Treatment Outcomes of Nilotinib as Second Line Therapy for Chronic Myeloid Leukemia Patients in Karbala Province of Iraq
Download
Abstract
Background: Second line treatment of chronic myeloid leukemia (CML) is crucial after imatinib therapy failure. In Iraq, nilotinib is the only available second line tyrosine kinase inhibitors (TKIs), making management of CML patients with failure response to 1st generation TKIs as a great challenge to the health system.
Objectives: Our study tries to evaluate nilotinib safety and efficacy among CML patients in Karbala province of Iraq as the only drug available as second line treatment for CML patients post imatinib failure.
Materials & Methods: This research was carried out in Al-Hussein cancer center in Karbala province of Iraq between January 2012 & December 2020. Nilotinib was used as a second-line treatment for 30 CML patients and their response were assessed by the level of BCR-ABL1 transcription in peripheral blood at 3 months, 6 months and 12 months from starting treatments.
Results: The median age was 42.5 years, included 16 males and 14 females with male to female ratio 1.14. According to Sokal score 15 patients were high risk, 11 patients were intermediate risk and 4 patients were low risk. More than 66% of our patients achieved major molecular response (MMR) after starting nilotinib as second line. The BCR- ABL transcription level had a significant reduction from baseline at 3 months, 6 months and 12 months respectively (P value <0.05). Male patients and those who received imatinib for ≥ 24 months were better survival.
Conclusion: Nilotinib is effective and safe drugs as second line treatment among Iraqi patients.
Introduction
Chronic myeloid leukemia (CML) is a hematopoietic disease that is characterized by bone marrow stem cells malignant expansion. It is characterized by t (9;22) (q34; q11.2) translocation which is known as Philadelphia (Ph) chromosome. This translocation leads to BCR/ABL1 gene that encodes an oncogene (P210, more rarely P230 or P190) which produces an abnormal tyrosine kinase activity that causes aberrant myelopoiesis [1].
The development of tyrosine kinase inhibitors (TKIs) has revolutionized CML treatment, resulting in significant improvements in prognosis, response rate, overall survival, and patient outcomes as compared to earlier therapeutic regimens [2].
Many prognostic scoring models for CML risk stratification have been developed throughout the years. Using clinical factors, the Sokal score was used to risk stratify patients at the time of presentation. This classification divides patients into three groups: low risk, intermediate risk and high risk [3].
Patients with an intermediate or high-risk score had a higher possibility of disease progression, therefore NCCN guidelines, recommend second generation TKIs for high- risk group to decrease the risk of disease progression [4]. Nilotinib is an orally bioavailable drug that is more effective and selective against BCR-ABL than first- generation TKIs (imatinib). It was first licensed in 2007 in the United States and other countries for patients with chronic or accelerated CML who had developed resistance to or were unable to tolerate imatinib [5]. Because of limited resources in our war-torn country all patients offered imatinib as frontline regardless score while second generation TKIs reserved to refractory patients [6].
In this study we trying to assessed the efficacy & safety of nilotinib as the only available second line treatment in CML patients after imatinib failure in Karbala province of Iraq.
Materials and Methods
Study design and participants
This study was conducted at Al-Hussein cancer center in Karbala, on 30 CML patients diagnosed between January 2012 and December 2020. Eligibility criteria for inclusion of patients were as follows: Patients ages ≥ 18 years old, good performance status and without any comorbidities with normal hepatic, renal, and cardiac functions. Resistance was defined as no complete hematological response (CHR) at or after 3 months; no minimal cytogenetic response by 6 months; no major cytogenetic response (MCyR) by 12 months; loss of CHR; loss of minor cytogenetic response; loss of MCyR or complete cytogenetic response (CCyR); or the development of clonal evolution. Imatinib intolerance was defined as discontinuation due to a Grade 3/4 imatinib- related adverse effects. Nilotinib was given to patients in doses of 400 mg twice daily and they were followed up for survival.
Follow up after initiating treatment was by complete blood count, blood smear, renal function tests, liver function tests, ECG, and reverse transcriptase polymerase chain reaction (RT-PCR) to establish the response according to BCR-ABL1 transcripts level which defined by the internal scale. No mutational screening was done before establishing of nilotinib therapy for failure responders CML patients, because of its unavailability.
All nilotinib adverse events was registered according to the severity that can be classified by the National Cancer Institute’s grading scale [7].
Definitions of endpoints
The primary objective of the research was to assess the MMRs incidence in patients who were intolerance or resistant to imatinib and using nilotinib as second line. The molecular status was evaluated at 3, 6, and 12 months in the first year and in the following years, every 3-6 months.
The secondary objectives were to calculate the overall survival (OS), which was calculated from the time that nilotinib was started till death from any reason, as well as to determine the safety profile of nilotinib. Duration of response was defined as the period of time between the onset of the response and the date when nilotinib is stopped due to progression or death.
Calculation of Sokal score was done by calculation of the proportion of peripheral blood blast cells, the number of platelets, the size of the spleen (measured in centimeters below the costal margin) and the patient’s age. The patients were classified into three risk categories according to their individual numerical value: low (<0.8), intermediate (0.8-1.2), and high (> 1.2) [8].
Ethical considerations
This research was guided by the Helsinki Declaration, and all patients signed a written informed consent form in accordance with the institution’s policies. Ethical approval was obtained from the ethics committee of Karbala teaching hospital in Karbala, Iraq.
Statistical analysis
All time-to-event analyses were performed with the use of Kaplan–Meier methods and presented by Kaplan-Meier curves SPSS 20.0.0 (Chicago, IL) Minitab 17.1.0 software packages were used for statistical analysis and a P < 0.05 was considered indicative of statistically significant difference.
Results
There were 30 CML patients on nilotinib as second line treatment enrolled in our study, the median age of our patients was 42.5 years, ranged (18-65) years, with a male: female ratio 1.14. The Median duration of imatinib treatment was 22 months. Median duration of nilotinib was 62.9 months. Half of patients were high risk in 15 patients (50 %), intermediate risk in 11 patients (36.67%) and low risk in 4 patients (13.33%).
The causes of starting nilotinib were primary failure to imatinib in 5 patients (16.67%), secondary failure in 24 patients (80%) and intolerance in 1 patient (3.33%).
Imatinib doses were 800 mg in 22 patients (73.34%), 600 mg in 4 patients (13.33%), 400 mg in 3 patients (10%) and 300 mg in 1 patient (3.33%). Seventeen patients (56.67%) received imatinib for less than 24 months, while 13 patients (43.33%) for ≥ 24 months.
Twenty patients (66.67 %) achieved MMR while 10 patients (33.33%) not achieved MMR, the median time to achieve MMR was 9 months. At the end of follow up, one patient (3.33%) had blast transformation and died (Table 1).
| Variables | Values (%) |
| Median age (year) | 42.5 |
| Median duration on imatinib therapy (months) | 22 |
| Median duration on nilotinib therapy (months) | 62.9 |
| Sex, n (%) | |
| Male | 16 (53.33) |
| Female | 14 (46.67) |
| Sokal score on diagnosis, n (%) | |
| Low risk | 4 (13.33) |
| Intermediate risk | 11 (36.67) |
| High risk | 15 (50.00) |
| Cause of shifting to nilotinib, n (%) | |
| Primary failure | 5 (16.67) |
| Secondary failure | 24 (80.00) |
| Intolerance | 1 (3.33) |
| Imatinib dose, n (%) | |
| 300 mg | 1 (3.33) |
| 400 mg | 3 (10.00) |
| 600 mg | 4 (13.33) |
| 800 mg | 22 (73.34) |
| Duration on imatinib, n (%) | |
| < 24 months | 17 (56.67) |
| ≥ 24 months | 13 (43.33) |
| MMR, n (%) | 20 (66.67) |
| No MMR, n (%) | 10 (33.33) |
| Median time to achieve MMR (months) | 9 |
| Blast transformation during nilotinib treatment, n (%) | 1 (3.33) |
| Death, n (%) | 1 (3.33) |
MMR, Major molecular response
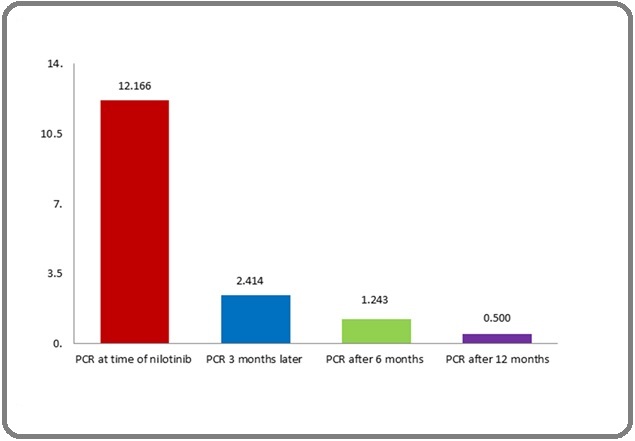
The BCR- ABL transcript level had a significant reduction from baseline at 3 months (P value 0.001), 3 months to 6 months (P value 0.016) and 6 months to 12 months (P value 0.019) (Figure 1).
Figure 1. Change in BCR-ABL Transcript Level During Nilotinib Therapy.
There was no significant association between sex, Sokal score, primary failure, secondary failure, intolerance and achieving MMR, P value > 0.05 for each (Table 2).
| Variables | MMR (20) (%) | No MMR (10) (%) | P value |
| Age (years) | 42.1 ± 12.09 | 47.4 ± 13.31 | 0.298 |
| Sex (%) | |||
| Female | 8 (57.14) | 6 (42.86) | 0.3 |
| Male | 12(75.00) | 4 (25.00) | |
| Sokal score (%) | |||
| Low risk | 4 (100.00) | 0 (0.00) | |
| Intermediate risk | 9 (81.82) | 2 (18.18) | 0.132 |
| High risk | 7 (46.67) | 8 (53.33) | |
| Duration of disease before starting nilotinib (months) | 31.55± 27.63 | 31.7 ± 29.99 | 0.989 |
| Causes of shifting to nilotinib (%) | |||
| Primary failure | 3 (60.00) | 2 (40.00) | |
| Secondary failure | 16 (66.67) | 8 (33.33) | 0.811 |
| Intolerance | 1 (100.00) | 0 (0.00) |
MMR, Major molecular response
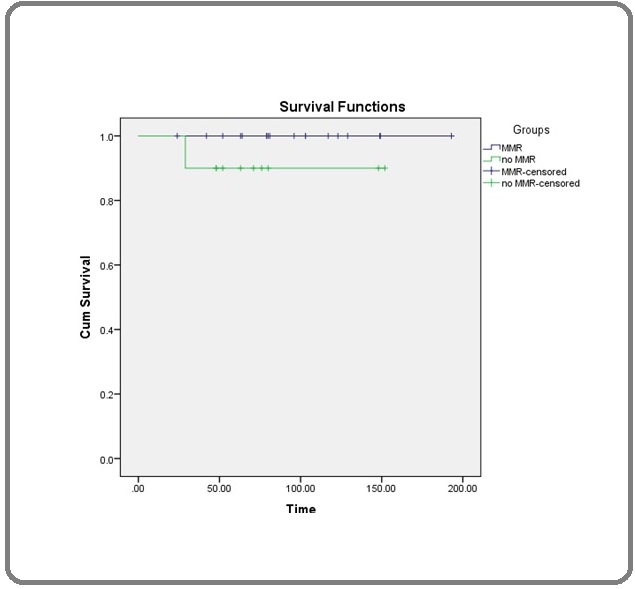
The estimated mean OS using Kaplan Meier survival formula was 94.5 months, males were better survival than females (100.06 versus 88.14 months, respectively), the hazard ratio of 1.15 (95% CI: 0.0126 – 104.71). Interestingly, patients who received imatinib for ≥ 24 months were better survival than those who received treatment < 24 months (135.07 versus 63.47 months, respectively), the hazard ratio of 2.349, (95% CI: 0.025 – 213.79). Patients who achieved MMR were higher overall survival (Figure 2 and 3) but this was not statistically significant (Table 3).
Figure 2. Overall Survival of all Patients.
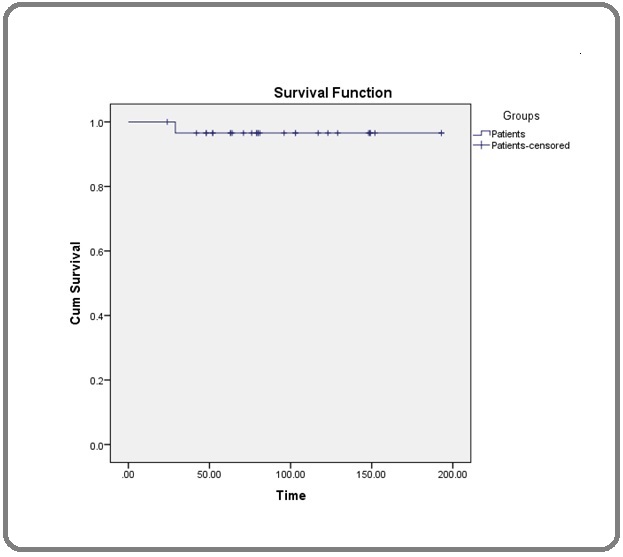
Figure 3. Overall Survival According MMR.
| Mean±SEM | 95% CI of Mean | HR | 95% CI | P value | |
| Overall | 94.5 ±8.440 | 78 – 111 | - | - | - |
| Sokal score | |||||
| Low | 89.66 ±12.461 | 63.3 – 114 | 1.074 | 0.012 – 97.7 | 0.108 |
| Intermediate | 108.636 ±14.808 | 79.6 – 138 | |||
| High | 73.75 ± 9.04 | 56 – 91.5 | |||
| Sex | 1.15 | 0.0126 – 104.71 | 0.011 * | ||
| Female | 88.142 ±11.947 | 64.7 – 112 | |||
| Male | 100.06 ±12.066 | 76.4 – 124 | |||
| Duration imatinib | |||||
| <24 | 63.470 ± 5.471 | 52.8 – 74.2 | 2.349 | 0.025 – 213.79 | 0.001 * |
| ≥24 | 135.076 ±10.149 | 115 – 155 | |||
| Causes of shifting | |||||
| Primary failure | 81.6 ± 11.156 | 59.7 – 104 | 1 | 0.010 – 91.201 | 0.46 |
| Secondary failure | 100.125 ± 9.792 | 80.9 ± 119 | |||
| Age at diagnosis | |||||
| <40 | 99.545 ± 16.275 | 67.6 – 131 | 1.025 | 0.011 – 93.32 | 0.657 |
| ≥40 | 91.578 ± 9.734 | 72.5 – 111 | |||
| MMR | |||||
| MMR | 95.95 ± 9.511 | 77.3 – 115 | 1.125 | 0.0141 – 102.56 | 0.812 |
| No MMR | 91.6 ± 17.474 | 57.4 – 126 |
* Means significant differences (P ≤ 0.05). HR, Hazard ratio; CI, Confidence interval; SEM, Standard error of the mean; MMR, Major molecular response.
Anemia was the most frequent hematological side effect in 3 patients (10%) followed by thrombocytopenia and leucopenia in 2 patients (6.67%) each. Regarding non hematological side effects, skin rash was the most common side effect in 4 patients (13.33 %) followed by join pain in 3 patients (10.00 %) and edema in 2 patients (6.67%), other side effects are explained in (Table 4).
| Hematological side effects | N (%) |
| Anemia | 3 (10.00) |
| Thrombocytopenia | 2 (6.67) |
| Leucopenia | 2 (6.67) |
| Non-Hematologic side effects | |
| Skin rash | 4 (13.33) |
| Join pain | 3 (10.00) |
| Edema | 2 (6.67) |
| Palpitation | 1 (33.33) |
| Headache | 1 (33.33) |
| Hair loss | 1 (33.33) |
| Jaundice | 1 (33.33) |
Discussion
In Karbala province of Iraq, leukemia accounts for more than 6% of cancer patients, whereas CML accounts for more than 24% of leukemia cases [9].
Actually, imatinib become the treatment of choice for newly diagnosed chronic phase CML patients. Despite this, a third of patients will have a poor response to imatinib, either due to primary failure or because they progressed after an initial response [10]. Several mechanisms can cause treatment resistance, such as point mutations in the BCR-ABL kinase domain, leading to poor response and inferior outcomes [11].
Imatinib dose escalation to a daily dose of 600 mg or 800 mg has shown to be effective in patients who have a poor response or disease progression [12]. In our center more than 86% of patients who had progressed disease received the escalating dose before shifting to second line TKIs.
In our study nilotinib therapy was given regardless of mutational analysis because of its unavailability as a screening test for the suboptimal response to or failure of imatinib treatment. Here 66.67% of our patients achieved MMR, which is higher than results in Latin America & Asia where MMR was 58%, 57% respectively [11,13]. The level of BCR-ABL transcription was significantly lower at 3 months, 6 months and 12 months respectively, same results by Yeung et al. where MMR deepen with time [14].
The median age in our patients was 42.5 years which close to previous studies in Karbala governorate but it is decade younger than in western countries, this may be explained by that only (3.4%) of the Iraqi population are above 65 years [6,15,16].
Sokal score is known as predictor to achieve MMR in CML patients [17,18], while in our study there was no correlation between achieving MMR and Sokal score. On the other hand, achieving MMR is a significant predictor for OS [14], but we couldn’t find any association between achieving MMR and OS. This is may be due to short follow up time and small sample.
Interestingly, males were better survival than females which is comparable to previous results in Africa although in Europe, females were better survival [18,19]. Meanwhile, patients who had long duration of response to imatinib were better outcomes this was compatible with previous studies in Asia [13].
Drugs side effects had poor impact on leukemia patients’ compliance, understanding these side effects and treat them urgently leading to improve outcomes [20]. In our study skin rash was the most common side effect, which is consistent with previous studies [13].
In conclusion, nilotinib is an effective & tolerable second line treatment for Iraqi CML patients regardless of mutational analysis. More than 66% of our patients achieved MMR. Future researches with a greater number of patients in various parts of Iraq are recommended to improve treatment outcomes for CML patients in our country.
Authorship
All the authors contributed equally to this paper.
Consent for publication
All authors have checked this manuscript and declared their consent for publication.
References
- Cytogenetic and molecular genetic evolution of chronic myeloid leukemia Johansson Bertil, Fioretos Thoas, Mitelman Felix. Acta Haematologica.2002;107(2). CrossRef
- BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review An Xin, Tiwari Amit K., Sun Yibo, Ding Pei-Rong, Ashby Charles R., Chen Zhe-Sheng. Leukemia Research.2010;34(10). CrossRef
- Clinico-pathologic features of chronic myeloid leukemia and risk stratification according to Sokal score Syed Naveen Naz, Usman Muhammad, Khaliq Gulnaz, Adil Salman N., Khurshid Muhamamd. Journal of the College of Physicians and Surgeons--Pakistan: JCPSP.2006;16(5). CrossRef
- National Comprehensive Cancer Network. Chronic myeloid leukemia [cited Jan 2021] Available from: https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf..
- Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia Saglio Giuseppe, Kim Dong-Wook, Issaragrisil Surapol, Coutre Philipp, Etienne Gabriel, Lobo Clarisse, Pasquini Ricardo, Clark Richard E., Hochhaus Andreas, Hughes Timothy P., Gallagher Neil, Hoenekopp Albert, Dong Mei, Haque Ariful, Larson Richard A., Kantarjian Hagop M.. The New England Journal of Medicine.2010;362(24). CrossRef
- Mjali A, Abbas SK. Imatinib Mesylate Adherence in Chronic Myeloid Leukemia Patients: Data from Middle Euphrates Region of Iraq. Sys Rev Pharm. 2021 Jan; 12(1): 83-87 .
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. United States Department of Health and Human services. National Institutes of Health. National Cancer Institute. [cited Jan 2021] Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf..
- Comparative analysis of the Sokal, Euro and European Treatment and Outcome Study score in prognostication of Indian chronic myeloid leukemia-chronic phase patients on imatinib Chhikara Sunita, Sazawal Sudha, Singh Kanwaljeet, Chaubey Rekha, Pati Haraprasad, Tyagi Seema, Mahapatra Manoranjan, Saxena Renu. South Asian Journal of Cancer.2018;7(4). CrossRef
- CHRONIC MYELOID LEUKEMIA PATIENT WITH ISOLATED CENTRAL NERVOUS SYSTEM BLAST CRISIS Mjali Ahmed, Kareem Yassmin, Hasan Jaleel Al-Shammari Haider, Abbas Nareen, Alnaqeeb Hussein, Al-Anssari Mohammed, Abbas Ghufran. WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES.2019;8. CrossRef
- Seeking the causes and solutions to imatinib-resistance in chronic myeloid leukemia Bixby D., Talpaz M.. Leukemia.2011;25(1). CrossRef
- Nilotinib As Second or Third-Line Therapy for Myeloid Chronic Leukemia Chronic-Phase in Mexican Patients Ayala Manuel, Domínguez Jacqueline, Chavez Antonieta. Blood.2016;128(22). CrossRef
- Efficacy of imatinib dose escalation in patients with chronic myeloid leukemia in chronic phase Kantarjian Hagop M., Larson Richard A., Guilhot Francois, O'Brien Stephen G., Mone Manisha, Rudoltz Marc, Krahnke Tillmann, Cortes Jorge, Druker Brian J.. Cancer.2009;115(3). CrossRef
- Nilotinib As Second-Line Therapy in Patients with Chronic Myeloid Leukemia in Chronic Phase: Thailand Experience Chansung Kanchana, Sirijerachai Chittima, Lekhakula Arnuparp, Viboonjuntra Pongtep, Niparuck Pimjai, Pauvilai Teeraya, Numbenjapon Tontanai, Bunworasate Udomsak, Nawarawong Weerasak, Tantiworawit Adisak, Suwanban Tawatchai, Jootar Saengsuree. Blood.2016;128. CrossRef
- TIDEL-II: first-line use of imatinib in CML with early switch to nilotinib for failure to achieve time-dependent molecular targets Yeung David T., Osborn Michael P., White Deborah L., Branford Susan, Braley Jodi, Herschtal Alan, Kornhauser Michael, Issa Samar, Hiwase Devendra K., Hertzberg Mark, Schwarer Anthony P., Filshie Robin, Arthur Christopher K., Kwan Yiu Lam, Trotman Judith, Forsyth Cecily J., Taper John, Ross David M., Beresford Jennifer, Tam Constantine, Mills Anthony K., Grigg Andrew P., Hughes Timothy P.. Blood.2015;125(6). CrossRef
- Management of chronic myeloid leukemia in France: a multicentered cross-sectional study on 538 patients Tardieu Sophie, Brun-Strang Catherine, Berthaud Patrice, Michallet Mauricette, Guilhot François, Rousselot Patrice, Sambuc Roland. Pharmacoepidemiology and Drug Safety.2005;14(8). CrossRef
- Classification of non-Hodgkin lymphoma in the Middle Euphrates Region of Iraq according to the World Health Organization classification Mjali Ahmed, Oudah Alyaa Hadi, Al-Shammari Haider Hasan Jaleel, Abbas Nareen Tawfeeq. Iraqi Journal of Hematology.2021;10(2). CrossRef
- Factors Affecting Early Molecular Response in Chronic Myeloid Leukemia Chikkodi Santosh V., Malhotra Pankaj, Naseem Shano, Khadwal Alka, Prakash Gaurav, Sahu Kamal Kant, Kumari Savita, Suri Vikas, Varma Neelam, Varma Subhash. Clinical Lymphoma, Myeloma & Leukemia.2015;15 Suppl. CrossRef
- Determinants of Overall and Progression-Free Survival of Nigerian Patients with Philadelphia-Positive Chronic Myeloid Leukemia Oyekunle AA, Bolarinwa RA, Oyelese AT, et al . Advances in hematology.2015;2015. CrossRef
- Gender aspects in chronic myeloid leukemia: long-term results from randomized studies Berger U., Maywald O., Pfirrmann M., Lahaye T., Hochhaus A., Reiter A., Hasford J., Heimpel H., Hossfeld D. K., Kolb H.-J., Löffler H., Pralle H., Queisser W., Hehlmann R.. Leukemia.2005;19(6). CrossRef
- Myeloid sarcoma as the presenting symptom of chronic myeloid leukemia chronic phase: A case report Mjali Ahmed, Hasan Doha, Al-Anssari Mohammed, Muhsin Rasha, Hamandi Ahmed, Hasan Jaleel Al-Shammari Haider. World Journal of Pharmaceutical Research.2019;7. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2022
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times