Evaluation of Haematological Parameters and Lymphocyte Monocyte Ratio as a Prognostic Marker in Diffuse Large B-cell Lymphoma and T Cell Lymphoma Patients- An Observational Study
Download
Abstract
Background: The present study is a retrospective observational analysis of patients diagnosed with DLBCL and T cell lymphoma during the period of January 2011 to December 2015 at Malabar Cancer Centre, Thalassery. Several studies have shown that ALC and LMR can be used as prognostic indicators for diagnosis, treatment and survival in DLBCL. But there were very few studies done on the correlation of these parameters with T cell lymphoma. So we analysed the influence of haematological parameters in both DLBCL and T cell lymphomas. The primary objective of the present study was to correlate haematological parameters and LMR as predictors of DLBCL and T cell lymphomas.
Methods: We analysed total cases of 108 with DLBCL and 25 with T cell lymphomas. The study was done to determine the changes in haematological parameters including WBC count, Platelet count, Hb, HCT, lymphocyte count, monocyte count, ALC, AMC and lymphocyte monocyte ratio in diffuse large B cell lymphoma and T cell lymphomas before and after chemotherapy.
Results: The results showed a significant decrease (p˂ 0.05) in the blood platelet counts after chemotherapy in DLBCL. AMC in female patients with DLBCL were decreased (0.69 ± 0.36 to 0.53 ± 0.31; p-value = 0.023) and this was highly significant. LMR was also increased (3.47 ± 3.05 to 5.22 ± 4.34; p-value = 0.048) in female patients with DLBCL.
Conclusion: Haematological parameters can be used as a prognostic marker in predicting the extent of disease progression and the effectiveness of the treatment adopted.
Introduction
Lymphoma is the general term for cancers that develop in the lymphatic system. The Non-Hodgkin’s lymphoma (NHL) and Hodgkin’s lymphoma (HL) are the most commonly occurring hematologic malignancies [1]. NHL represents a heterogeneous group of malignancies that arise from the lymphoid system. NHL can start almost anywhere in the body either in a single lymph node, a group of lymph nodes, or in another organ and can spread to other parts of the body, including the liver, bone marrow and Spleen (non-contiguous spread). Sub-types of NHLs are broadly divided into two main groups: B-cell lymphomas - those that arises from developing B-cells, called Diffuse large B-cell lymphoma (DLBCL) and T cell lymphomas – those that arises from developing T cells [2]. DLBCL was the most common type of lymphoma and constitutes about 30% of all the cases. Peripheral T-cell lymphomas accounted for only 6% of lymphomas, reflecting their rarity [3]. DLBCL is the most common lymphoid malignancy in adults accounting for approximately 30% to 40% of all cases. It is one of the most frequent subtypes of NHL, accounting for 25% of all newly diagnosed cases worldwide [4]. It is a large B lymphoid cell neoplasm with a diffuse growth pattern composed of large B-lymphocytes with nuclear size equal to or exceeding normal macrophage nuclei or more than twice the size of normal lymphocytes. Patients typically present with a rapidly enlarging symptomatic mass, with B cell symptoms in one third of the cases [5]. Localized (stage I or II) extra nodal disease occurs in up to 30%; bone marrow involvement was seen in 16%. Common extra nodal sites include the gastrointestinal tract, bone, and CNS. The prognosis was highly associated with the international prognostic index (IPI) score. DLBCL of certain extra nodal sites, such as the CNS, may be clinically distinctive and may have specific treatment protocols [6]. Peripheral T-cell lymphomas are a heterogeneous group of neoplasms presenting as advanced disease and are characterized by widespread dissemination, aggressive behaviour, and a very poor outcome despite aggressive therapy. The prognosis is dismal, with more than half the patients dying of their disease [7].
Patients with DLBCL should be staged according to both the Ann Arbor and IPI staging systems. The Ann Arbor staging system continues to be in use in DLBCL, provides limited prognostic information, but is of use in determining therapeutic management. The IPI provides more precise and reproducible prognostic information than the Ann Arbor stage, but does not distinguish patients eligible for combined modality therapy for localized disease [8, 9]. Treatment regimens rely up on the size and location of the tumor. It also depends on the purpose of the treatment, that is, whether it is intended to cure, to shrink the tumor prior to surgery or chemotherapy or to palliate an incurable tumor. Treatment for lymphoma may involve the use of chemotherapy, corticosteroid therapy, immunotherapy, radiotherapy, Stem cell transplant etc. [10, 11]. The US Food and Drug Administration (FDA) granted approval to rituximab and hyaluronidase as a subcutaneous injection for the treatment of diffuse large B cell lymphoma (DLBCL) in adult patients. Since 2002, the treatment has changed after addition of rituximab to CHOP chemotherapy (R-CHOP) which has led to advanced improvement in overall survival (OS) [12]. As a result, the IPI needed to be re described as to whether it still maintained its predictive value. Treatment regimens for peripheral T-cell lymphoma are the same as those used for DLBCL, but with the exclusion of rituximab [13, 14]. Overall survival in PTCL was very low when compared with DLBCL and so bone marrow transplantation was more frequently carried out. Bone marrow transplantation may be as effective in PTCL as in DLBCL [15, 16].
Prognostic factors in cancer patients provide information about possible clinical outcomes and help classify patients into different risk groups [17]. It is evident that components of the Complete Blood Cell (CBC) count can provide valuable prognostic information in solid tumours and hematologic malignancies and are important tools when evaluating response to treatment [18]. Anaemia is a common morbidity encountered in cancer patients and can be due to the malignancy itself or be a direct consequence of treatment. Therefore level of Hb and RBCs can provide relevant prognostic information in case of NHL and has also been studied as a prognostic factor in malignant disorders [19]. Chemotherapy drugs and radiation therapy can affect the bone marrow and lead to a low platelet count. Due to side effect there may be the destruction of some healthy cells and results in thrombocytopenia [20, 21]. The role of the WBC count has gone beyond the assessment of infectious processes and it has become an important prognostic measurement of outcomes in cancer treatment. The absolute WBC count has been historically used as a marker of infection and inflammation. It is a widely available tool for clinicians to identify the presence of infection and monitor the patient’s response to treatment, such as antibiotics. An absolute lymphocyte count (ALC) is known to be a strong prognostic factor in DLBCL and is proven to be an independent prognostic factor for survival, independent of cancer type. Lymphopenia is an adverse prognostic factor in NHL of various subtypes, including DLBCL [22, 23]. Monocytes, which are considered immunologically relevant, are regarded as a surrogate marker of the tumor microenvironment, were also recently reported to be a prognostic factor in DLBCL. It has been postulated that monocytes promote tumor progression and support host antitumor immunity [24].
The lymphocyte-to-monocyte ratio (LMR) is the ratio calculated by dividing the absolute lymphocyte counts by the absolute monocyte counts from the blood test. Studies have indicated that the LMR at diagnosis can predict long-term outcome in patients with DLBCL [25]. LMR at diagnosis, as a simple biomarker combining an estimate of host immune homeostasis and tumor microenvironment, was recently shown to be an independent prognostic indicator in DLBCL. LMR can present the status of pro- tumour and antitumor ability in response to inflammation, and its value combining with lymphocyte and monocyte counts index may reflect the pro tumour ability and antitumor capacity of the host more concisely [26]. In addition, it is convenient and inexpensive to measure the parameter of LMR in clinical application, which makes it a fascinating marker for the prediction of DLBCL. LMR at diagnosis showed promise as a prognostic factor of survival outcomes in DLBCL patients receiving R-CHOP therapy [27, 28]. However, the present study checked the level of blood parameters like WBC, platelet count, Hb and HCT, ALC and LMR in patients with DLBCL and T cell lymphoma in pre chemotherapy and post chemotherapy period. It also assessed the effectiveness of ALC and LMR in predicting extend of disease progression and the effectiveness of the treatment adopted.
Materials and Methods
The present study is a retrospective observational analysis of patients diagnosed with B cell and T cell lymphomas during the period of January 2011 to December 2015 at Malabar Cancer Centre, Kerala, India. Since there were no ethical issues in this study we could easily cope up with the further formalities. This study was approved by the Institutional Review Board of Malabar Cancer Centre and data was abstracted from those that were maintained by the Cancer registry and Medical records division. We consecutively analysed case reports of 201 patients who were diagnosed with and treated for DLBCL and T cell lymphomas during the period of 2011 – 2015. All patients underwent a physical examination, lymph node biopsy and bone marrow aspiration and biopsy. The disease stage was assessed using enhanced computerized tomography scans from the neck to the pelvis and whole- body positron emission tomography scans. We restricted the analysis to those patients with complete laboratory data in the medical records. Among the 201 case reports, we analysed the medical records of those patients who underwent chemotherapy as a part of their treatment. Those case reports with laboratory test results of the patients prior to chemotherapy and after chemotherapy were abstracted and thus we had to exclude 68 of them. It was found that 108 and 25 records maintained complete treatment data of the patients with DLBCL and T cell lymphomas respectively. The following clinical and laboratory parameters were available at the time of diagnosis: MRD No., age, gender, WBC count, platelet count, Hb and HCT, lymphocyte count and monocyte count. The Absolute Lymphocyte count (ALC) and Absolute monocyte Count (AMC) were derived. The Lymphocyte Monocyte ratio (LMR) was calculated using the equation
LMR= ALC / AMC
Recorded the haematological parameters prior to the initiation of chemotherapy and after chemotherapy and entered in a computerized database. Patient files with missing data on monocyte or lymphocyte counts or one of the above CBC parameters before treatment were excluded.
Criteria For Analysis
Inclusion Criteria – Subjects with B cell and T cell lymphoma with complete treatment records were included in the study. Patients with incomplete medical records were excluded from the study.
Statistical Analysis
Statistical analysis was done by IBM SPSS version 23.0. Paired t test was done to assess the changes in the B cell and T cell lymphoma before and after chemotherapy. P value <0.05 should be considered as statistically significant. Data was expressed n (%), Mean± SD, 95% CI etc.
Results
A total of 201 patients were diagnosed with DLBCL and T cell NHL during the period of January 2011 to December 2015. Among them 68 were excluded based on exclusion criteria. 36 of them dropped out treatment; 22 did not have complete treatment records; 2 died due to disease progression during treatment and 8 of them were sent to palliative centres. 108 cases with DLBCL and 25 with T cell lymphoma were enrolled. The changes of ALC, AMC and LMR and other haematological parameters before and after treatment were analysed in particular for DLBCL and T cell NHL. Then a comparison of all those parameters was made between the two NHLs to find out for significant changes.
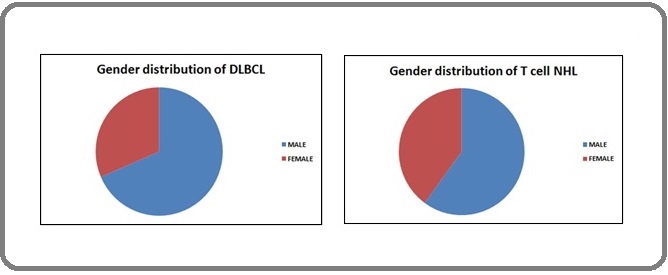
Gender distribution of DLBCL and T cell lymphoma
The DLBCL patient’s ages ranged from 10 to 77 years; the mean age was 55.12 years. Of these 68.51% were males and 31.48% were females and 5 patients were aged less than 30 years. The age of patients with T cell lymphoma ranged from 14 to 79 years and the mean age was 50.52 years. Of these 60% were males and 40% were females. 3 patients were aged less than 30 years (Figure 1).
Figure 1. Gender Distribution of DLBCL and T Cell NHL in Percentage.
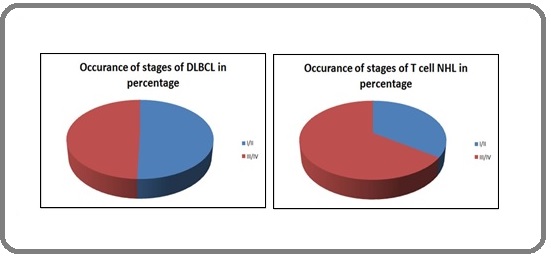
Occurrence of stages and Percentage of incidence of stage subtypes in DLBCL
In DLBCL cases, 50.5% were diagnosed in the early stages and 49.5% in the advanced stages of lymphoma (Figure 2).
Figure 2. Percentage Incidence of Stage in DLBCL and T Cell Lymphoma.
Out of the early diagnosed cases, 64.2% of them come under sub-type A and 31.5% under sub-type B. In the advanced stages, it was found that 35.8% was A and 68.5% was B sub-types (Table 1).
| DLBCL | T Cell Lymphoma | |||||
| Stage | I/II | III/IV | I/II | III/IV | ||
| A | 64.2 | 35.8 | 25 | 20 | ||
| B | 31.5 | 68.5 | 75 | 80 |
Occurrence of stages and Percentage of incidence of stage subtypes in T cell lymphoma
In case of T cell lymphoma, 34.8%NHL were diagnosed in the early stages and 65.2% in the advanced stages. Out of the early diagnosed cases, 25% of them come under sub-type A and 75% under sub-type B. In advanced stages, 20% are of A and 80% of B sub-types were observed. It is notable that large number of cases of T cell NHL is diagnosed only in the later stages (Table 1).
Effect of chemotherapy in haematological parameters in patients with DLBCL
In DLBCL, there were variations of haematological parameters before and after chemotherapy treatment. The WBC count before treatment was 8.75 ± 3.90 and after was 8.87 ± 8.52. The level of Hb before treatment was 12.03 ± 2.04 and after was 12.23 ± 2.15. The haematocrit value increased from 35.98 ± 6.19 to 37 ± 8.48. Since their p values were not ˂0.05, the result was not statistically significant. The platelet count decreased from 304.37 ± 111.21 to 263.56 ± 105.68 and this was a remarkable variation. This result was highly significant as the p values were ˂0.05. The values of lymphocyte count and ALC increased from 25.25 ± 13.21 and 2.21 ± 2.59 to 27.51 ± 14.91 and 2.64 ± 4.02 respectively. Monocyte count and AMC decreased from 8.61 ± 3.99 and 0.72 ± 0.37 to 7.79 ± 3.88 and 0.66 ± 0.65 respectively. There was an increase of lymphocyte monocyte ratio from 3.66 ± 3.70 to 4.23 ± 3.73 after treatment. These variations were insignificant (P ˃ 0.05) (Table 2).
| Parameters | Chemotherapy | 95% CI | P value | |
| Before | After | |||
| WBC count (cmm) | 8.75±3.90 | 8.87±8.52 | (-1.88, 1.64) | 0.893 |
| Platelet Count (cmm) | 304.37±111.21 | 263.56±105.68 | (18.59, 63.03) | 0* |
| Haemoglobin (g/dl) | 12.03±2.04 | 12.23±2.15 | (-0.59, 0.19) | 0.315 |
| Haemetocrit value | 35.98±6.19 | 37.00±8.48 | (-2.80, 0.75) | 0.256 |
| Lymphocyte (%) | 25.25±13.21 | 27.51±14.91 | (-5.43, 0.92) | 0.162 |
| Monocyte (%) | 8.61±3.99 | 7.79±3.88 | (-0.19, 1.82) | 0.113 |
| ALC | 2.21±2.59 | 2.64±4.02 | (-1.17, 0.31) | 0.258 |
| AMC | 0.72±0.37 | 0.66±0.65 | (-0.08, 0.18) | 0.437 |
| LMR | 3.66±3.70 | 4.23±3.73 | (-1.28, 0.14) | 0.117 |
Values are mean ± SD. Significance was calculated by paired t test. * P < 0.05 considered as significant
Statistical analysis of patients with DLBCL based on gender perspective also showed variations of parameters before and after treatment. A significant variation was observed in the platelet count of male and female patients, with P value of 0.005 and 0.039 respectively. A decrease in AMC from 0.69 ± 0.36 to 0.53 ± 0.31 was observed in female patients. Since the P value was 0.023, this was highly significant. LMR in female patients increased from 3.47 ± 3.05 to 5.22 ± 4.34 with a P value of 0.048, which is statistically significant.
Effect of chemotherapy in haematological parameters in patients with T cell lymphoma
In T cell NHL, there were variations of haematological parameters before and after chemotherapy treatment. The WBC count before treatment was 7.39± 4.32 and after was 7.75 ± 3.62. Level of Hb before treatment was 11.19 ± 2.11 and after was 11.27 ± 1.87. The haematocrit value increased from 33.44 ± 6.23 to 33.69 ± 6.02. The result was not statistically significant as their p values were not ˂0.05. The platelet count decreased from 274.08 ± 129.04 to 249.6 ± 182.29. The values of lymphocyte count and ALC decreased from 21.4 ± 10.99 and 1.43 ± 0.84 to 19.45 ± 9.80 and 1.37 ± 0.78 respectively. Monocyte count and absolute monocyte count decreased from 9.2 ± 5.88 and 0.67 ± 0.61 to 7.62 ± 4.54 and 0.59 ± 0.44 respectively. There was a decrease of lymphocyte monocyte ratio from 3.51 ± 3.45 to 3.05 ± 1.50 after treatment. Since their p values were not ˂0.05, variations were statistically in significant (Table 3).
| Parameters | Chemotherapy | 95% CI | P value | |
| Before | After | |||
| WBC count (cmm) | 7.39±4.32 | 7.75±3.62 | (-1.57, 0.85) | 0.549 |
| Platelet Count (cmm) | 274.08 ± 129.04 | 249.6 ± 182.29 | (-62.34, 111.30) | 0.566 |
| Haemoglobin (g/dl) | 11.19±2.11 | 11.27±1.87 | (-0.74, 0.58) | 0.805 |
| Haemetocrit value | 33.44±6.23 | 33.69±6.02 | (-2.28, 1.77) | 0.797 |
| Lymphocyte (%) | 21.40±10.99 | 19.45±9.80 | (-3.75, 7.65) | 0.488 |
| Monocyte (%) | 9.20±5.88 | 7.62±4.54 | (-0.76, 3.92) | 0.177 |
| ALC | 1.43±0.84 | 1.37±0.78 | (-0.32, 0.45) | 0.743 |
| AMC | 0.67±0.61 | 0.59±0.44 | (-0.14, 0.31) | 0.448 |
| LMR | 3.51±3.45 | 3.05±1.50 | (-0.82, 1.74) | 0.467 |
Values are mean ± SD. Significance was calculated by paired t test. * P < 0.05 considered as significant
Discussion
DLBCL, one of the most frequent subtypes of NHL, is an aggressive lymphoma, with a median survival of less than one year if left untreated, but is potentially curable. T-cell lymphomas are a heterogeneous group of neoplasms, accounting about 7-10% of NHL and presenting as an advanced disease with widespread dissemination, aggressive behaviour, and a very poor prognosis and outcome, with more than half the patients dying of their disease [29,16]. Haematologists along with the clinicians can provide an insight in the prompt and accurate diagnosis in NHL patients and proper treatment can be provided immediately. Numerous prognostic markers have been proposed for patients with DLBCL and T cell lymphoma. However, these can be expensive to test and are not always applicable in daily practice. Therefore, inexpensive and readily available parameters are more practical in a clinical context.
Several studies have explored the prognostic significance of LMR in a variety of solid cancers including DLBCL [30,31, 32]. But studies regarding the role of LMR and other haematological parameters as an integrated biomarker in the prognosis of T cell NHL are limited. Many of them had correlated the role of LMR as a prognostic indicator of DLBCL [33, 34]. But certain others did not reveal any remarkable variations in haematological parameters and LMR in both the lymphomas and those results were controversial. So we checked for the importance of variations in parameters and its role as prognostic markers in both lymphomas. We analysed various haematological parameters and determined ALC, AMC and LMR of the patients diagnosed with DLBCL and T cell lymphomas before and after chemotherapy.
In the present study, it was found that there was a variation in haematological parameters and LMR before and after chemotherapy in both the lymphomas. The frequency of anaemia in the patients was 60%. The criterion for anaemia in Bloomfield’s study was haemoglobin level below 12 g/dl for females and below 14 g/dl for males. Rosenberg et al. & Stein et al. have reported that results of hematologic studies are infrequently abnormal in patients with NHL at presentation [35, 36]. However, Bloomfield et al. reported that 57% of patients with NHL have some abnormality in haemoglobin level, leukocyte count, or platelet count at the time of diagnosis [37, 38]. Chen et al evaluated the correlation of the peripheral platelet count at disease onset with the therapeutic outcomes of patients with DLBCL treated with the R CHOP like regimen [20]. In our study we observed a significant decrease (P ˂ 0.05) of platelet count after chemotherapy in male and female patients with DLBCL. We also determined a significant decrease in AMC in female patients with DLBCL with a p value = 0.023. There was a marked increase in LMR after chemotherapy in female patients with DLBCL and this was highly significant (P value=0.048). Studies have indicated that LMR was an effective prognostic factor in patients with DLBCL treated with R-CHOP. Tadmor et al. assessed the prognostic significance of AMC in patients with DLBCL [39].
We also observed variations in haematological parameters and LMR before and after chemotherapy in patients with T cell lymphoma. Since their p values were not less than 0.05, those results were statistically insignificant.
The present retrospective study that was conducted had influence from various aspects. It depended upon the diversity and nature of patient populations and the factors such as their age group, varying lifestyles, and the unequal percentage of patient population that was considered in both the lymphomas and the various treatment regimens that were adopted during each period. Factors like whether they had undergone previous treatment for the same or for some other diseases might also hold some idiopathic role in the variations of their blood CBC parameters.
Our study had some limitations in finding a statistically significant result in the case of T cell lymphoma. It might be due to the scarcity in the number of patients who were treated during the period of 2011-2015 with T cell NHL and the unavailability of their overall treatment data during the entire period and their increased mortality rate. This may be one of the reasons why T cell NHL still remains as a less explored area of research. In this context, we realized the necessity and importance of further detailed prospective studies in future to explore the correlation of various haematological parameters as a prognostic factor in T cell lymphomas and its comparison with DLBCL.
The present study concludes in the requirement of further observational findings on a substantial number of people to reveal the importance of variations in LMR and haematological parameters as a useful prognostic biomarker in the early diagnosis and treatment in the upcoming era.
Acknowledgements
Authors are thankful to Dr. Sangeetha K Nayanar, HOD, Department of clinical laboratory services and translational medicine, Malabar Cancer Centre who provided valuable suggestions throughout the research.
This research work did not receive any specific research grant from funding agencies in the public commercial or not-for-profit sectors.
References
- Epidemiology of Non-Hodgkin's Lymphoma in India Nair R, Arora N, Mallath MK . Oncology.2016;91 Suppl 1. CrossRef
- [Reproducibility and prognostic value of histopathological classifications of malignant lymphomas. Prolegomena for the 1st international classification proposed by WHO. Group of the non-Hodgkin's Malignant Lymphoma Classification Project] Diebold J., Weisenburger D., MacLennan K. A., Müller-Hermelink H. K., Nathwani B. N., Harris N. L., Anderson J. R., Roy P., Armitage J. O.. Bulletin De l'Academie Nationale De Medecine.1998;182(7).
- Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011 Al-Hamadani M, Habermann TM , Cerhan JR , Macon WR , Maurer MJ , Go RS . American Journal of Hematology.2015;90(9). CrossRef
- World cancer report. World Health Organization. Lyon: IARC Press Stewart BW , Kleihues P. France .2003;:pp 237-41.
- New developments in the management of diffuse large B-cell lymphoma Habermann TM . Hematology (Amsterdam, Netherlands).2012;17 Suppl 1. CrossRef
- New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project Armitage J. O., Weisenburger D. D.. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.1998;16(8). CrossRef
- Peripheral T-cell lymphomas. Clinico-pathologic study of 168 cases diagnosed according to the R.E.A.L. Classification Ascani S., Zinzani P. L., Gherlinzoni F., Sabattini E., Briskomatis A., Vivo A., Piccioli M., Fraternali Orcioni G., Pieri F., Goldoni A., Piccaluga P. P., Zallocco D., Burnelli R., Leoncini L., Falini B., Tura S., Pileri S. A.. Annals of Oncology: Official Journal of the European Society for Medical Oncology.1997;8(6). CrossRef
- Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification Cheson BD , Fisher RI , Barrington SF , Cavalli F, Schwartz LH , Zucca E, Lister TA . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2014;32(27). CrossRef
- The lymphocyte to monocyte ratio improves the IPI-risk definition of diffuse large B-cell lymphoma when rituximab is added to chemotherapy Rambaldi A, Boschini C, Gritti G, Delaini F, Oldani E, Rossi A, Barbui AM , Caracciolo D, Ladetto M, Gueli A, De Crescenzo A, Passera R, Devizzi L, Patti C, Gianni AM , Tarella C. American Journal of Hematology.2013;88(12). CrossRef
- Individualized treatment of diffuse large B cell lymphoma Thanarajasingam G, Bennani-Baiti N, Nowakowski GS . Oncology & Hematology Review.2015;11(2):113-114. CrossRef
- Comparison of high-dose therapy and autologous bone marrow transplantation for T-cell and B-cell non-Hodgkin's lymphomas Vose J. M., Peterson C., Bierman P. J., Weisenburger D. D., Linder J., Harrington D., Vaughan W. P., Kessinger A., Armitage J. O.. Blood.1990;76(2).
- Lymphopenia assessed during routine follow-up after immunochemotherapy (R-CHOP) is a risk factor for predicting relapse in patients with diffuse large B-cell lymphoma Porrata L. F., Rsitow K., Inwards D. J., Ansell S. M., Micallef I. N., Johnston P. B., Habermann T. M., Witzig T. E., Colgan J. P., Nowakowski G. S., Thompson C. A., Markovic S. N.. Leukemia.2010;24(7). CrossRef
- T-cell lymphomas: immunologic, histologic, clinical, and therapeutic analysis of 63 cases Coiffier B., Berger F., Bryon P. A., Magaud J. P.. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.1988;6(10). CrossRef
- Peripheral T-cell lymphomas: initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the R.E.A.L. Classification López-Guillermo A., Cid J., Salar A., López A., Montalbán C., Castrillo J. M., González M., Ribera J. M., Brunet S., García-Conde J., Fernández de Sevilla A., Bosch F., Montserrat E.. Annals of Oncology: Official Journal of the European Society for Medical Oncology.1998;9(8). CrossRef
- Impact of high-dose chemotherapy on peripheral T-cell lymphomas Rodriguez J., Munsell M., Yazji S., Hagemeister F. B., Younes A., Andersson B., Giralt S., Gajewski J., Lima M., Couriel D., Romaguera J., Cabanillas F. F., Champlin R. E., Khouri I. F.. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2001;19(17). CrossRef
- Pathogenesis of non-Hodgkin's lymphoma Nogai H, Dörken B, Lenz G. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2011;29(14). CrossRef
- Prognostic significance of absolute lymphocyte count at diagnosis of diffuse large B-cell lymphoma: a meta-analysis Feng J, Wang Z, Guo X, Chen Y, Cheng Y, Tang Y. International Journal of Hematology.2012;95(2). CrossRef
- The Role of Complete Blood Cell Count in Prognosis- Watch this space! Rochet NM , Markovic SN , Porrata LF . Oncology & Hematology Review.2012;08(1):76-82. CrossRef
- Evaluation of Haematological Changes Associated to Non- Hodgkin Lymphoma in Subjects in Enugu State, South East, Nigeria Obeagu E, Obeagu G, Amilo G. Archives of Blood Transfusion & Disorders.2017;1.
- Prognostic value of platelet count in diffuse large B-cell lymphoma Chen L, Lin S, Yu M. Clinical Lymphoma, Myeloma & Leukemia.2012;12(1). CrossRef
- Platelets and cancer: a casual or causal relationship: revisited Menter DG , Tucker SC , Kopetz S, Sood AK , Crissman JD , Honn KV . Cancer Metastasis Reviews.2014;33(1). CrossRef
- Absolute lymphocyte counts predicts response to chemotherapy and survival in diffuse large B-cell lymphoma Kim D. H., Baek J. H., Chae Y. S., Kim Y.-K., Kim H. J., Park Y. H., Song H. S., Chung J. S., Hyun M. S., Sohn S. K.. Leukemia.2007;21(10). CrossRef
- Influence of low absolute lymphocyte count of patients with nongerminal center type diffuse large B-cell lymphoma with R-CHOP therapy Song M.-K., Chung J.-S., Seol Y.-M., Kim S.-G., Shin H.-J., Choi Y.-J., Cho G.-J., Shin D.-H.. Annals of Oncology: Official Journal of the European Society for Medical Oncology.2010;21(1). CrossRef
- Monocyte count at diagnosis is a prognostic parameter in diffuse large B-cell lymphoma: results from a large multicenter study involving 1191 patients in the pre- and post-rituximab era Tadmor T, Bari A, Sacchi S, Marcheselli L, Liardo EV , Avivi I, Benyamini N, Attias D, Pozzi S, Cox MC , Baldini L, Brugiatelli M, Federico M, Polliack A. Haematologica.2014;99(1). CrossRef
- The absolute monocyte and lymphocyte prognostic score predicts survival and identifies high-risk patients in diffuse large-B-cell lymphoma Wilcox R. A., Ristow K., Habermann T. M., Inwards D. J., Micallef I. N. M., Johnston P. B., Colgan J. P., Nowakowski G. S., Ansell S. M., Witzig T. E., Markovic S. N., Porrata L.. Leukemia.2011;25(9). CrossRef
- Clinical significance of hematologic parameters in non-Hodgkin's lymphoma at diagnosis Conlan M. G., Armitage J. O., Bast M., Weisenburger D. D.. Cancer.1991;67(5). CrossRef
- Management Strategies for Elderly Patients with Diffuse Large B-Cell Lymphoma Nastoupil LJ , Sinha R, Flowers CR . European Oncology & Haematology.2012;8(2). CrossRef
- Monocytosis has adverse prognostic significance and impacts survival in patients with T-cell lymphomas Bari A, Tadmor T, Sacchi S, Marcheselli L, Liardo EV , Pozzi S, Luminari S, Baldini L, Marmiroli S, Federico M, Polliack A. Leukemia Research.2013;37(6). CrossRef
- Comparison of prognostic impact of absolute lymphocyte count, absolute monocyte count, absolute lymphocyte count/absolute monocyte count prognostic score and ratio in patients with diffuse large B cell lymphoma Markovic O, Popovic L, Marisavljevic D, Jovanovic D, Filipovic B, Stanisavljevic D, Matovina-Brko G, Hajder J, Matkovic T, Živkovic R, Stanisavljevic N, Todorović M, Petrovic D, Mihaljevic B. European Journal of Internal Medicine.2014;25(3). CrossRef
- Prognostic performance of lymphocyte-to-monocyte ratio in diffuse large B-cell lymphoma: an updated meta-analysis of eleven reports Sun H, Pan Y, He B, Nie Z, Lin K, Peng H, Cho WC , Wang S. OncoTargets and therapy.2016;9. CrossRef
- Diffuse large B-cell lymphoma Martelli M, Ferreri AJM , Agostinelli C, Di Rocco A, Pfreundschuh M, Pileri SA . Critical Reviews in Oncology/Hematology.2013;87(2). CrossRef
- Histopathology and immunohistochemistry of peripheral T cell lymphomas: a proposal for their classification Suchi T., Lennert K., Tu L. Y., Kikuchi M., Sato E., Stansfeld A. G., Feller A. C.. Journal of Clinical Pathology.1987;40(9). CrossRef
- Peripheral blood lymphocyte/monocyte ratio predicts outcome for patients with diffuse large B cell lymphoma after standard first-line regimens Li Y, Pan Y, Jiao Y, Ning J, Fan Y, Zhai Z. Annals of Hematology.2014;93(4). CrossRef
- Peripheral blood absolute lymphocyte/monocyte ratio as a useful prognostic factor in diffuse large B-cell lymphoma in the rituximab era Watanabe R, Tomita N, Itabashi M, Ishibashi D, Yamamoto E, Koyama S, Miyashita K, Takahashi H, Nakajima Y, Hattori Y, Motohashi K, Takasaki H, Ohshima R, Hashimoto C, Yamazaki E, Fujimaki K, Sakai R, Fujisawa S, Motomura S, Ishigatsubo Y. European Journal of Haematology.2014;92(3). CrossRef
- Lymphosarcoma: a review of 1269 cases Rosenberg S. A., Diamond H. D., Jaslowitz B., Craver L. F.. Medicine.1961;40. CrossRef
- Bone marrow involvement in non-Hodgkin's lymphoma: implications for staging and therapy Stein R. S., Ultmann J. E., Byrne G. E., Moran E. M., Golomb H. M., Oetzel N.. Cancer.1976;37(2). CrossRef
- Significance of Haematological Parameters in the Non-Hodgkin's Malignant Lymphomas Bloomfield C. D., Mckenna R. W., Brunning R. D.. British Journal of Haematology.1976;32(1). CrossRef
- Improving outcomes for patients with diffuse large B-cell lymphoma Flowers cr , Sinha R, Vose jm . CA: a cancer journal for clinicians.2010;60(6). CrossRef
- Absolute monocytosis at diagnosis correlates with survival in diffuse large B-cell lymphoma-possible link with monocytic myeloid-derived suppressor cells Tadmor T, Fell R, Polliack A, Attias D. Hematological Oncology.2013;31(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2022
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times