Suprapubic Catheter in a Patient with Bladder Carcinoma, Against the Prohibition: A Systematic Review and Case Report
Download
Abstract
Background: Bladder tumors are the most common neoplasm of the lower urinary tract. Bladder carcinoma arising from a suprapubic cystostomy tract is a relatively rare. Some patients with bladder tumors have difficult urethral access for urinary retention due to inaccessible urethra. Most people think cystostomy in patient with bladder cancer can cause seeding and upstaging to suprapubic cystostomy tract. This study aim of suprapubic cystostomy can cause seeding and upstaging of bladder cancer.
Methods: Literature obtained from the search results of Pubmed, Medscape, Science Direct, Scopus, Cochrane Library, and Google Scholar electronic databases with the keywords used are “suprapubic catheter and bladder cancer”, “suprapubic catheter and bladder tumor”, “suprapubic catheter and bladder carcinoma”, “suprapubic cystotomy and bladder carcinoma”, and “suprapubic catheter and bladder cancer upstaging” with no time limits.
Results: Eighty two articles were obtained from the electronic database. Following all the inclusion and exclusion criteria, the final selection considered 5 literature with 16 patient eligible for this literature review. The five literature involved a total of 16 bladder cancer patients with a suprapubic catheter. The duration of suprapubic catheter insertion was between 1 to 3 months. As long as it is within the specified time and from the location of cancer, literature result there will be no seeding and upgrading in the suprapubic cystostomy tract. In our case report we have patient with duration suprapubic cystostomy 1 months wih no seeding and upgrading cancer.
Conclusion: Although SPC is an effective, inexpensive, easy mode of access for bladder tumors with difficult urethral access for urinary retention due to inaccessible urethra, it also presents a risk of SPC tract bladder cancer, mostly SCC and TCC. It is important to be aware of any suspicious signs and symptoms, duration time of use suprapubic cystostomy and location of the cancer.
Introduction
Bladder tumors are the most common neoplasm of the lower urinary tract. A majority of patients usually present with gross painless hematuria as the sole presenting symptom [1]. Incidence peaks in the sixth and seventh decades of life, although a trend toward presentation at younger ages has been suggested [2]. The lifetime risk for men is 3.4%, and for women, 1.2% [3]. Initial symptoms of urothelial carcinoma of the bladder (UCB) include microhematuria, painless macrohematuria, and/ or irritative voiding symptoms, and require further investigation. Carcinoma in situ of the bladder causes irritative lower urinary tract symptoms (LUTS) more often than papillary UCB. Histopathologic evaluation is necessary to assess the stage and grade with sufficient certainty after the appearance of bladder tumors [4].
Several causative risk factors are associated with urothelial carcinoma of the bladder, including smoking, genetic, and other carcinogenic exposure [5]. Other rarer tumor types include squamous cell carcinomas, which occur in the setting of chronic inflammation [6]. These were in line with the findings which determined that bladder tumors spread by implantation in abdominal wounds, denuded epithelium, resected prostatic fossa, or traumatized urethra; implantation occurs most often with high-grade tumors [7]. Squamous cell carcinoma arising from a suprapubic cystostomy tract (SCC-SCT) is a relatively rare bladder malignancy, which is known to have a close association with long-term inflammation and mechanical irritation from the suprapubic catheter [8]. The suprapubic catheter (SPC) is a non-continent, direct drainage of the bladder in cases of inaccessible urethra; it may be accomplished by an open or punch technique with a trocar or percutaneous methods using the Seldinger wire technique. The suprapubic catheter is a relatively safe and common procedure in urologic practice; it is also used in cases of genitourinary trauma, with few complications. It provides effective urinary diversion and drainage, which might be beneficial in cases of an inaccessible urethra [9]. The term inaccessible urethra means all prescribed endoscopic interventions have failed to relieve acute painful retention [9]. Inaccessible urethra with no retrograde endoscopic access due to multiple/diffuse strictures or multiple urethrocutaneous fistulas with acute urinary retention due to post-urethral instrumentation (transurethral resection of bladder tumor [TURBT], or TURBT with transurethral resection of the prostate [TURP]), is a rare entity. Management of such case with a bladder tumor for TURBT/surveillance cystoscopy poses a great challenge [9].
Therefore, this literature review aimed to investigate the utilization of SPC in a patient with bladder cancer against the prohibition.
Materials and Methods
Literature search strategy
Literature obtained from the search results of Pubmed, Medscape, Science Direct, Scopus, Cochrane library, and Google Scholar electronic databases with keywords: “suprapubic catheter and bladder cancer”, “suprapubic catheter and bladder tumor”, “suprapubic catheter and bladder carcinoma”, “suprapubic cystotomy and bladder carcinoma” and “Suprapubic catheter and bladder cancer upstaging”. References cited in the relevant literature were taken manually and only from full articles.
Inclusion and exclusion criteria
According to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines, the literature was obtained by reviewing PICOS (Population, Intervention, Comparator, Outcome, and Study design) to determine the feasibility of the study. Studies were considered feasible if they met the inclusion criteria as followings; 1) Patients of the studies underwent suprapubic catheter insertion; 2) Patients of the studies underwent the histopathological examination and physical examination leading to the diagnosis of bladder cancer; 3) Patients of the studies were examined for the following variables; i) Number of tumors, ii) Tumor diameter, iii) Localization of tumor, iv) Histology classification, v) Complication rate; 4) The studies consist of the outcome evaluation of suprapubic catheter in patients with bladder cancer.
Literature in the form of only abstracts, report meetings, conferences, editorial comments, reviews (systematic literature reviews and meta-analyses), irrelevant results, inaccessible literature, duplications from previous literature publications, and non-English studies were excluded.
Systematic literature review process
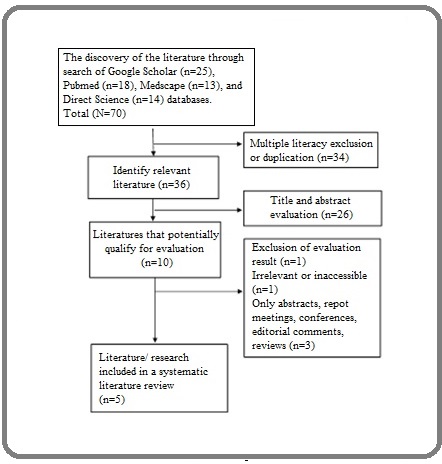
After excluding multiple literature, evaluation of the abstract and title of the relevant literature were carried out. After evaluating abstracts and titles, acquired literature that fulfilled the requirements for a full evaluation was selected. Data were then extracted and presented in a table. The table consisted of the author’s name, year of publication, country of origin, study design, number of samples, number of tumors, tumor diameter, localization of tumor, histopathological classification, and complication rate. The PRISMA diagram that describes the literature review process and literature selection are shown in Figure 1.
Figure 1. PRISMA Diagram that Describes the Search Process for Literature Review and Literature Selection.
Results
Since the beginning of 2021, 82 articles have been collected through the electronic database of Pubmed, Medscape, Science Direct, Scopus, Cochrane Library, and Google Scholar. After the exclusion of multiple literature, 36 relevant literature were obtained for the abstracts and titles evaluation. From the results of the evaluation, ten literature were eligible for a full evaluation. Based on all the inclusion and exclusion criteria mentioned earlier, the final selection considered 5 literature eligible with 16 patient for this literature review (Table 1).
| No | Author | Year, country | Type of research | Study population |
| 1 | Khawaja, et al.[9] | 2016, India | Case Series | 12 patients |
| 2 | Gusev, et al.[10] | 2020, United States | Case Report | 1 patient |
| 3 | Ito,et al.[11] | 2011, Japan | Case Report | 1 patient |
| 4 | Breul, et al.[12] | 1992, German | Case Report | 1 patient |
| 5 | Zhang, et al.[13] | 2015, China | Case Report | 1 patient |
The literature involved a total of 17 bladder cancer patients with a suprapubic catheter. Of those, there were 1 case series and 5 case report study aimed to investigate the outcome of suprapubic catheter insertion in bladder cancer patients.
Based on the demographic profile, all of the patients were male, with the age range of 38 to 80 years (Table 2).
| Study | Total Subjects | Age | Sex |
| Khawaja, et al.[9] | 12 patients | 38 – 68 years | Male (12 patients; 100%) |
| Gusev, et al.[10] | 1 patient | 65 years | Male |
| Ito,et al.[11] | 1 patient | 58 years | Male |
| Breul, et al.[12] | 1 patient | 80 years | Male |
| Zhang, et al.[13] | 1 patient | 61 years | Male |
In this review, the most frequent histopathological appearance in bladder cancer related to suprapubic catheter was TCC and SCC (Table 3).
| Study | Number of Tumors | Size of tumor | Localization of tumor | Histopathological Classification |
| Solitary (9; 75%) | Tumor diameter | Posterolateral wall | Low-grade transitional cell carcinoma (10; 83,33%) | |
| 0,5 mm – 1 cm (9; 75%) | (6; 50%) | |||
| Multifocality (2; 16,66%) | 1 – 3 cm (2; 16,66%) | Anterolateral (3; 25%) | ||
| Khawaja, et al.[9] | Focal bladder thickening (1; 8,33%) | 3 – 5 cm (1; 8,33%) | Dome (0) | High-grade transitional cell carcinoma (2; 16,66%) |
| Base of bladder (2; 16,66%) | ||||
| Bladder neck (1; 8,33%) | ||||
| Gusev, et al.[10] | N/A | N/A | Posterior bladder wall | High-grade, mus- cle-invasive urothelial carcinoma with glandular differentiation |
| Ito,et al.[11] | Solitary | 72 mm x 63 mm | An abdominal mass surrounding a suprapubic cystostomy | Stage IV (cT4N1M1) epidermal SCC |
| Breul, et al.[12] | Solitary | 4 x 4 x8 cm | Ventral to the bladder | poorly differentiated SCC with infiltration into the prostate (pT3, G3) |
| Zhang, et al.[13] | Solitary | (8 x 6 x 5) cm | Surrounding the suprapubic cystostomy and a space-occupying lesion | Squamous cell carcinoma |
| Study | Inaccesible | Type of SPC | Grade of the tumor | History of Operation |
| Khawaja, et al.[9] | Failed Catheterzation (9; 75%) | N/A | N/A | TURBT (12: 100%) |
| Urethrocutaneous fistula (2; 16,66%) | ||||
| Failed OIU with retention and urinary extravasation (1; 8,33%) | ||||
| Gusev, et al.[10] | Urethral stricture | Squamous cell carcinoma | N/A | Biopsi stricture, Biopsi suprapubic mass |
| Ito,et al.[11] | N/A | Squamous cell carcinoma | cT4N1M1 | N/A |
| Breul, et al.[12] | N/A | N/A | N/A | N/A |
| Zhang, et al.[13] | Urethral diverticulum with abscess formation perforated spontaneously | Moderately differen-tiated squamous cell carcinoma of the suprapubic tract but there was no bladder involvement | N/A | Operation on the spinal column (laminectomy T5 ± T9) resulted in a spinal cord injury, incomplete T4 and complete T7 (T4 ±T7 sensory/motor deficit). |
Most of the bladder cancer patients with suprapubic catheters presented with recurrent urinary retention, inflammation around the area of the suprapubic catheter tract, hydronephrosis, and neurogenic bladder. Other clinical presentations of SPC complications included urethrocutaneous fistula, urinary extravasation, nocturia, reduced urinary flow, and gross hematuria (Table 4).
| Study | Clinical Presentation of SPC Complication | Duration of SPC insertion | SPC Tract Histopathology |
| Acute urinary retention with failed catheterization | 1 – 2 months | Normal, only granulation tissue | |
| Khawaja, et al.[9] | Urethrocutaneous fistula | 3 months | Normal, only granulation tissue |
| Post urethral instrumentation (failed OIU) with retention and urinary extravasation | 3 months | Normal, only granulation tissue | |
| Gusev, et al.[10] | Inflammation of the left lower quadrant omentum around the area of the biopsy tract; Gross hematuria; Acute renal failure (serum creatinine 4.4 mg/dl); Severe bilateral hydronephrosis | N/A | high-grade, mus- cle-invasive urothelial carcinoma with glandular differentiation |
| Ito,et al.[11] | Severely inflamed abdominal mass; Absence of sensation below the waist; Chronic neurogenic bladder; An abdominal mass surrounding a suprapubic cystostomy, erythematous skin around the mass, edematous, foul-smelling, and purulent discharge | 1 month | Stage IV (cT4N1M1) epidermal SCC |
| Breul, et al.[12] | Repeated urinary retention; Nocturia of 3 times/night; Reduced urinary flow; Hydronephrosis; Filling defect of the bladder | N/A | poorly differentiated squamous cell carcinoma with infiltration into the prostate (pT3, G3) |
| Zhang, et al.[13] | Ulcerative bleeding with abnormal blisters surrounding SPC tract; Suprapubic catheter-related pain; Progressive lower extremity weakness; Neurogenic bladder; Bilateral hydronephrosis | 3 months | Squamous cell carcinoma |
The duration of suprapubic catheter insertion was between 1 to 3 months. Most histopathological appearance of suprapubic catheter-related bladder cancer was urothelial carcinoma and squamous cell carcinoma. We also reported our cases; a 55-years-old man presented to the emergency unit with a chief complaint of retension urine with bladder cancer and inaccessible urethra.
Case Presentation
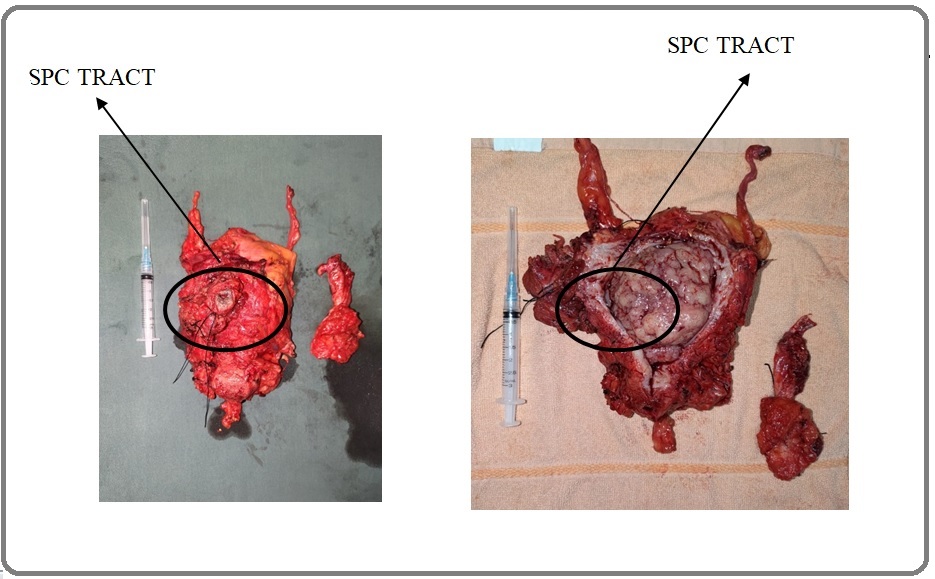
A 55-years-old man presented to the emergency unit with a chief complaint of retension urine. The patient has a history of TURBT with pathology anatomi non-muscle invasive bladder cancer results. Ultrasonography of the Kidney, Ureter, and Bladder (USG KUB) showed no right and left kidney abnormality. The urinary bladder was full of urine (approximate volume of 850 ccs) and mass was found in posteriorlateral of bladder shown in Figure 2.
Figure 2. Mass in Posteriolateral Aspect of the Bladder Found Intraoperatively.
Inserted a catheter does not work (inaccessible urethra) and decided to do SPC with Folley Catheter 16 fr.
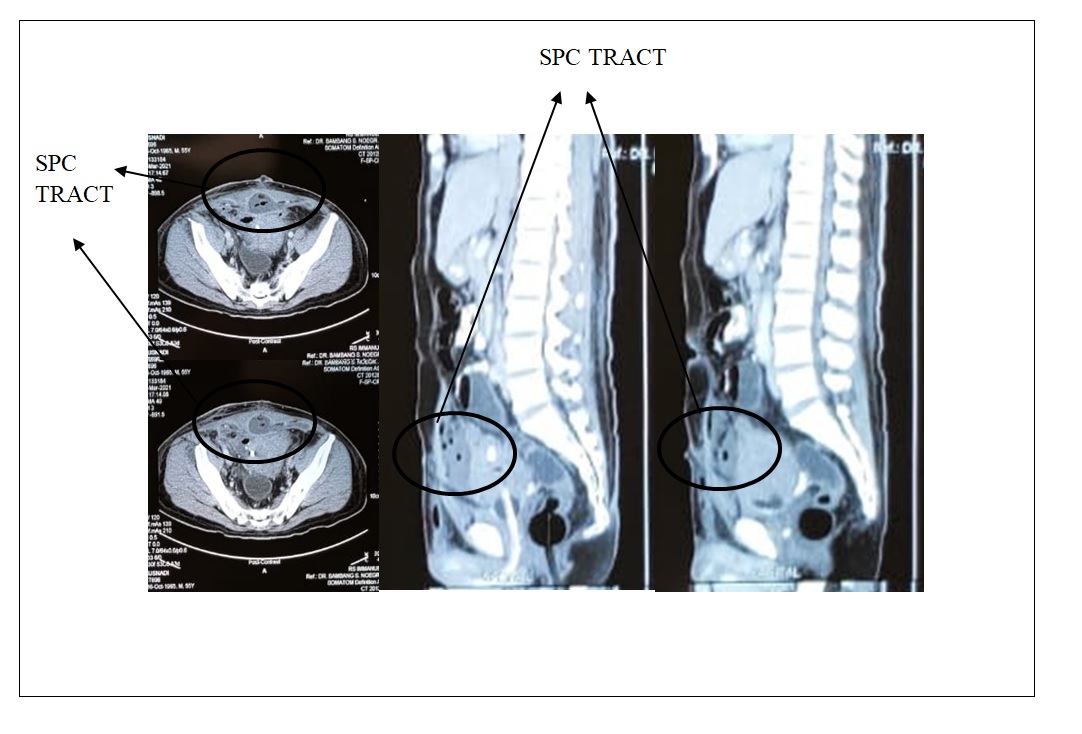
The patient come with CT Scan revealed single irregular mass within the bladder wall on the posteriorlateral part with calcification, extending from the mucosal layer to serosa layer, size 5,45 x 5,17 x 6,73 cm with post-contrast enhancement as shown in Figure 3.
Figure 3. SPC Tract Shown in CT Scan.
Patient have radical cystectomy and urinary diversion. Postoperation PA result found Low grade Urothelial carcinoma bladder (T2N0M0) without seeding and upstaging in SPC track. Figure 4 shows low-grade papillary bladder carcinoma compared to SPC tract without malignancy shown in Figure 5.
Figure 4. Low-Grade Papillary Bladder Carcinoma.
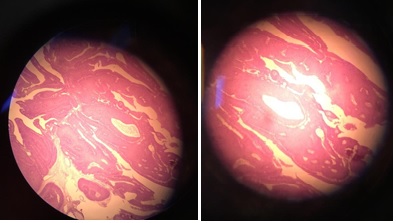
Figure 5. SPC Tract without Malignancy.
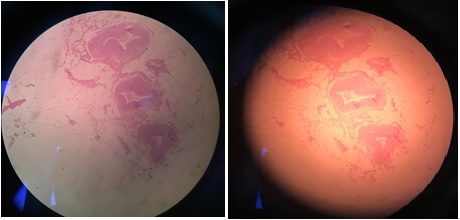
Discussion
Bladder cancer is the most common tumor of the urinary system, comprising 6% of all malignancies in men and 2% of those in women [1]. Incidence peaks in the sixth and seventh decades of life [2]. According to Pashos et al., the lifetime risk for men is 3.4%, and for women 1.2% [3]. In this review, all of the patients were male, with the age range of 38 to 80 years.
In this review, the most histopathological finding of suprapubic catheter-related bladder cancer was urothelial carcinoma and squamous cell carcinoma. Based on the result of this review, most of the patients presented with recurrent urinary retention, inflammation around the area of the suprapubic catheter tract, hydronephrosis, and neurogenic bladder. Other clinical presentations of SPC complications included urethrocutaneous fistula, urinary extravasation, nocturia, reduced urinary flow, and gross hematuria.
A suprapubic catheter is a non-continent, direct drainage of the bladder in cases of inaccessible urethra; it may be accomplished by an open or punch technique with a trocar or percutaneously using the Seldinger wire technique. A suprapubic catheter is a relatively safe and common procedure in urologic practice; it is also used in cases of genitourinary trauma, with few complications. It provides an effective urinary diversion and drainage, which might be beneficial in cases of an inaccessible urethra [9]. According to a study conducted by Mustofi et al., rarer tumor types include squamous cell carcinomas, which occur in the setting of chronic inflammation, and adenocarcinomas, which occur in the bladder in association with a persistent urachal remnant and in cystitis glandularis associated with bladder exstrophy. In this review, the SPCs have usually been applied for 1 to 3 months.
The advantages of suprapubic diversion are that it is easy, provides quick relief, is practiced by all urologists; it can also be performed under local anesthesia. In cases of suspected space-occupying lesions in the bladder, ultrasound can be helpful to avoid needle advancement into the tumor during the suprapubic diversion. Chemotherapy/ immunotherapy can be administered with surveillance cystoscopy in cases of inaccessible urethra via SPC. Moreover, the patient can examine the SPC tract site for any swelling and skin changes, and it is also accessible to general practitioners and treating urologists [9].
The five literature involved a total of 16 bladder cancer patients with a suprapubic catheter. The duration of suprapubic catheter insertion was between 1 to 3 months. As long as it is within the specified time and from the location of cancer, literature result there will be no seeding and upgrading in the suprapubic cystostomy tract. In our case report we have patient with duration suprapubic cystostomy 1 months wih no seeding and upgrading cancer.
In conclusion, although SPC is an effective, inexpensive, easy mode of access for bladder tumors with difficult urethral access for urinary retention due to inaccessible urethra, it also presents a risk of SPC tract bladder cancer, mostly SCC and TCC. It is important to be aware of any suspicious signs and symptoms, duration time of use suprapubic cystostomy and location of the cancer. Tumor location in posterior, low grade, use feeding tube 6-8 Fr, duration of SPC 1-2 months, and seeding or upgrading of bladder cancer was not found.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors. The authors declare no conflict of interest.
References
- Cancer statistics, 2001 Greenlee R. T., Hill-Harmon M. B., Murray T., Thun M.. CA: a cancer journal for clinicians.2001;51(1). CrossRef
- American Cancer Society. Cancer facts & figures, 2008. Atlanta, GA: American Cancer Society, 2008 .
- Bladder cancer: epidemiology, diagnosis, and management Pashos Chris Leo, Botteman Marc F., Laskin Benjamin Lewis, Redaelli Alberto. Cancer Practice.2002;10(6). CrossRef
- ICUD-EAU International Consultation on Bladder Cancer 2012: Pathology Amin Mahul B., McKenney Jesse K., Paner Gladell P., Hansel Donna E., Grignon David J., Montironi Rodolfo, Lin Oscar, Jorda Merce, Jenkins Lawrence C., Soloway Mark, Epstein Jonathan I., Reuter Victor E.. European Urology.2013;63(1). CrossRef
- Demographics and epidemiology of urothelial cancer of urinary bladder. In: Droller MJ, ed Fleshner N, Kondylis F. Urothelial tumors. Hamilton, ON: Decker.2004;:1-16.
- Imaging and Staging of Transitional Cell Carcinoma: Part 1, Lower Urinary Tract Vikram Raghunandan, Sandler Carl M., Ng Chaan S.. American Journal of Roentgenology.2009;192(6). CrossRef
- Campbell-Walsh Urology. 9th ed McDougal WS , Wein AJ , Kavoussi LR , et al, eds . Philadelphia, PA: Saunders Elsevier.2012;:2426.
- The method of bladder drainage in spinal cord injury patients may influence the histological changes in the mucosa of neuropathic bladder - a hypothesis Vaidyanathan Subramanian, Mansour Paul, Soni Bakul M., Singh Gurpreet, Sett Pradipkumar. BMC urology.2002;2. CrossRef
- Effectiveness of Antegrade Access in Bladder Tumors With Inaccessible Urethra Khawaja Abdul Rouf, Dar Tanveer Iqbal, Maik Sajad, Magray Javaid, Bhat Ashiq, Bhat Arif Hameed, Wani Mohd Saleem, Wazir Baldev Singh. Reviews in Urology.2015;17(4).
- Squamous cell carcinoma arising from suprapubic cystotomy site without bladder involvement Stroumbakis N., Choudhury M. S., Hernandez-Graulau J. M.. Urology.1993;41(6). CrossRef
- Transitional cell carcinoma in patients with spinal cord injury: a high risk malignancy? Pannek Jürgen. Urology.2002;59(2). CrossRef
- Histological typing of urinary bladder tumours, 2nd ed Mostofi F, Davis CJ , Sesterhenn I. Berlin, Germany: Springer-Verlag.1999;:3-27.
- Squamous cell carcinoma of suprapubic cystostomy tract without bladder involvement Schaafsma R. J., Delaere K. P., Theunissen P. H.. Spinal Cord.1999;37(5). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2022
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times