with surgery / cholecystectomy / extended cholecystectomy as indicated) and combination chemotherapy (400mg/m 5FU IV 1-4 days, 200 mg/m2 IV Leucovorin 14 days, mitomycin C (8 mg/ml IV one day) and repeat after 28 days into 6 cycles). Other chemotherapy like 5 FU and cisplatin was used in poor patients. Scheduling it prior or depending upon feasibility of the surgical procedures.
Statistical analysis
The results were analyzed using descriptive statistics and making comparisons among various groups. Categorical data were summarized as proportions and percentages (%) while discrete (quantitative) as mean (SD). All the associations were tested by using chi square test. Cox Regression analysis was performed for making model of treatment response outcome with general & clinical Profile of Patients. Statistical analyses were performed using SPSS version 23.0 (SPSS Inc., Chicago, IL, USA). A value of p<0.05 was considered statistically significant.
Results
Majority (29.3%) of the cases were aged 61-70 years just followed by the age group 41-50 years, females were in majority (73.2%). Adenocarcinoma was found in all the cases except one case where diagnosis was unspecified. Stage IV was the most common stage as found in 85.4% cases, while stage II/III was found in 12.2% cases respectively (Table 1).
| Variable | Total | |||
| No. | % | |||
| Age | 31 - 40 yr | 7 | 17.10 | |
| 41 - 50 yr | 10 | 24.40 | ||
| 51 - 60 yr | 8 | 19.50 | ||
| 61 - 70 yr | 12 | 29.30 | ||
| 71 - 80 yr | 4 | 9.80 | ||
| sex | Male | 11 | 26.80 | |
| Female | 30 | 73.20 | ||
| Histopathology | Adenocarcinoma | 40 | 97.60 | |
| Unspecified | 1 | 2.40 | ||
| Stage | Stage 0/I | 1 | 2.40 | |
| Stage II/III | 5 | 12.20 | ||
| Stage IV | 35 | 85.40 |
The analysis to find any association of treatment response with General & Clinical Profile of Patients (Table 2) revealed that the age group was not found to be significantly associated with response (p=0.414). Gender too not found to be associated significantly (p=0.482). Histopathology was near to significant level for association with response (p=0.050). However higher stage had significantly higher risk of partial or no response (p<0.001).
| Variable | Response | chi sq | p-value | ||||||
| CR (N=6) | PR (N=28) | NR (N=7) | |||||||
| No. | % | No. | % | No. | % | ||||
| Age | 31 - 40 yr | 2 | 28.6 | 2 | 28.6 | 3 | 42.9 | 8.21 | 0.414 |
| 41 - 50 yr | 2 | 20 | 7 | 70 | 1 | 10 | |||
| 51 - 60 yr | 1 | 12.5 | 6 | 75 | 1 | 12.5 | |||
| 61 - 70 yr | 1 | 8.3 | 9 | 75 | 2 | 16.7 | |||
| 71 - 80 yr | 0 | 0 | 4 | 100 | 0 | 0 | |||
| Sex | Male | 2 | 18.2 | 6 | 54.5 | 3 | 27.3 | 1.46 | 0.482 |
| Female | 4 | 13.3 | 22 | 73.3 | 4 | 13.3 | |||
| Histopathology | Adenocarcinoma | 5 | 12.5 | 28 | 70 | 7 | 17.5 | 5.98 | 0.05 |
| Unspecified | 1 | 100 | 0 | 0 | 0 | 0 | |||
| Stage | Stage 0/I | 1 | 100 | 0 | 0 | 0 | 0 | 41 | <0.001 |
| Stage II/III | 5 | 100 | 0 | 0 | 0 | 0 | |||
| Stage IV | 0 | 0 | 28 | 80 | 7 | 20 |
The distribution of toxicities with radiotherapy dose (Table 3) revealed that no. of patients with toxicities was highest at 4500 cGy dose (17.1%) followed by the 4000 cGy dose (12.2%). So the increasing trend of toxicities was observed with increasing dose.
| Dose | Total | ||
| No. | % | ||
| 1000 cGy | 0 | 0.00 | |
| 2000 cGy | 4 | 9.80 | |
| 3000 cGy | 3 | 7.30 | |
| 4000 cGy | 5 | 12.20 | |
| 4500 cGy | 7 | 17.10 |
The significant association was found between toxicities and treatment response at dose 2000 cGy (p<0.001) and 4500 cGy (p<0.001). At lower dose of 2000 cGy, toxicities was present among complete response cases, while at higher dose of 4000 cGy and 4500 cGy, toxicities were present among partial/no response cases (p<0.001).
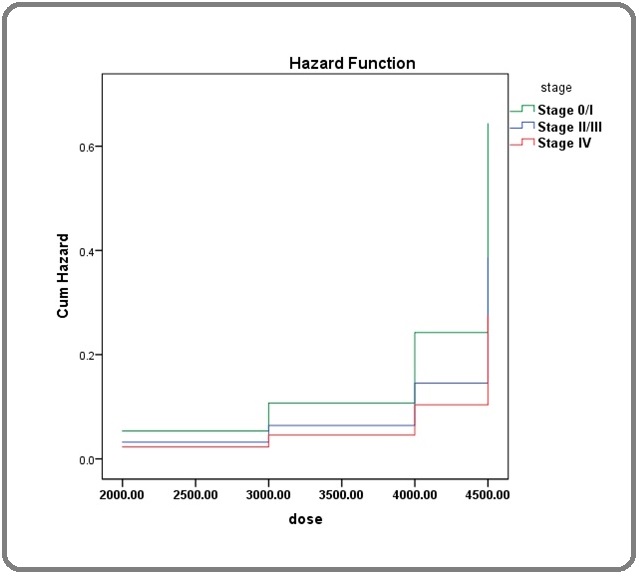
The Cox regression analysis showed higher hazard ratio for stage IV (HR=2.33 95% CI : 0.31-17.66) followed by stage II/III (HR=1.67 95% CI : 0.17-16.02) (Table 4 and 5) (Figure 1).
| Dose | No. of Toxicities | chi sq | p-value | |||||
| CR (N=6) | PR (N=28) | NR (N=7) | ||||||
| No. | % | No. | % | No. | % | |||
| 1000 cGy | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| 2000 cGy | 4 | 66.7 | 0 | 0 | 0 | 0 | 25.86 | <0.001 |
| 3000 cGy | 0 | 0 | 3 | 10.7 | 0 | 0 | 1.5 | 0.472 |
| 4000 cGy | 0 | 0 | 3 | 10.7 | 2 | 28.6 | 2.64 | 0.267 |
| 4500 cGy | 0 | 0 | 0 | 0 | 7 | 100 | 41 | <0.001 |
| Stage | B | SE | p-value | HR | 95% CIL for HR | 95% CIU for HR |
| Stage | 0.645 | |||||
| Stage 0/I | Ref. | |||||
| Stage II/III | -0.511 | 1.15 | 0.658 | 1.67 | 0.17 | 16.02 |
| Stage IV | -0.847 | 1.03 | 0.412 | 2.33 | 0.31 | 17.66 |
Figure 1. Hazard Ratio and Cite.
Discussion
GBC is the fifth most prevalent and one of the most malignant cancers of the gastrointestinal system. Because of their high prevalence, randomized controlled trials to determine the best therapy have been few and far between. Surgical excision has long been thought to be the sole curative therapy for these tumors. However, the outcome is still uncertain. The most common failure pattern is loco-regional, followed by systemic failure. As a result, many institutes have employed local adjuvant radiation in conjunction with contemporaneous and adjuvant chemotherapy. Patients with regional metastasis or tumors invading the liver who were treated with adjuvant radiation lived longer, notwithstanding the limitations of the large retrospective cohort.
With the accessibility of innovative refined radiation techniques it may be possible to deliver adequate dose to the target while keeping the organs at risk within tolerable limits. There is also a probability of increasing the dose to by using image guidance and improved radiation delivery techniques.
Majority (29.3%) of the cases were aged 61-70 years just followed by the age group 41-50 years, females were in majority (73.2%). Other literatures by Hundal R et al, Cavallaro A and Kim HJ were also found consistent in terms of age where mean age of GBC at diagnosis was found as 64–69.4 years [22-24]. Adenocarcinoma was found in all the cases except one case where diagnosis was unspecified. Study conducted by Singh SK et al revealed that females considerably outnumbered males with 80 females and 26 males (F: M = 3:1) [25]. Another study conducted by Dubey AP et al, also reported that 77.94% of the patients were females. Stage IV was the most common stage as found in 85.4% cases, while stage II/III was found in 12.2% cases respectively. Our result was consistent with the outcome of Dubey AP et al as he also found that (57/68) of patients had advanced stage disease. Singh SK et al also found concomitant results such that stage I (0%), stage II (4%), stage IIIA (10%), stage IIIB (8%), stage IVA (17%), and stage IVB (61%) [26].
The analysis to find any association of treatment response with General & Clinical Profile of Patients revealed that the age group was not found to be significantly associated with response (p=0.414). Gender too not found to be associated significantly (p=0.482). Histopathology was near to significant level for association with response (p=0.050). However higher stage had significantly higher risk of partial or no response (p<0.001). A Study on the Clinical Profile and Treatment Outcomes in Gallbladder Carcinoma from Northern India done by Sreen A et al also found that higher stage profile patients showed poor survival rate as well as treatment response [27].
The distribution of toxicities with radiotherapy dose revealed that number of toxicities was highest for higher 4500 cGy dose (17.1%) followed by the 4000 cGy dose (12.2%). So the increasing trend of toxicities was observed with increasing dose. The significant association was found between toxicities and treatment response at dose 2000 cGy (p<0.001) and 4500 cGy (p<0.001). At lower dose of 2000 cGy, toxicities was present among complete response cases, while at higher dose of 4000 cGy and 4500 cGy, toxicities were present among partial/ no response cases (p<0.001).Although similar study conducted by Tanguturi SK et al found no relationship between gallbladder dose and toxicity and did not reach the maximum tolerated gallbladder dose in this cohort treated with high-dose radiation [28]. Study conducted by Bosset et al and Czito et al where radiation dose of 45 Gy and 46 Gy was delivered produced moderate acute side effects in five patients however, higher doses 54 Gy (range 50.4–60.8 Gy) delivered as mentioned in study conducted by Kresl et al produced Chronic toxicity in two patients. These results unveiled that fact that higher doses could cause toxicity in patients [29-31].
In conclusion, healthcare professionals should discuss the indeterminate benefit and possible risks of adjuvant therapy when counseling their patients and reassure enrollment in clinical trials of novel regimens. To confirm the efficacy of adjuvant therapy, a major randomized controlled trial is required. Current adjuvant therapy for gallbladder cancer has unclear curative potential, necessitating a placebo-controlled study of innovative chemotherapy combinations or other techniques. Over the last decade the radiotherapy technique has witnessed a paradigm shift from 2D planning to conformal radiation with an aim to optimize tumor control and minimize both acute and chronic radiation morbidity. Modern radiation procedures and target delineation radiation may increase the impact without adding to the toxicity profile in today’s day. As a result, radiation in gall bladder cancer should be reconsidered in order to improve the treatment outcome of such a dangerous illness. The improvement in radiation delivery with intensity modulated radiotherapy (IMRT), image guided radiotherapy has further paved the way for exploration of adjuvant radiation for GBC.
References
- Cancer statistics, 2015 Siegel Rebecca L., Miller Kimberly D., Jemal Ahmedin. CA: a cancer journal for clinicians.2015;65(1). CrossRef
- Epidemiology and molecular pathology of gallbladder cancer Lazcano-Ponce E. C., Miquel J. F., Muñoz N., Herrero R., Ferrecio C., Wistuba I. I., Alonso de Ruiz P., Aristi Urista G., Nervi F.. CA: a cancer journal for clinicians.2001;51(6). CrossRef
- Gallbladder cancer worldwide: geographical distribution and risk factors Randi Giorgia, Franceschi Silvia, La Vecchia Carlo. International Journal of Cancer.2006;118(7). CrossRef
- Surgical treatment of primary carcinoma of the gallbladder based on the histologic analysis of 48 surgical specimens Matsumoto Y., Fujii H., Aoyama H., Yamamoto M., Sugahara K., Suda K.. American Journal of Surgery.1992;163(2). CrossRef
- Current Management of Gallbladder Carcinoma Zhu Andrew X., Hong Theodore S., Hezel Aram F., Kooby David A.. The Oncologist.2010;15(2). CrossRef
- Defining the role of adjuvant therapy: cholangiocarcinoma and gall bladder cancer Williams Terence M., Majithia Lonika, Wang Samuel J., Thomas Charles R.. Seminars in Radiation Oncology.2014;24(2). CrossRef
- Carcinoma of the gallbladder Misra Sanjeev, Chaturvedi Arun, Misra Naresh C., Sharma Indra D.. The Lancet. Oncology.2003;4(3). CrossRef
- Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies Jarnagin William R., Ruo Leyo, Little Sarah A., Klimstra David, D'Angelica Michael, DeMatteo Ronald P., Wagman Raquel, Blumgart Leslie H., Fong Yuman. Cancer.2003;98(8). CrossRef
- Recent advances in systemic therapies and radiotherapy for gallbladder cancer Caldow Pilgrim CH, Groeschl RT, Quebbeman EJ, Gamblin TC. Surg Oncol.2013;22(1):61-67.
- Irradiation therapy for gallbladder carcinoma: recent advances Houry S., Barrier A., Huguier M.. Journal of Hepato-Biliary-Pancreatic Surgery.2001;8(6). CrossRef
- Image-guided intensity-modulated radiotherapy (IG-IMRT) for biliary adenocarcinomas: Initial clinical results Fuller Clifton David, Dang Nguyen Dinh, Wang Samuel J., Desai Prashant, Choi Mehee, Thomas Charles R., Fuss Martin. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2009;92(2). CrossRef
- SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma Ben-Josef Edgar, Guthrie Katherine A., El-Khoueiry Anthony B., Corless Christopher L., Zalupski Mark M., Lowy Andrew M., Thomas Charles R., Alberts Steven R., Dawson Laura A., Micetich Kenneth C., Thomas Melanie B., Siegel Abby B., Blanke Charles D.. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2015;33(24). CrossRef
- Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer Wang Samuel J., Fuller C. David, Kim Jong-Sung, Sittig Dean F., Thomas Charles R., Ravdin Peter M.. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2008;26(13). CrossRef
- Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis Horgan Anne M., Amir Eitan, Walter Thomas, Knox Jennifer J.. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2012;30(16). CrossRef
- Extended operation with or without intraoperative (IORT) and external (EBRT) radiotherapy for gallbladder carcinoma Lindell G, Holmin T, Ewers SB, Tranberg KG, Stenram U, Ihse I. Hepatogastroenterology.2003;50:310-314.
- Impact of adjuvant external beam radiotherapy on survival in surgically resected gallbladder adenocarcinoma: a propensity score_matched Surveillance, Epidemiology, and End Results analysis. Hyder O, Dodson RM, Sachs T, Weiss M, Mayo SC, Choti MA, et al. . Surgery.2014;155(1):85-93. CrossRef
- Adjuvant radiation therapy is associated with improved survival for gallbladder carcinoma with regional metastatic disease Mojica Pablo, Smith David, Ellenhorn Joshua. Journal of Surgical Oncology.2007;96(1). CrossRef
- Magnitude of combination therapy of radical resection and external beam radiotherapy for patients with carcinomas of the extrahepatic bile duct and gallbladder Itoh H, Nishijima K, Kurosaka Y, Takegawa S, Kiriyama M, Dohba S, et al . Dig Dis Sci.2005;50:2231-2242. CrossRef
- Prognostic factors for adenocarcinoma of the gallbladder: an analysis of 162 cases North Jr JH,, Pack MS,, Hong , C, Rivera DE. Am Surg.1998;64:437-440.
- Improved survival in resected biliary malignancies Nakeeb Attila, Tran Khoi Q., Black Michael J., Erickson Beth A., Ritch Paul S., Quebbeman Edward J., Wilson Stuart D., Demeure Michael J., Rilling William S., Dua Kulwinder S., Pitt Henry A.. Surgery.2002;132(4). CrossRef
- Multidisciplinary treatment of biliary tract cancers Kraybill W. G., Lee H., Picus J., Ramachandran G., Lopez M. J., Kucik N., Myerson R. J.. Journal of Surgical Oncology.1994;55(4). CrossRef
- Gallbladder cancer: epidemiology and outcome Hundal Rajveer, Shaffer Eldon A.. Clinical Epidemiology.2014;6. CrossRef
- Managing the incidentally detected gallbladder cancer: Algorithms and controversies Cavallaro A, Piccolo G, Di Vita M, Zanghì A, Cardì F, Di Mattia P, et al . Int J Surg.2014;12(Suppl 2):S108-119. CrossRef
- Diagnostic role of endoscopic ultrasonography-guided fine needle aspiration of gallbladder lesions Kim Hong Jun, Lee Sung-Koo, Jang Ji Woong, Kim Tae Gyoon, Ryu Choong Heon, Park Do Hyun, Lee Sang Soo, Seo Dong Wan, Kim Myung-Hwan. Hepato-Gastroenterology.2012;59(118). CrossRef
- Patterns of Presentation, Treatment, and Survival Rates of Gallbladder Cancer: a Prospective Study at a Tertiary Care Centre Singh Santosh Kumar, Talwar Rajnish, Kannan Narayanan, Tyagi Arvind Kumar, Jaiswal Pradeep, Kumar Adarsh. Journal of Gastrointestinal Cancer.2018;49(3). CrossRef
- Carcinoma of Gall Bladder: Demographic and Clinicopathological Profile in Indian Patients Dubey AP, Rawat K, Pathi N, Viswanath S, Rathore A, Kapoor R, Pathak A. Oncology Journal of India.2018;2:3-6.
- A study on the clinical profile and treatment outcomes in gallbladder carcinoma from Northern India Sreen A, Anadure RK, Singh HP, Sharma R, Garg A. Oncol J India.2020;4:128-132. CrossRef
- Gallbladder toxicity and high-dose ablative-intent radiation for liver tumors: Should we constrain the dose? Tanguturi Shyam K., Niemierko Andrzej, Wo Jennifer Y., Nguyen Khanhnhat N., Prichard Hugh, Zhu Andrew X., Wolfgang John A., Hong Theodore S.. Practical Radiation Oncology.2017;7(5). CrossRef
- Primary carcinoma of the gallbladder. Adjuvant postoperative external irradiation Bosset J. F., Mantion G., Gillet M., Pelissier E., Boulenger M., Maingon P., Corbion O., Schraub S.. Cancer.1989;64(9). CrossRef
- Adjuvant external-beam radiotherapy with concurrent chemotherapy after resection of primary gallbladder carcinoma: a 23-year experience Czito BG, Hurwitz HI, Clough RW, Tyler DS, Morse MA, Clary BM, et al . Int J Radiat Oncol Biol Phys.2005;62(4):1030-1034. CrossRef
- Adjuvant external beam radiation therapy with concurrent chemotherapy in the management of gallbladder carcinoma Kresl John J., Schild Steven E., Henning George T., Gunderson Leonard L., Donohue John, Pitot Henry, Haddock Michael G., Nagorney David. International Journal of Radiation Oncology, Biology, Physics.2002;52(1). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2022
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times