Long Term Clinical Outcome and Prognostic Factors after Treatment of Patients Diagnosed with Ductal Carcinoma in Situ of Breast
Download
Abstract
Objectives: The retrospective study evaluated the clinical outcome after treatment of patients diagnosed with ductal carcinoma in situ of breast and reanalyzed the prognostic factors related to recurrence rate and disease free survival(DFS) using long-term follow-up.
Material & Methods: Between January 2008 and July 2021, 130 patients previously diagnosed ductal carcinoma in situ underwent surgery. We collected retrospective data characteristic data, radiology data, operative data, pathology data, clinical outcome and time to breast tumor recurrence. Median follow-up time was 51.5 months.
Results: The 12-year cumulative incidence of tumor recurrence and re- excision in 130 patients were 6.92%(9 patients) and 12.31%(16 patients). Among 9 patients, 5 patients had locoregional recurrence, 3 patients had distant metastasis recurrence and 1 patient had both. Ki-67(OR, 1.06;95% CI 1.00 – 1.11); p-value = 0.045) was associated with an increase risk of recurrence tumor in multivariable analysis. Simple mastectomy(41.54%) and wide excision (38.46%) were the most surgery in this study.
Conclusion: The retrospective study showed the 12-year cumulative incidence of recurrence tumor. Although Ki-67 increased risk of recurrence tumor.
Introduction
Ductal carcinoma in situ (DCIS) of the breast is commonly found in women. In the United States (US), the incidence of DCIS marked increased from 5.8 per 100,000 women in the 1970s to 32.5 per 100,000 women in 2004 [1]. The mortality for women with ductal carcinoma in situ(DCIS) is low, regardless of whether breast-conserving therapy(BCT) or mastectomy is performed [2-4]. However, women with DCIS will develop invasive ductal carcinoma(IDC). The risk of death from breast cancer increases greatly after recurrence [5,6].
The standard treatment goals after diagnosed ductal carcinoma in situ are local control and preventing invasive local recurrence. Primary treatment is surgery [7,8]. The surgery options suggested simple mastectomy or breast- conserving therapy with or without postoperative radiation therapy. Some studies examining the postoperative radiation therapy (RT) following breast-conserving therapy (BCT) for patients with ductal carcinoma in situ (DCIS) showed that radiation therapy reduced the risk of local recurrence by approximately half [9-13].
In the developing country, the women with diagnosed DCIS mostly presented with abnormal calcification from the breast screening program[9],[14,15]. On the contrary, women are unable to access the breast screening program in our country [16]. As the consequence, the common presentation of DCIS is palpable breast mass.
We aim to perform this long-term analysis to improve the understanding of the patient with diagnosed DCIS in this patient population.
Materials and Methods
Study design
We undertook a retrospective review of all patients who had surgery for DCIS at Srinagarind hospital of Khon Kaen University between January 2008 and July 2021. This study has been approved by the appropriate local ethical committees (HE641035).
Population
All patients who had surgery for DCIS and follow-up more than 1 year at Srinagarind hospital of Khon Kaen University between January 2008 and July 2021.
Characteristic data such as age at diagnosis, sex, underlying disease, family history of breast and ovarian cancer, BMI, oral contraceptive used, menopausal status, laterality, mode of detection, date at diagnosis were aggregated. Radiology was reviewed and data collected on the report such as BIRADS Operation notes were reviewed and data collected on report such as type of surgery, sentinel lymph node biopsy, date of surgery. Pathology reports were reviewed and data were collected on reports such as histology, tumor grade, size, tumor margin, immunohistochemistry including estrogen receptor(ER), progesterone receptor(PR), human epidermal growth factor(HER-2), Ki-67. The presence of comedone necrosis or microinvasion was recorded.
Clinical notes were reviewed to collected adjuvant therapy, radiation data, follow-up data, recurrence data. When recurrences did occur, we gathered information on the location of recurrences such as ipsilateral breast tumor recurrence(IBTR) or contralateral breast tumor recurrence(CBTR), which was recorded as locoregional or distant metastatic, histopathological data.
Follow up
All patients underwent clinical examination and radiological (x-rays, ultrasonography, and mammography) at least 1 year after surgery until death or endpoints of data collection (July 2021).
Locoregional recurrence was confirmed by a pathological report. Distant metastasis recurrence was confirmed by pathological report or radiological report (Computerized tomography or Bone scan). The date at recurrence was studied date of pathological report or date of radiological.
Statistical analysis
The data were collected and analyzed using STATA version 10. All continuous variables are reported as mean ± standard deviation (SD) or median with interquartile range (IQR), and categorical variables were summarized as counts and percentages. The ANOVA test was performed to evaluate the normal distribution of continuous variables. Conversely, if continuous samples did not show a normal distribution, the Kruskal-Wallis test was used to compare quantitative parameters. Categorical variables were analyzed using the Chi-square test or Fisher exact test.
The primary outcome is a 12-year cumulative incidence. The secondary outcome was simple and multiple logistic regression analysis for analysis of prognostic factors.
Disease free survival (DFS) was evaluated using Kaplan–Meier survival curves and the log-rank test was used to demonstrate survival. Results with a P-value <0.05 were considered statistically significant.
Results
In total, 138 patients were diagnosed with ductal carcinoma in situ at the first pathological report. Eight patients (5.8%) were upstaged to invasive ductal carcinoma after surgery. One hundred and thirty patients were included in this study. Median follow-up time was 51.5 months. Patient characteristics are listed (Table 1).
| Total (n=130) | |
| Sex | |
| - female | 130 (100) |
| Age at diagnosis | 52.09 (8.76) |
| Underlying disease | |
| - DM | 13 (10) |
| - HT | 28 (21.54) |
| - DLP | 13 (10) |
| - CKD | 1 (0.77) |
| - Others | 27 (20.77) |
| Family history of breast + ovarian cancer | |
| - No | 122 (93.85) |
| -Yes | 8 (6.15) |
| BMI | 23.68 (21.29 - 26.83) |
| OC used | |
| - No | 109 (84.5) |
| - Yes | 20 (15.5) |
| Menopausal status | |
| - No menopause | 68 (52.31) |
| - Menopause | 62 (47.69) |
| Follow up time(months) | 51.5 (31 - 70) |
| tumor size(cm) | 2 (1 - 4) |
| laterality | |
| - Right | 66 (50.77) |
| - Left | 64 (49.23) |
| Mode of detection | |
| - mass | 85 (65.89) |
| - nipple discharge | 9 (6.98) |
| - breast pain | 4 (3.10) |
| - calcification | 29 (22.48) |
| - breast ulcer | 2 (1.55) |
| Tumor margin status | |
| - free | 116 (89.23) |
| - involved | 14 (10.77) |
| free (cm) | 0.4 (0.1 - 1) |
| Lymph node | |
| - negative | 129 (99.23) |
| - positive | 1 (0.77) |
| Postoperative radiation | |
| - No | 86 (66.15) |
| - Yes | 44 (33.85) |
| Radiation technique | |
| - 2D | 2 (5.56) |
| - 3D wedge pair | 2 (5.56) |
| - Forward IMRT | 32 (88.89) |
| Pathology report DCIS before surgery | |
| - No | 10 (7.69) |
| - Yes | 120 (92.31) |
| Upstaging | |
| - No | 119 (91.54) |
| - Yes | 11 (8.46) |
| Surgery | |
| - Simple mastectomy | 54 (41.54) |
| - Nipple sparing mastectomy | 12 (9.23) |
| - wide excision or NLE | 50 (38.46) |
| - lumpectomy | 11 (8.46) |
| - quadrantectomy | 1 (0.77) |
| - modified radical mastectomy | 2 (1.54) |
| Sentinel lymph node biopsy | |
| - No | 28 (21.54) |
| - Yes | 102 (78.46) |
| DCIS grade | |
| - low | 15 (12.2) |
| - intermediate | 23 (18.7) |
| - high | 85 (69.11) |
| ER | 70 (0 - 90) |
| PR | 5 (0 - 70) |
| HER2 | |
| 0 | 29 (24.79) |
| - 1+ | 25 (21.37) |
| - 2+ | 24 (20.51) |
| - 3+ | 39 (33.33) |
| Ki67 | 20 (5 - 30) |
| Comedone necrosis/microinvasion | |
| - No | 73 (56.59) |
| -Yes | 56 (43.41) |
| Hormonal therapy | |
| - No | 38 (29.69) |
| - Yes | 90 (70.31) |
| Tamoxifen | 78 (60.94) |
| Aromatase inhibitor | 16 (12.5) |
| Anti- HER2 | |
| - No | 123 (96.09) |
| - Yes | 5 (3.91) |
| Adjuvant CMT | |
| -No | 113 (88.28) |
| - Yes | 15 (11.72) |
In the patients with DCIS, those who underwent a mastectomy, nipple-sparing mastectomy and modified radical mastectomy have 4 patients (5.88%) to tumor recurrence and 2 patients (2.94%) to re-operation. The patients with DCIS who underwent wide excision, needle localized excision, lumpectomy and quadrantectomy have 5 patients (8.06%) to tumor recurrence and 14 patients (22.58%) to re-operation. Mostly case that reoperation were involved tumor margin.
There was a 12-year cumulative incidence of tumor recurrence and re- excision in 130 patients were 6.92% (9 patients) and 12.31% (16 patients). Among the 9 patients with tumor recurrence, five patients had a locoregional recurrence, three patients had distant metastasis recurrence, one patient had both. There were IBTR 5 patients and CBTR 1 patients (Table 2).
| Total (n= 9) | RT(1) (n=4) | no RT(0) (n=5) | p-value | |
| Local recurrence | >0.999 | |||
| IBTR | 5 (83.33) | 2 (100.00) | 3 (75.00) | |
| CBTR | 1 (16.67) | - | 1 (25.00) | |
| distant recurrence | 3 (50.00) | 2 (100.00) | 1 (25.00) | 0.4 |
Fisher’s exact test, ipsilateral breast tumor recurrence (IBTR), contralateral breast tumor recurrence (CBTR)
The pathological report in IBTR is 3 DCIS and 2 IDC. The pathological report in CBTR is one DCIS.
On univariate analysis, family history of breast or ovarian cancer, Menopause status, large tumor size, involved tumor margin, high Ki-67, high grade tumor, and seen comedone necrosis/microinvasion were predictive factors for tumor recurrence, but no statistical difference (Table 3).
| Univariate | ||
| OR (95%CI) | p-value | |
| Family history of breast or ovarian cancer | 2.5 (0.26 – 24.07) | 0.428 |
| BMI | 0.92 (0.78 – 1.09) | 0.348 |
| oral contraceptive used | 0.75 (0.09 – 6.44) | 0.793 |
| Menopausal status | 0.61 | |
| -No menopause | 1 | |
| -Menopause | 1.43 (0.36 – 5.62) | |
| Tumor size(cm) | 1.01 (0.78 – 1.31) | 0.919 |
| Mode of detection | ||
| - mass | 1 | |
| -nipple discharge | 1 | |
| -breast pain | 3.29 (0.30 – 35.97) | 0.33 |
| -calcification | 0.43 (0.05 – 3.67) | 0.439 |
| -breast ulcer | 1 | |
| BIRADS | ||
| 2 | 1 | |
| 3 | 1 | |
| 4 | 1 | |
| 4a | 1 | |
| 4b | 0.45 (0.03 – 5.87) | 0.542 |
| 4c | 0.21 (0.01 – 4.48) | 0.321 |
| 5 | 0.45 (0.03 – 5.87) | 0.542 |
| 6 | 1 | |
| Tumor margin status | 0.145 | |
| -free | 1 | |
| -involved | 6.44 (0.53 – 78.89) | |
| Lymph node | NA | |
| - none | 1 | |
| - positive | 1 | |
| Sentinel lymph node biopsy | 0.489 | |
| -No | 1 | |
| -Yes | 2.12 (0.25 -17.87) | |
| DCIS GRADE | ||
| -Low | 1 | |
| -Intermediate | 1 | |
| -High | 1.78 (0.21 – 15.38) | 0.601 |
| ER | 0.99 (0.98 – 1.01) | 0.313 |
| PR | 0.99 (0.96 – 1.01) | 0.231 |
| HER2 | ||
| 0 | 1 | |
| - 1+ | 0.63 (0.05 – 7.40) | 0.709 |
| - 2+ | 0.63 (0.05 – 7.40) | 0.709 |
| - 3+ | 2.23 (0.40 – 12.54) | 0.362 |
| KI-67 | 1.04 (1.00 – 1.09) | 0.056 |
| Comedone Necrosis/Microinvasion | 0.48 | |
| -No | 1 | |
| -Yes | 1.64 (0.42 – 6.45) |
P-value < 0.05
On multivariate analysis, Ki-67 was associated with an increased risk of recurrence tumor (OR, 1.06;95% CI 1.00 – 1.11; p-value = 0.045). There was no statistical difference in tumor margin status (Table 4).
| Multivariate | ||
| OR (95%CI) | p-value | |
| Tumor margin status | 0.428 | |
| - Free | 1 | |
| - Involved | 11.49 (0.61 – 215.55) | |
| Ki-67 | 1.06 (1.00 – 1.11) | 0.045 |
P-value < 0.05
Disease free survival listed (Figure 1).
Figure 1. Kaplan Meier Curve for Free vs Involved Margin.
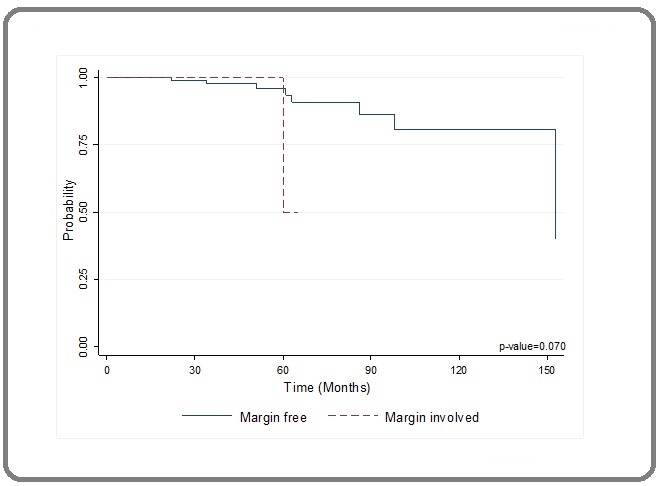
On Kaplan Meier analysis, the recurrence probability in margin free groups is better than involved margin. But not statistical significant (Figure 1).
Discussion
This study has demonstrated long-term clinical outcomes of patients with diagnosed DCIS of the breast. The 12-year cumulative incidence of tumor recurrence in 130 patients was 6.92% (9 patients). The ECOG-ACRIN E5194 study [14] show 12-year rates of developing ipsilateral breast event is 14.88% for women with ductal carcinoma in situ (DCIS) of the breast treated with surgical excision without radiation. It is possible that the breast cancer specialization of all of the multidisciplinary teams at our tertiary care institution, including pathology, radiology, and surgery, may lead to earlier detection of DCIS, therefore resulting in the lower overall volume of disease, more complete pathologic evaluation of the surgical margins, and therefore low 12-year recurrence rates. Warren et al. [17] show 10 years cumulative incidence for DCIS with breast conserving therapy with RT is 1.5%.
We found 138 patients with diagnosed ductal carcinoma in situ at the first pathological report. Eight patients (5.8%) were upstaged to invasive ductal carcinoma after surgery. Yoo et al. [18] study shows the 175 breast lesions diagnosed as DCIS on core-needle biopsy. Fifty-eight lesions (33.1%) were confirmed to be invasive breast cancer after the final surgical approach. That may be caused by the progression of the tumor or the invasive part not located at the biopsy area. In our study, the patients detected by palpable breast mass are 65.89% (85/130). Compare with the other study the most common presentation is abnormal calcification in mammograms [9],[14,15]. Kerlikowske et al. [19] show initial detection by palpation was increased the risk for subsequent invasive cancer. But our results show palpable breast mass does not increase the risk for tumor recurrence. Similar to another study, palpable breast mass does not increase the risk for tumor recurrence [20,21].
In this multivariate analysis, the predictive factor associated with increased risk of tumor recurrence is high Ki-67 (OR, 1.06;95% CI 1.00 – 1.11); p-value = 0.045).
Kerlikowske et al.(19) show biomarkers that p16 positive, Ki-67 positive, and COX-2 positive were statistically significantly associated with subsequent invasive cancer. In the others, Nobuko et al. study [22] found younger age was a risk factor for invasive breast tumor recurrence, whereas the HR+/HER2− tumor subtype and a family history of breast cancer were risk factors for contralateral breast tumor recurrence.
In the EORTC trial 10853 [21], they found factors associated with an increased risk of local recurrence in the multivariate analysis were involved margins (hazard ratio, 2.07; P = .0008), compared to this study the patients who had involved tumor margin did not increase the risk for tumor recurrence. In our study, involved margin tumor status wasn’t statistical significant to increase tumor recurrence. Because there was incidence of tumor recurrence.
In part of postoperative radiation. In the large prospective randomized control trial, NSABP B-17 and B-24 trials [9] show radiation reduced invasive ipsilateral breast tumor recurrence by 52% in the lumpectomy followed by radiation group compared with lumpectomy only (B-17, hazard ratio = 0.48, 95% confidence interval = 0.33 to 0.69, P < .001). In our study, we found postoperative radiation was protective risk for tumor recurrence in patient with breast conservative therapy (OR 0.23; 95%CI 0.034-1.56 ; p-value = 0.066). But we did not find significant benefit from postoperative radiation. It can cause by a few incidences of tumor recurrence in this report. Additionally, It is also likely that genomic tests, such as Oncotype DCIS [23] to predict a local recurrence (HR 2.31, p = 0.02) and an invasive local recurrence(HR 3.68, p = 0.01). It helps risk stratify patients and better estimate the benefts of post-operative radiation.
With respect to limitations, this retrospective study was conducted using data from only one tertiary care center. This retrospective design introduces selection bias regarding which patients were included in this study population. Some variables were missing and not included. Moreover, the BIRADS graded from 2008 to 2012 were not specifically classified into 4a, 4b, or 4c by the reading radiologist. Another limitation, the very small numbers of events limit the statistical power to determine risk factors for local or contralateral recurrences.
The strengths of this study included long-term median follow-up. Our data shows another different presentation of DCIS. Most patients were surgery by specialty breast surgeons in our center. Additionally, we recorded the hormonal receptor in the numeric data. That can increase utility for analysis.
In conclusion, the retrospective study showed the 12- year cumulative incidence of recurrence tumor. Although high Ki-67 significantly increased risk of recurrence tumor.
Acknowledgements
Piyanan Suparattanagool conducted the data analysis.
Invitation research from faculty of medicine research grant Khon Kaen University.
Conflict of interest
We declared no conflict of interest.
This study has been approved by the appropriate local ethical committees (HE641035)
References
- Finding the balance between over- and under-treatment of ductal carcinoma in situ (DCIS) Groen Emma J., Elshof Lotte E., Visser Lindy L., Rutgers Emiel J. Th, Winter-Warnars Hillegonda A. O., Lips Esther H., Wesseling Jelle. Breast (Edinburgh, Scotland).2017;31. CrossRef
- Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes Virnig Beth A., Tuttle Todd M., Shamliyan Tatyana, Kane Robert L.. Journal of the National Cancer Institute.2010;102(3). CrossRef
- Comparison of Local Recurrence After Simple and Skin-Sparing Mastectomy Performed in Patients with Ductal Carcinoma In Situ Timbrell Simon, Al-Himdani Sarah, Shaw Oliver, Tan Kian, Morris Julie, Bundred Nigel. Annals of Surgical Oncology.2017;24(4). CrossRef
- Cause-specific Mortality in a Population-based Cohort of 9799 Women Treated for Ductal Carcinoma In Situ Elshof Lotte E., Schmidt Marjanka K., Rutgers Emiel J.Th., Leeuwen Flora E., Wesseling Jelle, Schaapveld Michael. Annals of Surgery.2018;267(5). CrossRef
- Breast Cancer Mortality After a Diagnosis of Ductal Carcinoma In Situ Narod Steven A., Iqbal Javaid, Giannakeas Vasily, Sopik Victoria, Sun Ping. JAMA oncology.2015;1(7). CrossRef
- Incidence and Mortality and Epidemiology of Breast Cancer in the World Ghoncheh Mahshid, Pournamdar Zahra, Salehiniya Hamid. Asian Pacific journal of cancer prevention: APJCP.2016;17(S3). CrossRef
- Controversies in the Treatment of Ductal Carcinoma in Situ Barrio Andrea V., Van Zee Kimberly J.. Annual Review of Medicine.2017;68. CrossRef
- Long-term outcomes of ductal carcinoma in situ of the breast: a systematic review, meta-analysis and meta-regression analysis Stuart Kirsty E., Houssami Nehmat, Taylor Richard, Hayen Andrew, Boyages John. BMC cancer.2015;15. CrossRef
- Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS Wapnir Irene L., Dignam James J., Fisher Bernard, Mamounas Eleftherios P., Anderson Stewart J., Julian Thomas B., Land Stephanie R., Margolese Richard G., Swain Sandra M., Costantino Joseph P., Wolmark Norman. Journal of the National Cancer Institute.2011;103(6). CrossRef
- Breast-conserving treatment with or without radiotherapy in ductal carcinoma In Situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial Donker Mila, Litière Saskia, Werutsky Gustavo, Julien Jean-Pierre, Fentiman Ian S., Agresti Roberto, Rouanet Philippe, Lara Christine Tunon, Bartelink Harry, Duez Nicole, Rutgers Emiel J. T., Bijker Nina. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2013;31(32). CrossRef
- A Biological Signature for Breast Ductal Carcinoma In Situ to Predict Radiotherapy Benefit and Assess Recurrence Risk Bremer Troy, Whitworth Pat W., Patel Rakesh, Savala Jess, Barry Todd, Lyle Stephen, Leesman Glen, Linke Steven P., Jirström Karin, Zhou Wenjing, Amini Rose-Marie, Wärnberg Fredrik. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2018;24(23). CrossRef
- Radiation therapy for ductal carcinoma in situ: a decision analysis Punglia Rinaa S., Burstein Harold J., Weeks Jane C.. Cancer.2012;118(3). CrossRef
- Association of Radiotherapy With Survival in Women Treated for Ductal Carcinoma In Situ With Lumpectomy or Mastectomy Giannakeas Vasily, Sopik Victoria, Narod Steven A.. JAMA network open.2018;1(4). CrossRef
- Surgical Excision Without Radiation for Ductal Carcinoma in Situ of the Breast: 12-Year Results From the ECOG-ACRIN E5194 Study Solin Lawrence J., Gray Robert, Hughes Lorie L., Wood William C., Lowen Mary Ann, Badve Sunil S., Baehner Frederick L., Ingle James N., Perez Edith A., Recht Abram, Sparano Joseph A., Davidson Nancy E.. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2015;33(33). CrossRef
- Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS Trial Wärnberg Fredrik, Garmo Hans, Emdin Stefan, Hedberg Veronica, Adwall Linda, Sandelin Kerstin, Ringberg Anita, Karlsson Per, Arnesson Lars-Gunnar, Anderson Harald, Jirström Karin, Holmberg Lars. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2014;32(32). CrossRef
- Breast cancer screening and prevention system in Thailand from the perspective of service providers Pongthavornkamol K, Watthayu N, Khuhaprema T. Thai Cancer J.2019;39(3):77-92.
- Long-term outcomes of breast-conserving therapy for women with ductal carcinoma in situ Warren Laura E. G., Chen Yu-Hui, Halasz Lia M., Brock Jane E., Capuco Alexander, Punglia Rinaa S., Wong Julia S., Golshan Mehra, Bellon Jennifer R.. Breast Cancer Research and Treatment.2019;178(3). CrossRef
- Predictive significance of breast-specific gamma imaging for upstaging core-needle biopsy-detected ductal carcinoma in situ to invasive cancer Yoo Jang, Kim Bom Sahn, Yoon Hai-Jeon. Annals of Nuclear Medicine.2018;32(5). CrossRef
- Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis Kerlikowske Karla, Molinaro Annette M., Gauthier Mona L., Berman Hal K., Waldman Fred, Bennington James, Sanchez Henry, Jimenez Cynthia, Stewart Kim, Chew Karen, Ljung Britt-Marie, Tlsty Thea D.. Journal of the National Cancer Institute.2010;102(9). CrossRef
- Long-term outcome after breast-conservation treatment with radiation for mammographically detected ductal carcinoma in situ of the breast Solin Lawrence J., Fourquet Alain, Vicini Frank A., Taylor Marie, Olivotto Ivo A., Haffty Bruce, Strom Eric A., Pierce Lori J., Marks Lawrence B., Bartelink Harry, McNeese Marsha D., Jhingran Anuja, Wai Elaine, Bijker Nina, Campana Francois, Hwang Wei-Ting. Cancer.2005;103(6). CrossRef
- Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in-situ: analysis of European Organization for Research and Treatment of Cancer Trial 10853 Bijker N., Peterse J. L., Duchateau L., Julien J. P., Fentiman I. S., Duval C., Di Palma S., Simony-Lafontaine J., Mascarel I., Vijver M. J.. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2001;19(8). CrossRef
- Clinicopathological predictive factors for ipsilateral and contralateral events following initial surgery to treat ductal carcinoma in situ Tamura Nobuko, Tsuda Hitoshi, Yoshida Masayuki, Hojo Takashi, Akashi-Tanaka Sadako, Kinoshita Takayuki, Sugihara Kenichi. Breast Cancer (Tokyo, Japan).2016;23(3). CrossRef
- A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast Solin Lawrence J., Gray Robert, Baehner Frederick L., Butler Steven M., Hughes Lorie L., Yoshizawa Carl, Cherbavaz Diana B., Shak Steven, Page David L., Sledge George W., Davidson Nancy E., Ingle James N., Perez Edith A., Wood William C., Sparano Joseph A., Badve Sunil. Journal of the National Cancer Institute.2013;105(10). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2022
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times