Morphologic and Vascular Changes in Treated Brain Arteriovenous Malformation by Stereotactic Radiosurgery; A Pilot Study
Download
Abstract
Objective: Goal of stereotactic radiosurgery in brain arteriovenous malformation (AVM) is complete obliteration of the nidus to prevent risk in intracranial hemorrhage. This study was to evaluate morphologic and vascular parameters of treated brain AVM by radiosurgery in follow-up MR studies, which might predict the outcome.
Methods: This retrospective single-centered study included 34 patients with brain AVMs treated by radiosurgery in Srinagarind Hospital during January 2013 to December 2017. We excluded patients without treatment information, no follow-up imaging, or first follow-up images longer than 24 months. One neuroradiologist reviewed brain MRI/MRA and measured parameters. Baseline characteristic of patients and nidi were collected. Overall change in parameters was calculated and comparison between radiographically complete obliteration and residual disease at post treatment 36 months were reported with 95%CI.
Result: Of 24 nidi, most were compact nidus, average 3.16 ml, deep venous draining (72%), and 1-2 draining veins (80%). 52% were non-eloquent region. Spletzler-Martin scores 2 was 48% and 3 was 40%. Follow-up images from 3 to 70 months showed reduction in 2.93 ml (95%CI [1.07, 4.81]), 13.48 mm (95%CI [11.14, 15.81]) in maximal diameter, 0.82 mm (95%CI [0.63, 1.01]) and 0.695 mm (95%CI [0.51, 0.89]) in bifurcation and prenidal arterial feeder diameters, and 1-2 draining vein(s) (95%CI [1.06, 1.85]). There is no statistically significant between radiographically complete obliteration and residual disease at 36 months.
Conclusion: There are parameter changes of brain AVMs in follow-up MR images after radiosurgery. However, no statistical significance to predict obliteration at 36 months.
Introduction
Brain arteriovenous malformation (BAVM) is an abnormal connection between intracranial arterial and venous systems in compaction of vascular structure, called nidus, without normal intervening capillary plexus, which results in escalation of circulating blood volume and decrease in vascular resistance.
Because of variation in shape, size or volume, and location of AVM nidus, each patients with different nidal characters were grouped and planned in choices of treatment individually, such as, conservative treatment, microscopic surgery, embolization with agent, stereotactic radiosurgery, or in combination [1].
Stereotactic radiosurgery in treatment for BAVM aims to occlude groups of nidus and results in complete obliteration of abnormal vascular connection. Previous studies [1] found that treated BAVMs via stereotactic radiosurgery had successful obliteration in 50 - 90%, depending on size or volume of nidus. Gradual process in obliteration was averagely taken about 2-3 years [2]. However, risk of intracranial hemorrhage persists unless complete obliteration occurs. The diagnosis of complete obliteration is decided on cerebral angiogram, which is the current reference standard modality, and has risk of future intracranial bleeding less than 1%.
Successful response of stereotactic radiosurgery treatment for brain AVM obliteration depends on dose and accuracy of procedure through the targeted nidus. Size and volume of nidus are corresponding to occlusion of the nidus. Post treatment follow-up studies with MRI of the brain have a crucial role for evaluation in treatment outcome and complete obliteration at the end of latent period, supported by some studies [3-5]. However, there is some limitation to accurately evaluate obliteration in case of subcentimeter nidus size (<10 mm) with a recommendation of diagnostic confirmation via cerebral angiogram [2].
Some studies, supporting benefits of MR follow-up in treated brain arteriovenous malformation via radiosurgery, found each MR sequences, such as 3D time-of-flight MR angiography, susceptibility weighted image, or contrast-enhanced MR image, helped in evaluation of post treatment [5-7]. Some studies also show benefits of perinidal T2 high signal intensities in prediction of complete obliteration [2][8] Currently, there is no definite guideline for follow-up imaging in post treatment of brain AVM. Some studies recommend regular interval for follow-up imaging, including 6-month MRI scan for detection of complication and annual scans to assess radiosurgical effect. If at the end of 3 years MRI suggests complete obliteration, confirmation with repeated DSA is requested. If MRI clearly defines residual nidus, angiography is delayed, and the patient is contacted to suggest the possible need for repeat radiosurgery to achieve the final obliteration response.
This thesis was conducted to evaluate morphologic change and vascular parameters of post treated brain AVM by stereotactic radiosurgery in subsequent follow-up MRI studies of the brain. Moreover, probable correlation of these changes for prediction of complete obliteration in early follow-up MRI studies was assessed.
Materials and Methods
Study population
The study protocol was approved by the Ethic Committee for Human Research, Khon Kaen University and was exempted from informed consent. The study was a retrospective descriptive study, carried out at Srinagarind Hospital, Faculty of Medicine (Thailand). We collected datas from the electronic hospital system (Health Object) and PACS system, which were recorded between January 2013 to December 2020. For inclusion, the subjects consist of 34 patients with diagnoses as brain arteriovenous malformation and obligatorily treated with stereotactic radiosurgery between January 2013 to December 2017. 5 patients were excluded from study due to no recording data about date of stereotactic radiosurgical treatment, 1 patient was excluded due to no available post treatment image, and 4 patients were excluded because the first post treatment MRIs were later than 2 years from treatment date (Figure 1).
Figure 1. Workflow of the Study.
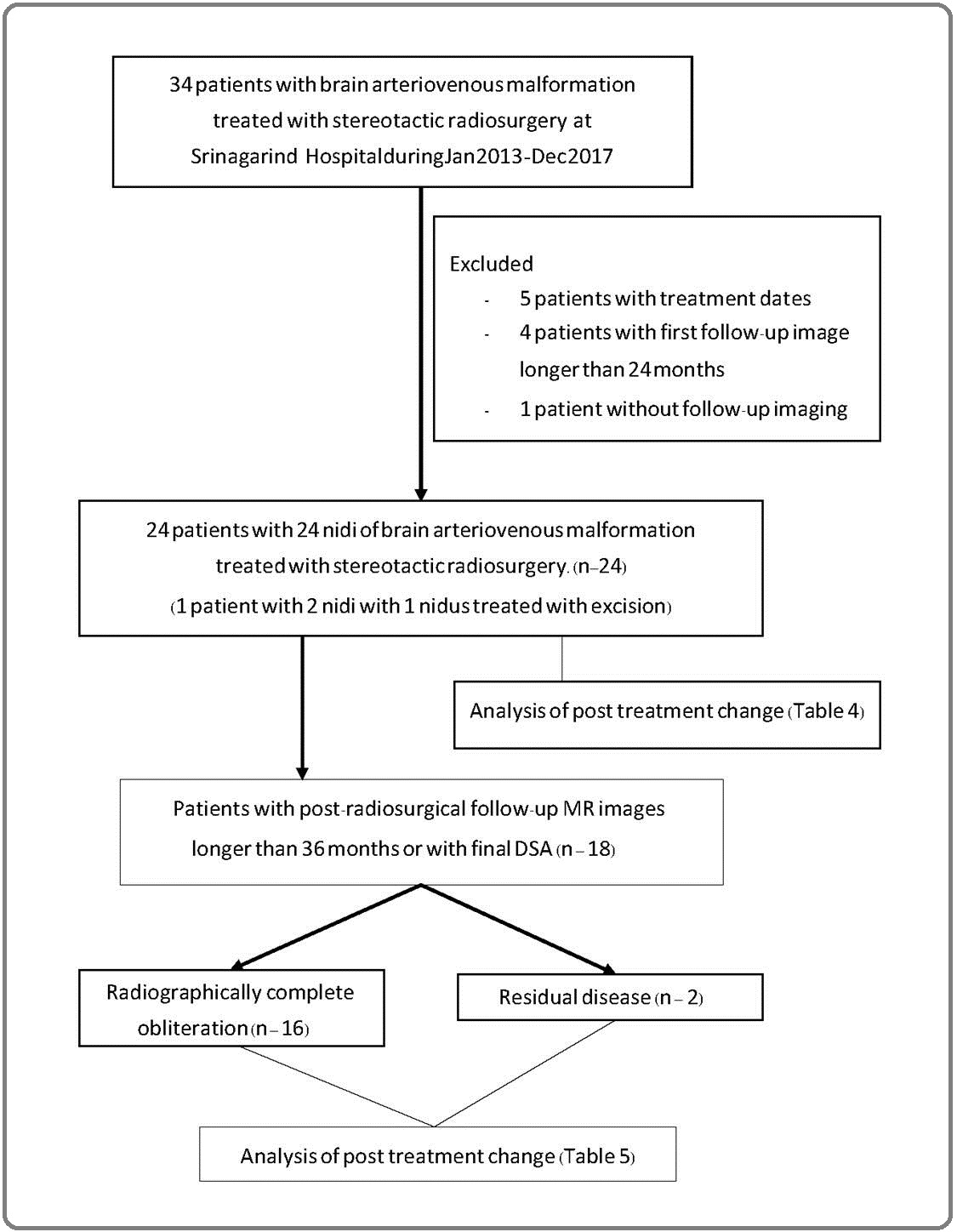
We collected patients’ clinical datas from Health Object, which were recorded by the primary physicians, mostly neurosurgeons, and from PACS, recorded by radiologists.
Imaging technique and analysis
All patients were performed standard digital subtraction cerebral angiograms, prior to treatment, and discussed in choices of treatment by monthly conference between radiotherapy physicians, neurosurgeons, and neurointerventionists. There are 7 patients, who received pre-stereotactic embolization including 6 targeted embolization and a partial embolization, with post embolization cerebral angiogram: 6 sessions of embolization using NBCA and a session using detachable coil.
Digital subtraction cerebral angiograms in standard four vessels were performed by neurointerventionists using iodinated contrast agents with injector approximately 2 ml/kg (Iopamidolum, Iopamiro 300 mg/ml; Bracco Suisse SA).
Post treatment imaging with MRI of the brain using routine brain protocol (sagittal T1WI, axial T1W, T2W, FLAIR, coronal T1W, T2 gradient image, SWI, and DWI/ ADC mapping) and MR angiography in 3D time-of-flight (TOF) technique and contrast-enhanced technique in axial and coronal views and volume rendering technique (VRT). Administration of gadolinium-based contrast was 0.1 mmol/kg (Gadobutrol, Gadovist; Bayer Healthcare Pharmaceuticals).
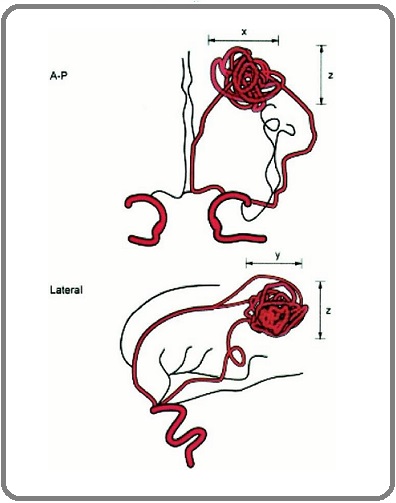
We measured the size or volume of nidus based on Reporting Terminology for Brain Arteriovenous Malformation Clinical and Radiographic Features for Use in Clinical Trials by Joint Writing Group of the Technology Assessment Committee (Figure 2).
Figure 2. Demonstrate How to Measure Nidus Volume in Coronal and Sagittal Views of Cerebral Angiography Based on Reporting Terminology for Brain Arteriovenous Malformation Clinical and Radiographic Features for Use in Clinical Trials.
For measurement in vascular diameter, we measured the maximal diameter of the largest arterial feeder in the same views between pre-stereotactic cerebral angiogram and post-treatment MR angiography at two points; post bifurcation site, approximately 1-2 cm distal to circle of Willis or vertebrobasilar artery, and prenidal site (Figure 3).
Figure 3. Demonstrate How to Measure Maximal Diameter of Feeding Artery (left MCA) at Bifurcation site and Prenidal Site in the Same View of Coronal DSA and Coronal MIP MR Angiography.
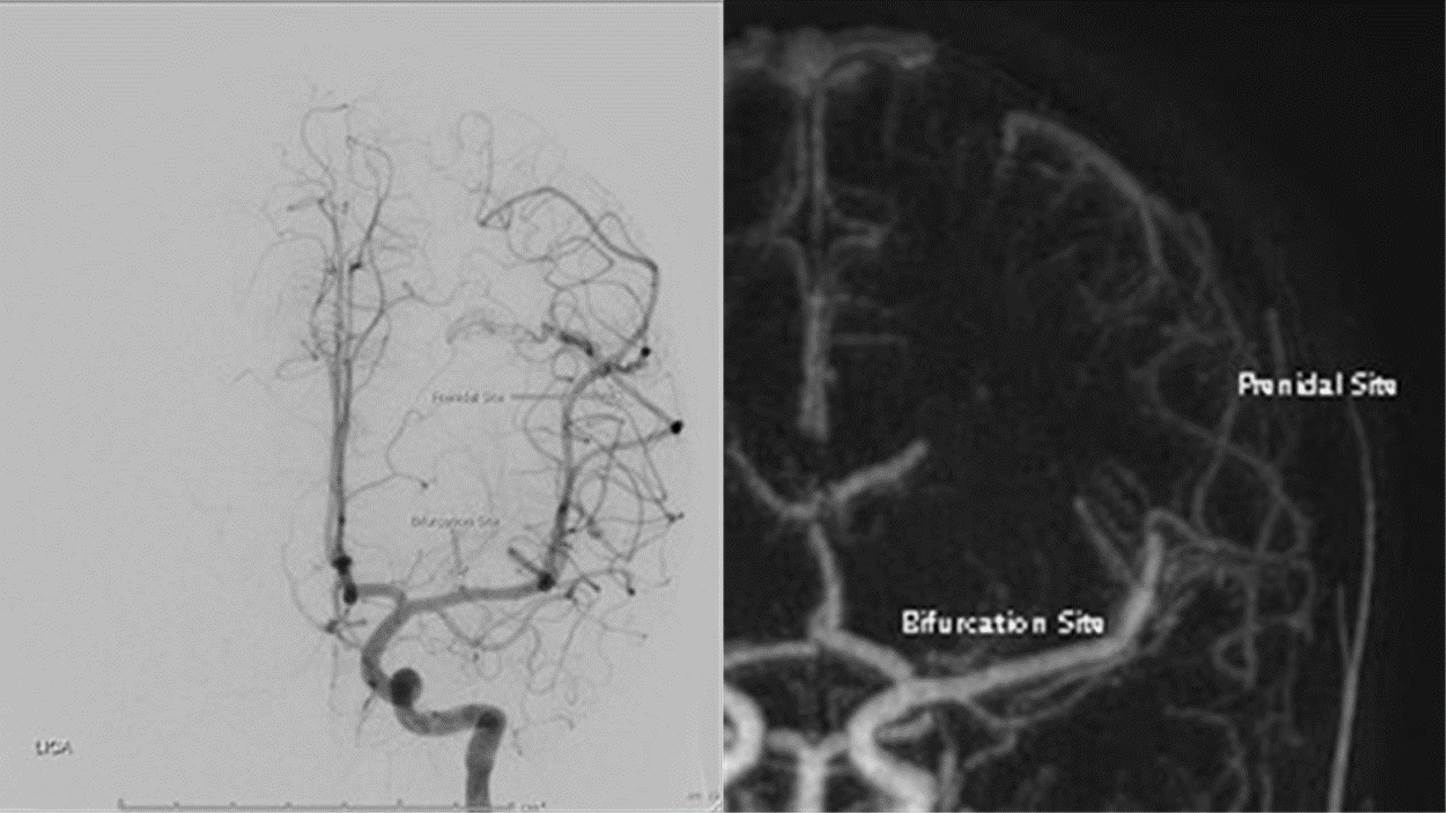
In evaluation of perinidal high T2WI signal intensity, we categorized into two groups (persistent and reversible changes in comparison to latest previous study) in T2W by one neuroradiologist. In additional analysis of high- resolution black-blood vascular wall image, we divided into two groups (positive and negative) of vascular wall thickening and enhancement by one neuroradiologist.
Data and statistical analysis
Characteristic and clinical datas of the patients were collected via hospital recording system, Health Object, and PACs.
Qualitative datas were presented in number and percentage. Quantitative data were presented as maximum, minimum, median, mean, and standard deviation (SD).
Baseline characteristic of BAVM nidus were collected in pretreatment studies, including in those received prior to pre-stereotactic embolization.
In analysis of treated nidus with stereotactic radiosurgery, we assessed in prestereotactic (post- embolized) cerebral angiographic studies to subsequent and the latest poststereotactic follow-up MR angiograms with available post treatment cerebral angiography, using two-sample Wilcoxon (Mann-Whitney) test in between groups of zero nidal volume and measurable volume in the last study.
Results
This study included 24 in total patient numbers with 25 nidus of brain arteriovenous malformation and 24 nidi treated with stereotactic radiosurgery (1 patient with 2 nidus, which one nidus treated with radiosurgery and another nidus was excised).
Table 1 in characteristics of the 24 patients, 17 patients (70.83%) presented with evidence of intracranial hemorrhage.
| Patient age at treatment (years): Mean, SD, Min, Max | 32.42, 13.39, 11, 56 n (%) |
| Male: Female | 10 (41.67): 14 (58.33) |
| Prior surgery | 0 (0) |
| Prior embolization | 7 (29.17) |
| Presentation | |
| Incidental | 1 (4.17) |
| Hemorrhagen | 17 (70.83) |
| Non-hemorrhagen | 6 (25.00) |
| Hemorrhage | |
| New hemorrhagen | 17 (70.83) |
| New hemorrhage with symptomn | 17 (70.83) |
| Old hemorrhagen | 2 (8.33) |
| Old hemorrhage with symptom | 0 (0) |
| Location of hemorrhage (all applicable) | |
| Ventricular | 12 (50) |
| Parenchymal | 13 (54.17) |
| Subarachnoid | 1 (4.17) |
| Hemorrhage volume (ml): Mean, SD, Min, Max | 12.39, 33.38, 14.80, 62 |
All of them were symptomatic from new hemorrhagic events with findings of parenchymal hemorrhage (13 patients, 54.17% with average 12.4 ml in volume), ventricular hemorrhage (12 patients, 50%) and subarachnoid (1 patient, 4.17%). 6 patients (25%) came with non-hemorrhagic presentation, such as, headache, seizure, and spastic gait. Incidentally found BAVM nidus in 1 patient who presented with traumatic brain injury was noted. Of these study patients, most of them were female (14, 58.3%) with mean age of treatment in 32.42 years (SD = 13.39 years). None of them got pre-stereotactic surgical excision and 7 of them got embolization (6 were targeted embolization and 1 was partial embolization) prior to radiosurgery.
All 25 nidus in 24 patients were shown in Table 2.
| Compact, n (%): Diffuse, n (%) | 22 (88%), 3 (12%) n (%) |
| Side | |
| Right, n (%) | 10 (40) |
| Left, n (%) | 12 (48) |
| Midline, n (%) | 3 (12) |
| Size of nidus in volume (ml): Mean, SD, Min, Max | 3.02, 4.65, 0.10, 20.42 |
| Maximal diameter of nidus (mm): Mean, SD, Min, Max | 18.70, 7.70, 8.80, 36.00 |
| Location of nidus (all applicable), n (%) | |
| Cortical | 8 (32) |
| Subcortical | 11 (44) |
| Ventricular | 6 (24) |
| Corpus callosum | 5 (20) |
| Frontal | 3 (12) |
| Temporal | 7 (28) |
| Parietal | 5 (20) |
| Occipital | 3 (12) |
| Basal ganglia | 1 (4) |
| Internal capsule | 1 (4) |
| Intraventricular | 2 (8) |
| Cerebellar hemisphere | 4 (16) |
| Vermian | 1 (4) |
| Deep cerebellar nuclei | 3 (12) |
| Brainstem | 1 (4) |
| Eloquence (all applicable), n (%) | |
| None | 13 (52) |
| Sensorimotor | 3 (12) |
| Visual | 2 (8) |
| Language | 2 (8) |
| Thalamus/ hypothalamus/ basal ganglia | 2 (8) |
| Internal capsule | 1 (4) |
| Cerebellar peduncle | 2 (8) |
| Deep cerebellar nuclei | 2 (8) |
| Brainstem | 1 (4) |
| Venous drainage, n (%) | |
| Superficial | 7 (28) |
| Deep | 10 (40) |
| Superficial and deep | 8 (32) |
| Periventricular, n (%) | 8 (32) |
| Number of draining vein (s) (n) | |
| =1 | 13 (52) |
| =2 | 7 (28) |
| =3 | 3 (12) |
| =4 | 2 (8) |
| Number of draining vein (s) to sinus (n) | |
| =1 | 12 (48) |
| =2 | 5 (20) |
| =3 | 0 (0) |
| =4 | 1 (4) |
| Venous reflux, n (%) | 2 (8) |
| Feeding artery (all applicable), n (%) | |
| Anterior choroidal artery | 1 (4) |
| ACA | 9 (36) |
| MCA | 10 (40) |
| PCA | 13 (52) |
| SCA | 2 (8) |
| AICA | 2 (8) |
| PICA | 1 (4) |
| Aneurysm | |
| None | 17 (68) |
| One aneurysm | 7 (28) |
| Two aneurysms | 1 (4) |
| Location of aneurysm(s), n (%) n = 8 | |
| Flow-related 0 (0) | |
| Not flow-related | |
| Nidal | 7 (87.5) |
| Proximal | 1 (12.5) |
| Distal | 0 (0) |
| ial-pial collateralization, n (%) | |
| Same territory 4 (16) | |
| Between territories | 8 (32) |
| None | 13 (52) |
| Spletzler-Martin, n (%) | |
| =1 | 2 (8) |
| =2 | 12 (48) |
| =3 | 10 (40) |
| =4 | 1 (4) |
| =5 | 0 (0) |
Reference, Reporting Terminology for Brain Arteriovenous Malformation Clinical and Radiographic Features for Use in Clinical Trials by Joint Writing Group of the Technology Assessment Committee
Most of them (22, 88%) were compact nidus with 18.7 mm in average maximal diameter of nidus (SD = 7.70). The three most supplying arteries are PCA (13, 52%), MCA (12, 48%), and ACA (9, 36%) in order. More than half BAVM nidus (13, 52%) were in non-eloquent regions and others were scattered in each eloquent location. 18 of the nidi (72%) drained into deep venous system. Overall Spetzler-Martin scales are 2 (48%) and 3 (40%) with a few of 1 (8%) and 4 (4%). There were 8 aneurysms with all non-flow-related aneurysms in 7 patients who got embolized sessions prior to being treated with stereotactic radiosurgery.
In post embolization, results of 24 nidus prior to stereotactic radiosurgical treatment and average change after treatment are shown in Table 3 and Figure 4.
| Pretreatment | Post treatment change (Overall; 3-70 months) | |||
| Mean | SD | 95% CI | ||
| Size of nidus in volume (ml): Mean, SD | 3.16, 4.74 | 2.93 | 4.43 | [1.07, 4.81] |
| Maximal diameter of nidus (mm): Mean, SD | 17.83, 7.20 | 13.48 | 5.54 | [11.14, 15.81] |
| Maximal diameter of feeding artery (mm): Mean, SD, Min, Max | ||||
| Bifurcation site | 1.95, 0.68, 0.91, 3.40 | 0.82 | 0.094 | [0.46, 0.63] |
| Prenidal site | 1.39, 0.68, 0.44, 3.47 | 0.695 | 0.092 | [0.43, 0.51] |
| Total draining vein (s): N (%) | 1.46 | 0.93 | [1.06, 1.85] | |
| =1 | 13 (54.17) | |||
| =2 | 6 (25.00) | |||
| =3 | 3 (12.50) | |||
| =4 | 2 (8.33) | |||
| Total draining vein (s) to sinus: N (%) | 0.75 | 0.85 | [0.39, 1.11] | |
| =0 | 9 (37.50) | |||
| =1 | 10 (41.67) | |||
| =2 | 4 (16.67) | |||
| =3 | 0 (0) | |||
| =4 | 1 (4.17) |
Figure 4. Four Graphs Demonstration each Parameter of all 24 Nidi Treated with Stereotactic Radiosurgery and Post Treatment Follow-up Imaging (MR and DSA),Iincluding A) BAVM Maximal Nidus Diameter (mm), B) BAVM Nidus Volume (ml), C) Arterial Feeder Diameter at Bifurcation Site (mm), and D) Arterial Feeder Diameter at Prenidal Site.
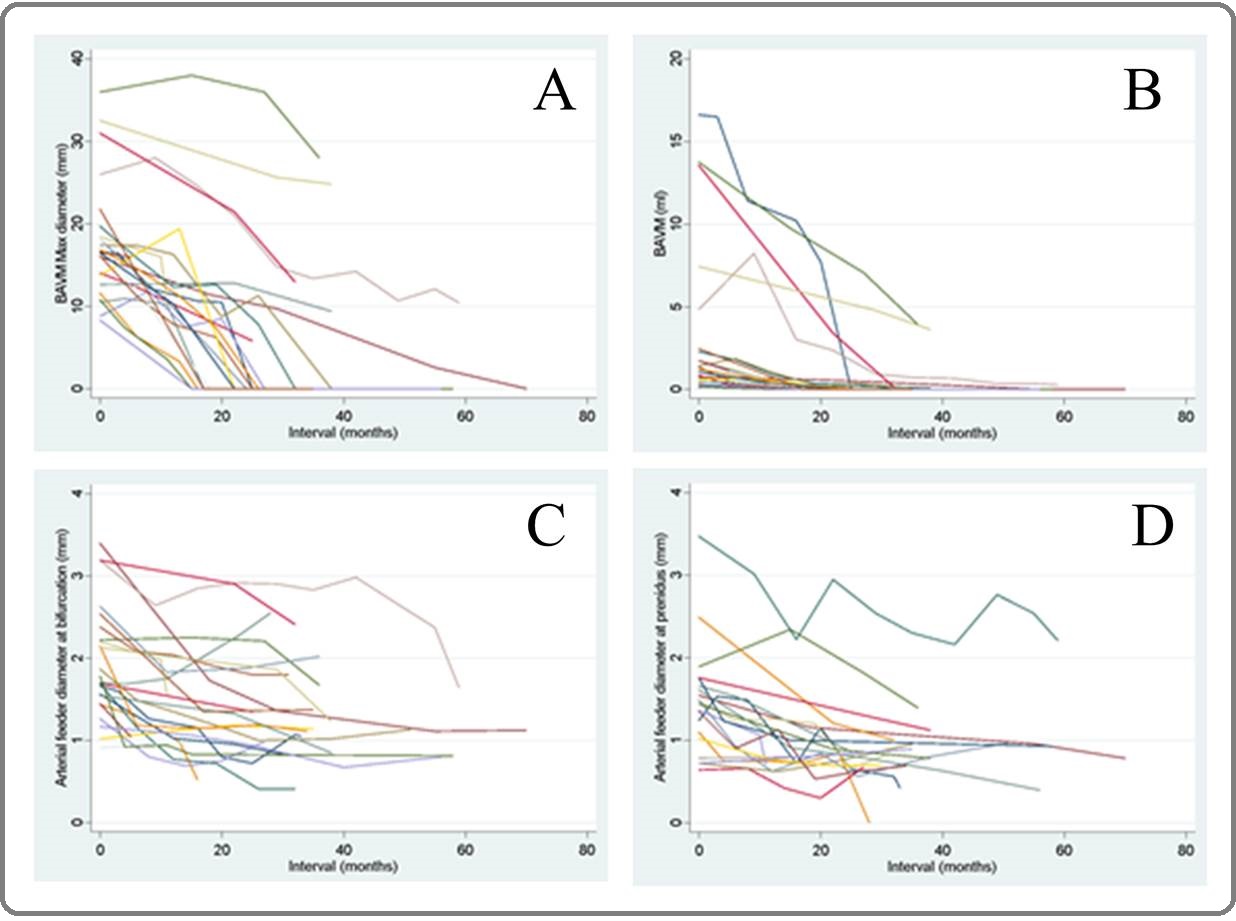
If following up to 70 months, we found that size of nidus would decrease about 2.93 ml (SD 4.43, 95%CI [1.07, 4.81]) and reduction about 13.48 mm in maximal nidal diameter (SD 5.54, 95%CI [11.14, 15.81]) after follow-up radiosurgical treatment. The diameters of the largest feeding artery would also decrease 0.82 mm in bifurcation site (SD 0.46, 95%CI [0.63, 1.01]) and 0.695 mm in prenidal site (SD 0.429, 95%CI [0.51, 0.89]). Disappearance in the number of total draining veins was about 1-2 veins (95%CI [1.06, 1.85]).
In additional evaluation of perinidal T2 high signal intensity, the study shows 17 patients with persistent high T2 signal (13 of them (76.4%) were classified into radiographically complete obliteration) and 7 patients with reversible high T2 signal (3 of them were with nonmeasurable nidus volume at 36 months follow-up and other two of them were with residual disease). In 17 patients with persistent perinidal T2 high signal intensity, two of them showed increased degree of surrounding high signal during follow-up; one was diagnosed as radiation- induced necrosis (peripheral enhancement without demonstrable MR perfusion study) and another was in suspicion for radiation-induced necrosis.
5 in 24 patients (12.5%) had post radiosurgical follow- up MR studies with high resolution black-blood vessel wall images. 4 of 5 patients found positive thickening and enhancing wall of nidus during obliteration (2 of 4 were in complete obliteration group and 1 of 4 was in residual disease). In 1 of 5 patient with negative thickening and enhancing wall of nidus was in residual disease group.
Of 24 patients with 24 treated nidi with radiosurgery, 16 patients were classified into a group of radiographically complete obliteration and 2 patients into residual disease (Table 4).
| Pretreatment | Post treatment change in 36 months (n=18) | |||||||
| Radiographically complete obliteration (n=16) | Residual disease (n=2) | Group difference in 95% CI | ||||||
| Mean | SD | 95% CI | Mean | SD | 95% CI | |||
| Size of nidus in volume (ml): Mean, SD, Min, Max | 2.79, 4.67, 0.10, 16.6 | 2.01 | 3.96 | [0.10, 4.12] | 8.89 | 1.32 | [2.99, 20.77] | [0.76, 12.99] |
| Maximal diameter of nidus (mm): Mean, SD, Min, Max | 16.83, 6.60, 8.30, 36.00 | 15.63 | 3.82 | [13.59, 17.66] | 13.8 | 5.37 | [-34.38, 62.08] | [-8.07, 4.42] |
| Maximal diameter of feeding artery (mm): Mean, SD, Min, Max | ||||||||
| Bifurcation site (n=18) | 1.92, 0.70, 0.91, 3.40 | 0.86 | 0.5 | [0.59, 1.13] | 1.07 | 0.69 | [-5.10, 7.22] | [-0.62, 1.02] |
| Prenidal site (n=16) | 1.32, 0.71, 0.44, 3.47 | 0.63 | 0.42 | [0.39,0.87] | 1.13 | 0.25 | [-1.16, 3.42] | [-0.16,1.16] |
| Total draining vein (s): N (%) | ||||||||
| =1 | 10 (55.56) | |||||||
| =2 | 4 (22.22) | -1.56 | 0.89 | [-2.04, -1.09] | -1 | 0 | [-1, -1] | [-0.81,1.94] |
| =3 | 3 (16.67) | |||||||
| =4 | 1 (5.56) | |||||||
| Total draining vein (s) to sinus: N (%) | ||||||||
| =0 | 7 (38.89) | |||||||
| =1 | 7 (38.89) | |||||||
| =2 | 4 (22.22) | |||||||
| =3 | 0 (0) | |||||||
| =4 | 0 (0) |
1 patient in the complete obliteration group was observed with newly developed evidence of arteriovenous shunting adjacent to the treated nidus (Figure 5).
Figure 5. Demonstration in a Case of BAVM Post Radiosurgery with Complete Obliteration in the Previous Nidus with New Evidence of Arteriovenous Shunting in Post Treatment DSA (right: pre-treatment DSA, middle: post-treatment MRA, left: post-treatment DSA).
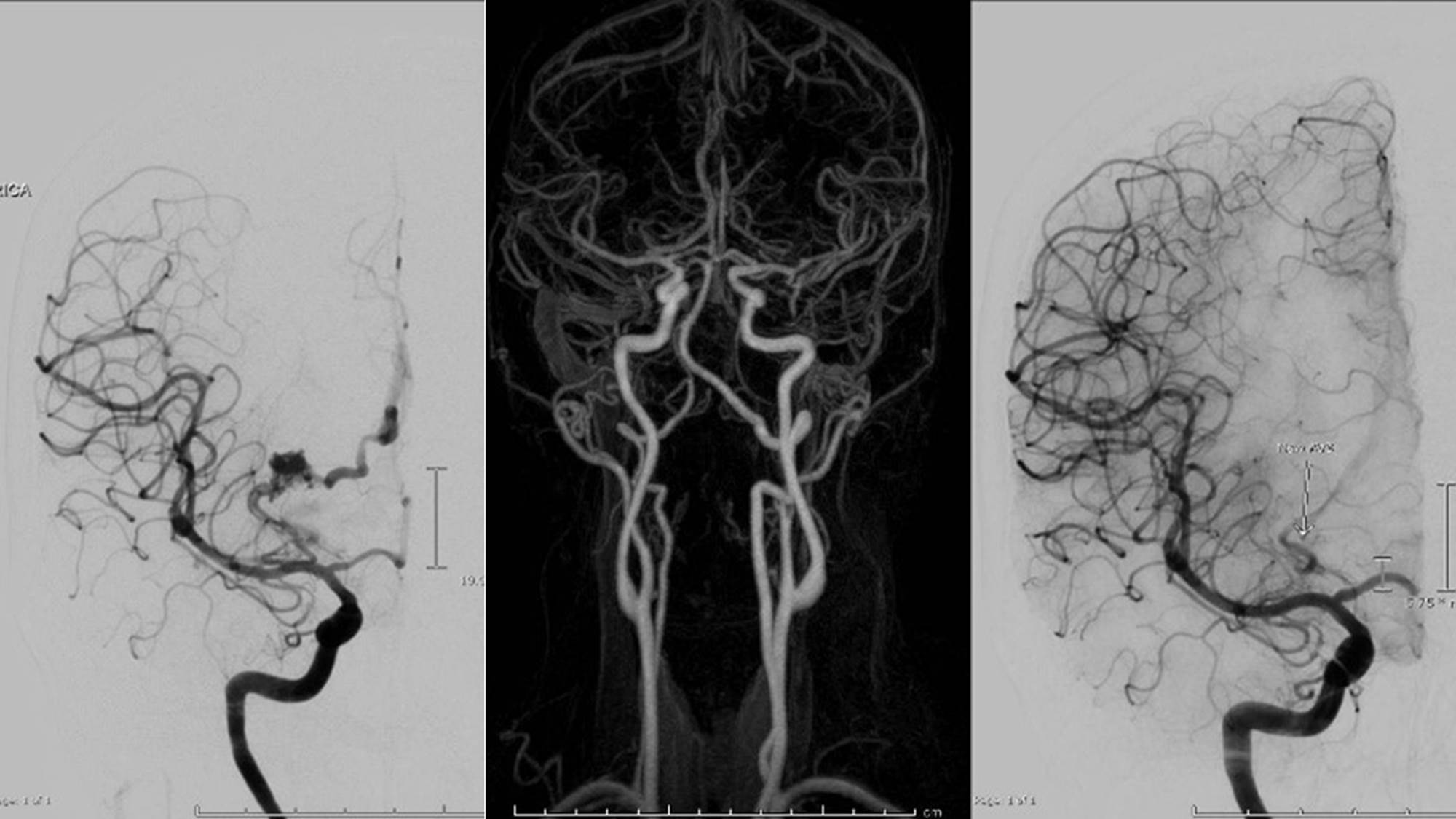
7 patients of 24 were performed with cerebral angiogram in evaluation of obliteration and one of them was with residual disease.
Discussion
This study was single-centered retrospective study of stereotactic radiosurgery treatment for brain arteriovenous malformation (BAVM), which tried to identify parametric factors for evaluation in treatment response during latent period, especially changes in vascular parameters. There are only a few studies mentioning about feeding artery diameter and number of draining veins, which were focused in this study [9,10].
Major findings of treated nidus by stereotactic radiosurgery in follow-up MR imaging in this study found that average reduction of nidus volume was 2.93 ml (95%CI [1.07, 4.81]) and decrease of maximal nidus diameter was 13.48 mm (95%CI [11.14, 15.81]). These findings correlates to other previous studies [1][8][11], which found that successful obliteration of BAVM nidus after stereotactic radiosurgery depended on multiple factors, including smaller AVM volume. Moreover, a scientific statement for management in brain arteriovenous malformation from the American Heart Association/ American Stroke Association (AHA/ASA) also indicates small to moderate volume of BAVMs, that are generally <12 ml in volume or <3 cm in maximal diameter [2].
However, the degree of reduction in volume or maximal diameter of nidus in this study is relatively too low to other studies. This could be due to baseline pretreatment characteristic of BAVM nidi, averagely 3.02 ml and 18.70 mm in maximal diameter. Another possibility is supposedly due to low number of recruited patients in this study.
In analysis between group of radiographically complete obliteration (BAVM nidus is equal to zero in either DSA or MRA) and residual disease at 36 months (3 years), we found that complete obliteration group was average 2.01 ml in volume reduction, which was lower than 8.89 ml in residual group. This finding emphasized prediction in obliteration was affected by pretreatment nidus volume, favorably for small volume. However, there was not statistically significant, due to low power of the study.
Disappearance of draining vein after treatment in this study is averagely 1.46 (95%CI [1.06, 1.85]). This finding is correlate to Pollock BE et al study [11] that low number of draining veins is a factor associated with complete obliteration. This finding is possibly described by radiation-induced endothelial injury causes gradual luminal narrowing and final nidus obliteration [12]. Possibly corresponding to high-resolution black- blood vascular wall image in this study, 2 of 4 patients in complete obliteration groups showed positive inflammatory vascular wall change and 1 of 2 patients with residual disease showed negative vascular wall change. Currently, there is no available study mentioning about benefits of vascular wall image in BAVM, except to identify site of rupture [13]. Further study of this vessel wall change might predict successful obliteration rate.
Most of patients in this study were with small volume of nidi (mean 3.02 ml with 4.65 ml in SD) and venous drainage into deep system (18, 72%), which suited to stereotactic radiosurgery treatment. 12 of 25 nidi (48%) were in eloquent region. Overall Spletzler-Martin in this study were 2 (48%) and 3 (10%), which were possible factors for BAVM obliteration as Osama et al. [8]. Nonetheless, analysis of baseline nidus characteristic was not the main purpose in this study.
There are some limitations in this study. First, this study with non-regular interval in follow-up MRI imaging is possibly due to number of cases in this hospital and recent quite new modality of treatment. As a result, low power effect of this study is acclaimed. Secondly, due to low spatial resolution of MR study, lower accuracy of vascular parameters is another limitation, such as, diameter of arterial feeder or nidus size, especially in smaller number. No inter- and intra- observer agreement was not evaluated in this study.
In conclusion, there are some vascular parameter change in follow-up MR images after treatment of brain arteriovenous malformation by stereotactic radiosurgery. However, no statistic significance in this study to predict obliteration at 36 months is possibly from low population.
References
- Brain arteriovenous malformations Lawton MT , Rutledge WC , Kim H, Stapf C, Whitehead KJ , Li DY , Krings T, terBrugge K, Kondziolka D, Morgan MK , Moon K, Spetzler RF . Nature Reviews. Disease Primers.2015;1. CrossRef
- Management of Brain Arteriovenous Malformations: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association Derdeyn CP , Zipfel GJ , Albuquerque FC , Cooke DL , Feldmann E, Sheehan JP , Torner JC . Stroke.2017;48(8). CrossRef
- The predictive value of 3D time-of-flight MR angiography in assessment of brain arteriovenous malformation obliteration after radiosurgery Buis DR , Bot JCJ , Barkhof F, Knol DL , Lagerwaard FJ , Slotman BJ , Vandertop WP , Berg R. AJNR. American journal of neuroradiology.2012;33(2). CrossRef
- The predictive value of magnetic resonance imaging in evaluating intracranial arteriovenous malformation obliteration after stereotactic radiosurgery Lee CC , Reardon MA , Ball BZ , Chen CJ , Yen CP , Xu Z, Wintermark M, Sheehan J. Journal of Neurosurgery.2015;123(1). CrossRef
- Predictive Value of MRI in Diagnosing Brain AVM Recurrence after Angiographically Documented Exclusion in Children Jhaveri A, Amirabadi A, Dirks P, Kulkarni AV , Shroff MM , Shkumat N, Krings T, Pereira VM , Rea V, Muthusami P. AJNR: American Journal of Neuroradiology.2019;40(7). CrossRef
- Accuracy of susceptibility-weighted imaging for the detection of arteriovenous shunting in vascular malformations of the brain Jagadeesan BD , Delgado Almandoz JE , Moran CJ , Benzinger TLS . Stroke.2011;42(1). CrossRef
- Application of susceptibility weighted imaging (SWI) for evaluation of draining veins of arteriovenous malformation: utility of magnitude images Miyasaka T, Taoka T, Nakagawa H, Wada T, Takayama K, Myochin K, Sakamoto M, Ochi T, Akashi T, Kichikawa K. Neuroradiology.2012;54(11). CrossRef
- Correlation of Appearance of MRI Perinidal T2 Hyperintensity Signal and Eventual Nidus Obliteration Following Photon Radiosurgery of Brain AVMs: Combined Results of LINAC and Gamma Knife Centers Abdelaziz O, Shereen A, Inoue T, Hirai H, Shima A. Journal of Neurological Surgery. Part A, Central European Neurosurgery.2019;80(3). CrossRef
- A proposed radiosurgery-based grading system for arteriovenous malformations Pollock BE , Flickinger JC . Journal of Neurosurgery.2002;96(1). CrossRef
- A modified radiosurgery-based arteriovenous malformation grading scale and its correlation with outcomes Wegner RE , Oysul K, Pollock BE , Sirin S, Kondziolka D, Niranjan A, Lunsford LD , Flickinger JC . International Journal of Radiation Oncology, Biology, Physics.2011;79(4). CrossRef
- Factors associated with successful arteriovenous malformation radiosurgery Pollock BE , Flickinger JC , Lunsford LD , Maitz A, Kondziolka D. Neurosurgery.1998;42(6). CrossRef
- Morphological observations in brain arteriovenous malformations after gamma knife radiosurgery Szeifert GT , Levivier M, Lorenzoni J, Nyáry I, Major O, Kemeny AA . Progress in Neurological Surgery.2013;27. CrossRef
- High-resolution Vessel Wall Magnetic Resonance Imaging in Intracranial Aneurysms and Brain Arteriovenous Malformations Matouk CC , Cord BJ , Yeung J, Malhotra A, Johnson MH , Minja FJ . Topics in magnetic resonance imaging: TMRI.2016;25(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2023
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times