Survival Outcomes of Patients with Locally Advanced Breast Cancer after Neoadjuvant Systemic Therapy in St. Luke’s Medical Center
Download
Abstract
Background: In locally-advanced breast cancers (LABC), the previous primary goal of neoadjuvant systemic therapy was to improve resectability and achieve better margins. Currently, it can also provide important prognostic information based on actual response to therapy. Achieving pathologic complete response (pCR) after neoadjuvant therapy is targeted because it is associated with favorable disease-free survival (DFS) and overall survival (OS). The purpose of this study is to report the 5-year disease-free survival rate and overall survival rate among patients with LABC treated with neoadjuvant treatment followed by surgery.
Methods: All patients with histologically diagnosed locally advanced breast cancer, who completed neoadjuvant therapy and surgery at St. Luke’s Medical Center from January 2007 to December 2017 where included. Response to neoadjuvant therapy were categorized using post-surgical pathologic reports. Survival curves were generated by the Kaplan-Meier method and compared using the log-rank test.
Results: The cohort included 231 women, most were diagnosed with stage III disease (n=151). Pathologic complete response to neoadjuvant treatment had a significantly greater 5-year disease-free survival at 73.17% compared to those with residual disease after neoadjuvant treatment at 51.25% (Log-rank test p=0.0107). The locoregional recurrence-free survival and distant metastasis-free survival were 83.20% and 62.0% at 5 years, respectively. No deaths were recorded at 5 years for patients who achieved pathologic complete response after neoadjuvant chemotherapy.
Conclusion: Pathologic complete response has better outcomes in terms of disease-free survival compared to those who did not achieve complete pathologic response after neoadjuvant therapy. Disease-free survival among patients who achieved pathologic complete response does not significantly differ among the different molecular phenotypes. The overall survival of the patients in this cohort does not differ in terms of phenotypic subtypes and mortality is seen among patients with disease progression.
Introduction
Background of the Study
Patients with early breast cancer are typically subjected to definitive surgery first (mastectomy or lumpectomy) followed by adjuvant systemic therapy in the form of endocrine therapy, cytotoxic chemotherapy and anti-HER2 therapy, depending on certain tumor and patient factors. Radiation therapy is also an important adjuvant treatment for early breast cancer with tumors more than 5 centimeters or those with node-positive disease to complete the curative treatment.
Some patients, however, present with large and fixed tumors or with heavy axillary nodal disease such that upfront surgery is not possible or the chances of a positive margin is very likely. Thus, preoperative or neoadjuvant systemic therapy is given, typically in intravenous form, to patients who have locally advanced cancer to render unresectable tumors operable.
These patients are considered to have locally advanced breast cancer (LABC), referring to a heterogeneous group of breast cancers without evidence of distant metastasis (M0) and represent only 2% to 5% of all breast cancers in first world countries like the United States, however, based on a 14-year study of 4,260 patients from the Hospital Tumor Registry of SLMC-QC by the Breast Cancer Working group, the incidence may be as high as 20% with the rest being early stage (45%), stage 4 (25%) and pure DCIS or stage 0 (6%) [1,2].
A recent descriptive study entitled “Neoadjuvant Systemic Therapy for Locally Advanced Breast Cancer in St. Luke’s Medical Center: 10-year local experience and response rates”, included 259 patients who underwent neoadjuvant systemic therapy followed by definitive surgery and described the preoperative treatment given and postoperative results. Our aim is to follow up these patients and determine long-term outcomes of their previous treatment [3].
Statement of the Problem
According to international guidelines from the National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology (ASCO), and European Society for Medical Oncology (ESMO), preoperative systemic therapy is indicated in women with locally advanced or inoperable breast cancer particularly those with inflammatory breast cancer, those with N2 and N3 regional lymph node disease; and T3 and T4 tumors. More importantly, one of the goals of neoadjuvant systemic therapy for triple negative, HER2 Amplified, and Luminal B type breast cancers is to provide early and immediate systemic control with the main goal of achieving pathologic complete response (pCR), a treatment outcome associated with a more favorable disease free and overall survival [4].
Significance of the Study
The primary goal of neoadjuvant systemic therapy used to be just to improve resectability and achieve better margins. Currently, neoadjuvant treatment can provide important prognostic information based on actual response to therapy. Studies have shown that for patients with inflammatory breast and locally advanced breast cancer, it is now of prime importance to achieve pCR after neoadjuvant therapy because it is associated with favorable disease-free survival (DFS) and overall survival (OS) [4]. Patients who experience pCR have better long- term outcomes with lower risk of cancer recurrence than women with residual cancer following neoadjuvant chemotherapy [1].
A recent meta-analysis which identified 49 eligible studies involving 18, 772 patients on neoadjuvant therapy trials show that the response rates and clinical outcome (DFS and OS) are very much dependent on the molecular subtype of the patient, i.e., luminal, HER2 positive, triple negative. The rate of Pathologic complete response was 21.5% and among patients who achieved pathologic complete response an odds ratio of 0.33 (CI 0.28-0.39; p<0.001) for recurrence and 0.28 for mortality (CI 0.21- 0.36; p <0.001) was observed. The following pathologic complete response rates were found for the three breast cancer subtypes: Hormone Receptor-Positive disease, 8.3%; HER2- Positive disease, 70.3%; and triple-negative disease, 38.7%. It concluded that pathologic complete response was associated with significantly reduced disease recurrence and mortality; and can be used as a prognostic marker, which seem to be most useful among the triple negative subtypes and the ER negative/PR negative, HER2 positive (clinically HER2 enriched) subtypes and are less useful in predicting outcome for the luminal tumors (ER and PR positive) [5].
The results of this study will show our local experience in the real world, survival outcomes of patients who previously underwent neoadjuvant treatment and definitive surgery and describe factors which may have affected said outcomes.
Objectives of the Study
a. General Objective
Determine the 5-year disease-free survival (DFS) rate among patients with locally-advanced breast cancer after neoadjuvant systemic therapy.
b. Specific Objectives
i. Determine the 5-year DFS rate among patients with LABC after neoadjuvant systemic therapy who achieved pCR stratified according to phenotype
ii. Determine the 5-year DFS rate among patients with LABC after neoadjuvant systemic therapy who did not achieve pCR stratified according to phenotype
iii. Determine the 5-year DFS rate among patients with LABC who received neoadjuvant systemic therapy who achieved pCR from those who did not achieve pCR stratified according to phenotype
iv. Determine the locoregional recurrence-free survival rate among patients with LABC after neoadjuvant systemic therapy
v. Determine the distant metastasis-free survival rate among patients with LABC after neoadjuvant systemic therapy
vi. Determine the overall survival (OS) rate of patients with LABC who received neoadjuvant systemic therapy stratified according to pathologic response and phenotype
Scope and Limitation
Patients included in the study were those aged 18 years old and above, histopathologically diagnosed with locally advanced breast cancer who completed neoadjuvant therapy and underwent surgical management at St. Luke’s Medical Center from January 2007 to December 2017.
Likewise, patients with metastatic disease upon diagnosis were excluded from the study
Definition of Terms
● Age at diagnosis – age at histopathologic diagnosis
● Concurrent – different treatment molecules given at the same time
● Disease free survival – time interval from treatment initiation and first documented relapse (local, regional, or metastatic), death, or last follow-up
● Histologic Subtype – classification of breast cancer patients based on the morphologic appearance of the cancer as seen by light microscopy [1].
o Invasive Ductal – infiltrative proliferation of malignant-appearing mammary ductal epithelial cells
o Invasive Lobular - infiltrative proliferation of generally small and often loosely cohesive cells originating in the terminal duct-lobular unit, with or without pagetoid involvement of terminal ducts
o Others – all histologic subtypes not classified as Invasive ductal, Invasive lobular, mixed or inflammatory
● Inflammatory - is a clinical diagnosis that requires erythema and derma edema (peau d’ orange) of a third or more of the skin of the breast.1
● Ki67 – cellular marker for proliferation [2].
a. Low Ki67 – defined as Ki67 ≤ 10%
B. High Ki67 – defined as Ki67 ≥ 25%
● Locally advanced breast cancer – refers to a heterogeneous group of breast cancers without evidence of distant metastasis, this includes (1) operable disease at presentation (clinical stage T3N1), (2) inoperable disease at presentation (clinical stage T4 and/or N2-3) and (3) Inflammatory Breast Cancer (IBC) (Clinical stage T4dN0-3) [1].
● Lymphovascular Invasion (LVI) – invasion of cancer cells into the blood vessels of lymphatic system
● Neoadjuvant Systemic Therapy – refers to any systemic treatment prior to surgery in the form of either cytotoxic chemotherapy, anti-estrogen therapy and/or anti-Her2 targeted therapy
● Overall Survival -time elapsed from treatment initiation until death
● 5-year disease free survival – percentage of patients without radiologically confirmed disease from treatment initiation until 5 years after
● Locoregional recurrence – reappearance of cancer in the ipsilateral chest wall
● Distant recurrence – reappearance of cancer in other organs other than the ipsilateral chest wall
● Pathologic Complete Response (pCR) – defined as absence of invasive residual disease in breast or nodes; non-invasive breast residuals allowed or ypT0/is ypN0; used by MD Anderson Cancer center, Austrian Breast and Colorectal Study Group and Colorectal Cancer Study group and Neo-Breast International Group.
● Phenotypic Subtype – classification of breast cancer patients based on IHC and/or staining of molecular markers namely Estrogen Receptor (ER), Progesterone Receptor (PR), Her2Neu and Ki67. In patients with no available Ki67, the tumor grade was used to capture cell proliferation.
o Luminal A – patients with ER positive and/or PR positive, Her2 negative, Ki67<30%
o Luminal B - patients with ER positive and/or PR positive, Her2 negative, Ki67>30%
o Luminal B, Her2 Overexpressed – patients with ER positive and/or PR positive, Her2 positive
o Her2 Overexpressed/Enriched - ER negative, PR negative, Her2 positive o Triple negative – ER negative, PR negative, Her2 negative
● Postmenopausal – after permanent cessation of menses; prior bilateral oophorectomy, age ≥ 60, Age <60 and amenorrheic for 12 or more months in the absence of chemotherapy, tamoxifen, toremifene, or ovarian suppression and follicle-stimulating hormone (FSH)and estradiol in the postmenopausal range; if taking tamoxifen or toremifene, and age <60 y, then FSH and plasma estradiol in the postmenopausal ranges [4].
● Premenopausal - before permanent cessation of menses; without prior bilateral oophorectomy [4].
● Sequential - Treatment molecules given following a logical order
● Surgical Treatment – refers to surgery in the form of breast conservation surgery (i.e. lumpectomy) or modified radical mastectomy with axillary surgical evaluation
● Target lesions – measurable lesion defined by RECIST 1.1 Criteria (appendix A).
● Tumor infiltrating lymphocytes – presence of infiltrating lymphocytes within or around the tumor and an important biomarker linked with clinical outcome [6].
● Tumor necrosis – Death of cancer cells within the tissue sample suggestive of aggressiveness in which the tumor outgrows its blood supply Recurrence free interval - time interval from treatment initiation and first documented local or regional relapse.
Review of Related Literature
Breast Cancer is a major public health problem for women throughout the world. In the United States, breast cancer remains the most common malignancy in women and the second most frequent cause of cancer death [4].
Multiple factors are associated with an increased risk of developing breast cancer, but the majority of these factors convey small to moderate increase in risk for any individual woman. It has been estimated that approximately 50% of women who develop breast cancer have no identifiable risk factor beyond increasing age and female gender [1].
The evaluation of the patient newly diagnosed with breast cancer begins with a determination of operability. Patient known to have metastatic disease and those with locally advanced breast cancer are not candidates for surgery as the first therapeutic approach and should be treated with neoadjuvant therapy [4].
The term LABC encompasses patients with (1) operable disease at presentation (clinical stage T3N1),
(2) inoperable disease at presentation (clinical stage T4 and/or N2-3) and (3) Inflammatory Breast Cancer (IBC) (Clinical stage T4dN0-3). These groups of patients carry a substantial risk for metastasis, and as such, should be evaluated by a multidisciplinary team. Treatment includes neoadjuvant chemotherapy, surgery and radiation therapy. Long-term survival was improved with the use of neoadjuvant therapy as part of a trimodal treatment [1].
In the Philippines, the identified risk factors for breast cancer are being overweight, having no children at the age of 30, having a family history of breast cancer, drinking excessive alcohol, and having early menstruation and later menopause, among others [6].
According to the Philippine Cancer Society and DOH data as well as the Philippine Society of Medical Oncology, breast cancer is so common in the Philippines that one in every 13 Filipinas is expected to develop it in her lifetime. Moreover, the Philippines has been identified as among the having the highest incidence rate of breast cancer in Asia [6].
There is limited published local data describing the response rates of breast cancer patients to neoadjuvant treatment. The response rates to neoadjuvant systemic therapies are affected by and dependent on multiple factors of a patient’s clinical profile at presentation:
● Patient factors: age, menopausal status, tumor size, nodal involvement, performance score, presence of co- morbidities, educational attainment and financial status;
● Tumor factors: specific histology, the histologic and nuclear grade, the presence or absence of Estrogen Receptor (ER) and Progesterone Receptor (PR) and HER2/neu overexpression, proliferative markers like ki67 and the specific clinical molecular subtype for which the tumor is classified and other tumor factors like presence of Tumor Infiltrating Lymphocytes (TILs) and presence of tumor necrosis.
The overall response rates to neoadjuvant cytotoxic chemotherapy alone, given in combination using non cross-resistant drugs, whether sequentially administered or not, are high, as breast carcinoma is a generally chemosensitive tumor. After neoadjuvant therapy however, histopathologic residual invasive cancer in the breast and the axilla is very common and the rate of pathologic Complete Response (pCR) rate have been historically low at 20-25%.
The role of neoadjuvant therapy in locally advanced breast cancer was studied by Akhtar M, et al in 2015. Results in this prospective comparative study showed that neoadjuvant chemotherapy aids in downstaging LABC and offers surgical treatment options. However, of the 31 patients enrolled with a mean follow-up of 24 months there was no significant difference in disease free survival (p=0.73), overall survival (p=0.67), and post-operative complications between the neoadjuvant and adjuvant group. It concluded that neoadjuvant chemotherapy does not offer survival advantage over adjuvant chemotherapy [7].
In an RCT by Corben AD, et al (2013), which involved 62 patients randomized to receive sequential neoadjuvant chemotherapy with Doxorubicin and Paclitaxel. Residual disease in breast and nodes (RBDN) predicted distant disease-free survival (P = 0.01; HR, 2.54; 95% CI, 1.36- 5.08). More so, lymph node status predicts survival after neoadjuvant chemotherapy [8]. More lymph nodes after neoadjuvant chemotherapy correlated with worse outcomes. The size of the largest lymph node deposit, measured microscopically predicted distant disease-free survival (P=0.01; HR, 1.1; 95% CI, 1.04-1.33).
To our knowledge, there is very limited local data on neoadjuvant therapy in the Philippines. The use of other forms of neoadjuvant systemic treatments such as endocrine therapy in our institution and globally is also very limited and is not a popular approach locally.
In a local cohort study by Acidera et al, (2004) among 26 patients who underwent neoadjuvant therapy, there was no (0%) pathologic complete response noted using the standard anthracycline and taxane-based cytotoxics [9]. In another study done by Fournier et al, (2015) on 76 patients who underwent pre-operative treatment, residual invasive cancer was present in almost 90% of these patients, either in the breast, axillary lymph nodes or both [10].
In a cohort study done by Macalindong et al., (2015), among the 63 patients, 54% had local recurrence at 2 years with 263 days mean time to recurrence. Age, pathologic nodes (pN), percent positive pN, pStage, lymphovascular invasion (LVSI), and RT were significant LR predictors on simple logistic regression. Pathologic nodes (OR 1.31, p= 0.01) and radiotherapy (OR 0.14, p= 0.004) were independent predictors on multiple logistic regression. In patients without radiotherapy, no independent predictor was found [11].
A recent local descriptive study entitled “Neoadjuvant Systemic Therapy for Locally Advanced Breast Cancer in St. Luke’s Medical Center: 10 year local experience and response rates” by Ordinario, et al., (2017), showed that the most common chemotherapy regimen used in locally advanced breast cancer was sequential Anthracycline and Taxane (AC/EC/FEC then Taxane with or without Trastuzumab and Docetaxel with or without Trastuzumab followed by FEC) at 31%. pathologic complete response (pCR) was achieved in 18% of the total subjects using standard chemotherapy. Among the patients who achieved pCR, the most common subtype is Luminal/HER2 negative at 17% and the most common regimen used was Docetaxel followed by FEC (5FU+EC) at 39% [3]. Some of the recent landmark trials that has improved pathologic complete response rates were seen among HER2 expressing breast cancer subtypes, incorporating anti-HER2 therapies like Trastuzumab, Pertuzumab and Lapatinib. One study is the NOAH trial where the addition of neoadjuvant and adjuvant Trastuzumab to neoadjuvant chemotherapy significantly improved 3-year event-free survival in patients with HER2-positive breast cancer by 71% (95% CI 61-78); n=36 events]; hazard ratio 0.59 [95% CI 0.38-0.90]; p=0.013) as well as survival, and clinical and pathological tumor responses [12].
Another study is the Neosphere trial which showed the combination of Pertuzumab, Trastuzumab and Docetaxel preoperatively led to a statistically significant increase in pCR in the breast to 16.8 % (95% CI 3.5-30.1; P=.0141) [13]. In the TRYPHAENA trial, the use of Pertuzumab and Trastuzumab given along with anthracycline containing or anthracycline-free standard chemotherapy regimens to patients with operable, locally advanced or inflammatory Her2 positive breast cancer showed pCR rates in all treatment arms ranging from 57 to 66% [14]. The NEOALLTO trial showed that among 455 patients randomized to receive either Lapatinib, Trastuzumab or both, the 3.77 years of follow up showed 84% event free survival in the combination group, which is more superior when either was used alone. The result did not differ between the Lapatinib and Trastuzumab groups nor between the combination and trastuzumab group, which is also true with overall survival. Landmark analyses showed that 3-year event-free survival was significantly improved for women who achieved pathological complete response compared with those who did not (HR 0.38, 95% CI 0.22–0.63, p=0.0003), as well as 3-year overall survival (0.35, 0.15–0.70, p=0.005) [15].
The National Comprehensive Cancer Network panel recommends that tumor response should be routinely assessed by clinical exam during the delivery of preoperative systemic therapy. Imaging during preoperative systemic therapy should not be done routinely, but may be considered if tumor progression is suspected [4].
Materials and Methods
Research Design
We conducted a retrospective cohort study including patients who are aged 18 years old and above, histopathologically diagnosed with locally advanced breast cancer who completed neoadjuvant therapy and underwent surgical management at St. Luke’s Medical Center from January 2007 to December 2017. Patients with metastatic disease upon diagnosis were excluded from the study.
Sources of Data
Data were collected through chart review, clinic records; and telephone or personal interviews of attending physicians.
Sampling Technique
The study included patients in a previous retrospective cohort study done by Ordinario, et al., [3] with locally advanced breast cancer identified from but not limited to available census, tumor registry, surgical logbooks, clinic logbooks, and Ambulatory Care Unit database.
Data Gathering Procedure
Charts of all patients diagnosed with Breast cancer were reviewed and all patients who meet the Inclusion Criteria were included in the study.
Patients were then followed up via chart review, clinic records; and telephone or personal interviews of attending physicians.
Outcomes were characterized as primary and secondary. The Primary outcome was the 5-year disease free survival (DFS) rate measured in months of patients who received neoadjuvant systemic therapy. For the secondary outcomes, the 5-year DFS rate among patients with LABC after neoadjuvant systemic therapy who achieved and did not achieve pCR was stratified according to phenotype; and lastly, the overall survival (OS) of patients with LABC who received neoadjuvant systemic therapy was stratified according to pathologic response and phenotype. In addition, included were the locoregional recurrence-free survival and distant metastasis-free survival rate determination among patients with LABC who received neoadjuvant systemic therapy.
Sample Size Calculation
Sample size was calculated based on the disease-free survival among breast cancer patients who underwent neoadjuvant systemic therapy assumed to be 21.5% (Spring et al., 2016), with the maximum allowable error of 5% and reliability of 95%, sample size required is 257 [5].
Statistical Analysis
Data were encoded in MS Excel by the researcher. Stata MP version 14 software was used for data processing and analysis. Continuous variables were presented as median, standard devation (SD) and interquartile range (IQR) depending on data distribution. Categorical variables were presented as frequency and percentage. Independent t-test was used to analyze continuous data, while Chi square test or Fisher’s exact test was used for categorical data.
Survival analyses were performed to examine the disease-free survival and overall survival probabilities. Date of treatment initiation served as the Time 0. Log- rank test was used to compare the survival probabilities by phenotypic subtype. P values <0.05 were considered statistically significant. Charts and graphs were created using MS Excel and Stata software.
Ethical Consideration
The clinical protocol and all relevant documents were reviewed and approved by the SLMC Institutional Ethics Review Board Committee. Patient confidentiality was respected by ensuring anonymity of patient records. All study data were recorded and investigators are responsible for the integrity of the data (i.e. accuracy, completeness, legibility).
The manner of disseminating and communicating the study results guarantees the protection of the confidentiality of patient’s data. Data were stored in file cabinets with locks that can only be accessed by the investigators. Data gathered will be kept for a minimum of 5 years and will be shredded once for disposal.
Results and Discussion
Characteristics of the population study
A total of 231 patients, who met the inclusion criteria, were included in the study. Baseline demographic and clinical characteristics of included patients are shown in Table 1.
| Characteristics | n (%) |
| Age (in years), median | 50.39 ± 11.23 |
| <60 years old | 180 (78) |
| ≥60 years old | 51 (22) |
| Menopausal status | |
| Pre-menopausal | 150 (65) |
| Post-menopausal | 81 (35) |
| AJCC staging# | |
| IIA | 25 (11) |
| IIB | 50 (22) |
| IIIA | 59 (26) |
| IIIB | 79 (35) |
| IIIC | 13 (6) |
| Histologic type# | |
| Invasive ductal carcinoma | 217 (94) |
| Invasive lobular carcinoma | 1 (1) |
| Others | 12 (5) |
| Estrogen receptor (ER) status# | |
| Positive | 171 (74) |
| Negative | 57 (25) |
| Unknown | 3 (1) |
| Progesterone receptor (PR) status# | |
| Positive | 157 (68) |
| Negative | 70 (30) |
| Unknown | 4 (2) |
| HER-2 | |
| Positive | 85 (37) |
| Negative | 136 (59) |
| Unknown | 10 (4) |
| Ki67 | |
| Positive | 59 (26) |
| Negative | 11 (5) |
| Unknowns | 161 (69) |
#Missing data
The median age at diagnosis was 50 years old, range: 23-82 years old. Most of the patients were premenopausal (65%) while 35% were postmenopausal. The most common histology was Invasive Ductal Carcinoma at 94% with a higher proportion of patients at stage IIIB followed by stage IIIA. The Majority of patients diagnosed with Stage III disease were 151 (65%). Among the 50 stage IIB patients, 37 (74%) were T2N1M0 and 13 (26%) were T3N0M0. While among the 59 stage IIIA patients 4 (7%) were T0N2M0, 6 (10%) were T1N2M0, 10 (17%) were T2N2M0, 31 (53%) were T3N1M0, and 8 (14%) were T3N2M0. In addition, among the 79 stage IIIB patients—22 (28%) were T4N0M0, 44 (56%) were T4N1M0, and 11 (14%) T4N2M0. However, there were 5 patients that were not properly staged since the available data and work-up were not available at the time of data gathering.
The majority of the patients were positive for ER (74%) and PR (68%), and only 85 (37%) were positive for HER-2. Her2 testing was not routinely done for breast cancer patients in developing countries [16]. Around 2010, with the establishment of the Scientific Partnership for HER2 testing Excellence (SPHERE) it promoted and facilitated HER2 testing for both breast and gastric cancer across Asia Pacific [17]. Seventy patients were tested for ki67—59 (26%) of which were positive (>=20%). Ki-67 is a marker for cancer proliferation although not routinely done due to a lack of a standardized procedure for Ki67 assessment.
The most common phenotypic subtype was Luminal/ HER2 negative (53%) more than half of the total patients as shown in Figure 1.
Figure 1. Phenotypic Subtype of Patients (n=219).
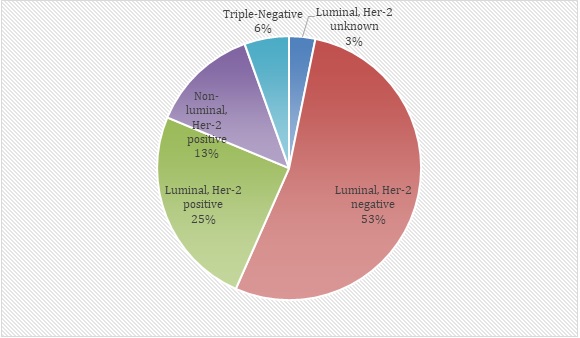
Around twenty-five 25% were Luminal/HER2 positive and thirteen 13% were Non-Luminal/HER2 positive. Only 6% were triple negative breast cancers.
Neoadjuvant Treatment
The most common neoadjuvant chemotherapy regimen used was Cytotoxic treatment 221(98%). Four (2%) patients received endocrine treatment with aromatase inhibitors. Majority of patients were given concurrent anthracycline and taxane (N=96, 43%) and sequential anthracycline and taxane among forty-seven (21%) patients. Anthracycline-based treatment was given in 19% of patients while 16% received Taxane only. Around thirty-seven patients (16%) received anti-Her2 treatment (Table 2).
| n (%) | |
| Endocrine treatment (n=4) | |
| Aromatase inhibitor | 4 (2) |
| Cytotoxic treatment (n=221) | |
| Anthracycline only | 42 (19) |
| Taxane only | 36 (16) |
| Concurrent anthracycline and taxane | 96 (43) |
| Sequential anthracycline and taxane | 47 (21) |
| Anti-Her-2 treatment, %yes | 37 (16) |
In our institution, the use of endocrine therapy as neoadjuvant chemotherapy is very limited and is not a popular approach in the local setting. Endocrine therapy emerged in the early 1980’s as a treatment option for elderly women unfit to be treated with chemotherapy or ineligible for surgery [18]. The 3rd generation aromatase inhibitors like anastrozole, letrozole, and exemestane are currently considered standard treatment for postmeniopausal women with early or advanced breast cancer. In a double-blinded study by Ellis et al. 2001, 337 women with LABC (clinical stage II or III) ineligible for BCS were randomized to either 4 months of Letrozole (2.5mg daily) or tamoxifen (20mg daily) followed by surgery. Letrozole was found to be superior to tamoxifen in terms of clinical response rates as assessed by palpation, ultrasound, mammography, and breast conservation rate. The response to tamoxifen was inferior 41% (P=0.004) [19]. In our study, 4 patients who received Endocine treatment had residual disease.
Likewise, of the 85 patients with Her2 positive disease, 37 (16%) received anti-Her2 blockade as part of their neoadjuvant regimen. The efficacy of anti-Her2 treatment has been observed in landmark trials including Neosphere, Tryphaena, NEOALLTO and NOAH which showed significant improvement in event-free survival and increase in complete pathologic response. In our study, not all Her2 positive patients were given anti-Her2 blockade. One possible reason being the added financial burden associated with targeted therapy.
Adjuvant Treatment
The most common adjuvant treatment used was radiotherapy (44%) followed by combined treatment (43%) (Figure 2).
Figure 2. Adjuvant Treatment Received by Patients (n=164).
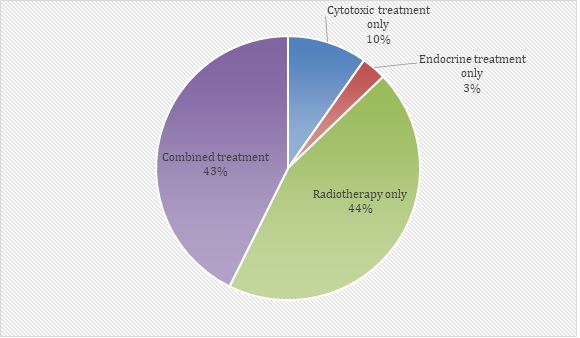
Only three percent (3%) of patients were given Endocrine treatment alone. Of the 164 patients who received adjuvant treatment, 70 patients were given combined therapy. Combined therapy given are as follows: Cytotoxic + Anti-her2 (n=6; 9%), Cytotoxic followed by endocrine therapy and radiotherapy (n=38; 54%), Cytotoxic followed by endocrine therapy (n=3; 4%), Cytotoxic plus anti-Her2 followed by endocrine therapy (n=3; 4%), and Endocrine therapy plus radiotherapy (n=20; 29%).
Adjuvant treatment is the administration of additional therapy after primary surgery to treat or inhibit micrometastases. Modalities include: Local irradiation after mastectomy, systemic therapy with cytotoxic chemotherapy and endocrine therapy [20]. In our study, majority of patients had radiotherapy as adjuvant treatment. Adjuvant postmastectomy radiotherapy is recommmended among patients with a high risk of local and regional relapse which incudes patients with large primary tumors (>5cm) and with 4 or more involved lymph nodes. Adjuvant radiotherapy has been shown to improve local control and decrease the risk of systemic recurrence [21]. Meanwhile, among patients given combined modality most of the patients were given Cytotoxic followed by Endocrine and radiotherapy (54%). Adjuvant chemotherapy are given among patients with tumors greater than 1cm, node-positive disease, or ER- negative cancers [22]. In addition, there were 20 (29%) patients given Endocrine therapy plus radiotherapy. In a meta-analysis by the Early Breast Cancer Trialists‘ Collaborative group, 5 years of adjuvant therapy with Tamoxifen reduced the 10-year proportional risk of recurrence by 47% and proportional risk of mortality by 26% [23].
Almost all patients (N=230, 99%) underwent modified radical mastectomy with axillary lymph node dissection post neoadjuvant chemotherapy (Table 3).
| n (%) | |
| Surgical management (n=231) | |
| MRM with ALND | 230 (99) |
| BCS with ALND | 1 (1) |
| Treatment response (n=231) | |
| Pathologic complete response | 33 (14) |
| Residual disease | 198 (86) |
| Disease progression (n= 216) | |
| Stable | 139 (64) |
| Progressive | 77 (36) |
There was one patient who opted for Breast conservation surgery (lumpectomy). Among patients given neoadjuvant chemotherapy, modified radical mastectomy is still the widely accepted surgical treatment.
In our study, pathologic complete response (pCR) which is defined as absence of invasive residual cancer in the breast and axillary lymph nodes, with or without ductal carcinoma-in-situ (DCIS) was seen in 33 patients (14%). This coincides with a study done by the National Cancer Database involving 13,939 breast cancer patients who underwent neoadjuvant chemotherapy wherein pCR was achieved in 19% of all patients [24]. In addition, among the 216 patients followed up for disease progression around 77 (36%) patients had local or distant metastases.
Disease recurrence
There were 79 patients that exhibited disease recurrence with an incidence of 35.59% (95% CI: 29.52- 42.15%). Most of the patients with disease recurrence had a significant difference as compared to those patients with no recurrence as they were classified as having Stage IIIB disease, negative for progesterone receptor, positive for Ki67, received combined adjuvant treatment, showed incomplete treatment response, and exhibited disease progression. Other demographic and clinical variables were comparable between patients with and without recurrence. Patients with disease recurrence had a greater proportion of more advanced cancers such as Stage III (p=0.050) compared to those without recurrence. There is a significant difference in the proportion of Stage IIIB patients, all of which are T4 in the TNM staging which translates to a tumor that has grown to the chest wall, skin, both, or are inflammatory (Table 4).
| Characteristics | YES | NO | P value |
| (n=79) | (n=143) | ||
| n (%) | n (%) | ||
| Age (in years), median | 52.24 ± 12.62 | 49.34 ± 10.33 | 0.0663 a |
| <60 years old | 59 (75) | 115 (80) | 0.320 b |
| ≥60 years old | 20 (25) | 28 (20) | |
| Menopausal status | |||
| Pre-menopausal | 50 (63) | 95 (66) | 0.661 b |
| Post-menopausal | 29 (37) | 48 (34) | |
| AJCC staging # | |||
| IIA | 5 (6) | 20 (14) | 0.050 *b |
| IIB | 12 (15) | 38 (27) | |
| IIIA | 24 (30) | 33 (23) | |
| IIIB | 34 (43) | 42 (29) | |
| IIIC | 4 (5) | 8 (6) | |
| Histologic type | |||
| Invasive ductal carcinoma | 73 (92) | 136 (95) | 0.585 c |
| Invasive lobular carcinoma | 0 | 1 (1) | |
| Others | 6 (8) | 6 (4) | |
| Estrogen receptor (ER) status# | |||
| Positive | 56 (72) | 108 (77) | 0.433 b |
| Negative | 22 (28) | 33 (23) | |
| Progesterone receptor (PR) status # | |||
| Positive | 45 (58) | 105 (75) | 0.008 *b |
| Negative | 33 (42) | 35 (25) | |
| HER-2 # | |||
| Positive | 26 (34) | 57 (42) | 0.289 b |
| Negative | 50 (66) | 80 (58) | |
| Ki67 # | |||
| Positive | 26 (96) | 33 (77) | 0.041 *c |
| Negative | 1 (4) | 10 (23) | |
| Phenotypic subtype # | |||
| Luminal, Her-2 unknown | 2 (3) | 5 (4) | 0.480 c |
| Luminal, Her-2 negative | 41 (53) | 71 (53) | |
| Luminal, Her-2 positive | 16 (21) | 37 (27) | |
| Non-Luminal, Her-2 positive | 10 (13) | 18 (13) | |
| Triple negative | 8 (10) | 4 (3) | |
| Adjuvant treatment # , %yes | |||
| Cytotoxic only | 8 (12) | 8 (8) | 0.005 *b |
| Endocrine only | 2 (3) | 2 (2) | |
| Radiotherapy only | 19 (28) | 54 (56) | |
| Combined | 38 (57) | 31 (33) | |
| Surgical management # | |||
| MRM with ALND | 77 (100) | 133 (99) | 1.000 c |
| BCS with ALND | 0 | 1 (1) | |
| Treatment response # | |||
| Complete | 5 (6) | 27 (18) | 0.012 *b |
| Residual | 73 (94) | 116 (81) | |
| Disease progression # | |||
| Stable | 5 (7) | 131 (96) | <0.0001 *b |
| Progressive | 72 (94) | 5 (4) |
#Missing data; aIndependent t test was used; bChi square test was used ;cFisher’s exact test was used
In a retrospective cohort by Soo Youn Bae 2015, single hormone receptor positive breast tumors without Her2 overexpression were associated with poorer survival. Survival outcomes were comparable to those with triple negative breast cancers [25]. In our study there were 18 patients that had ER+ PR- tumor. Among the 18 patients, 11 (61%) demonstrated disease recurrence. In the 99 patients with both ER and PR expression without Her2 overexpression, only 30 patients (30%) had disease recurrence. In addition, Ki67 which is a marker for cellular proliferation was also positive in a greater proportion of patients (96%) who had disease recurrence.
Figure 3 shows a Kaplan-Meier curve for locoregional recurrence free-survival (LRRFS).
Figure 3. Disease-free Survival (Locoregional) among Patients.
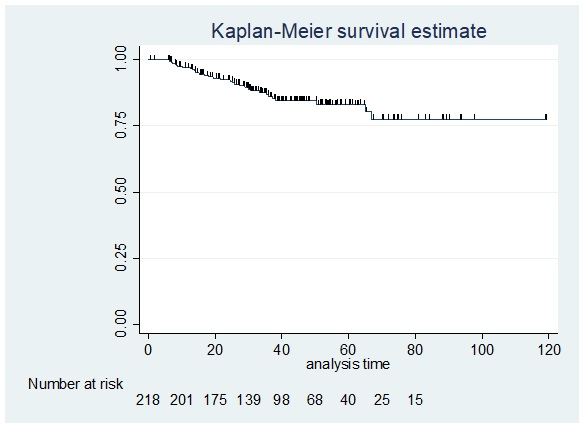
Thirteen [13] patients were lost to follow up (no date of last follow up) and were excluded. Of the 218 patients, there were 30 patients who were identified to have locoregional recurrence, with an incidence of 12.99% (95% CI: 9.21-18.01%). Recurrences were noted in the chest wall of 25 patients and in the axilla of 2 patients.
The 5-year locoregional recurrence free survival was 83.20%. Among the 30 patients who had locoregional recurrence, the median disease-free interval (DFI) was 701.5 days (IQR: 403-1014 days; Range:195-2031 days). Results of our study was consistent with a study done by Li et al. 2014 which showed that among 439 breast cancer patients after postmastectomy radiotherapy with a median duration of 54 months the 5-year rates of locoregional recurrence-free survival was 87.8%. Likewise, a Kaplan- Meier analysis showed that the 5-year rates of LRRFS were 92.0% and 82.9% for breast cancer stages pN2 and pN3 respectively (p=0.005). Cox regression analysis showed that pN3 stage is an independent predictor for LRRFS [hazard ratio: 2.241; 95% CI: 1.270 to 3.957; p = 0.005] [26].
For distant metastasis-free survival (DMFS), there were 61 patients who developed distant metastasis in the entire study, with an incidence of 26.41% (95% CI: 21.09-32.51%). Most of the patients developed distant metastasis to the lung and pleura (n=28), bone (n=21), and brain (n=12).
The 5-year overall distant metastasis-free survival was 62.00%. Among the 61 patients who had distant metastasis, the median disease-free interval (DFI) was 766 days (IQR: 482-1291 days; Range:10-2504 days) (Figure 4).
Figure 4. Disease-free Survival (distant) among Patients.
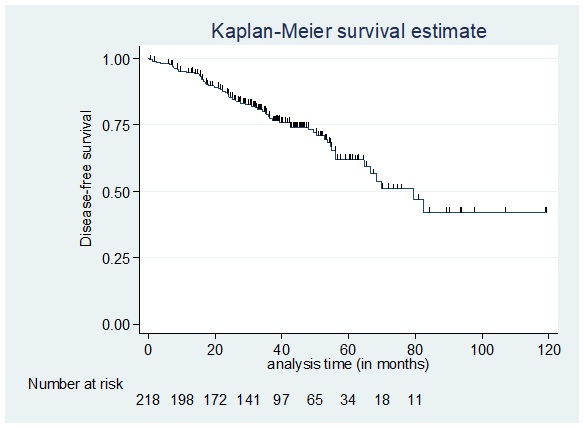
In the same study by Li et al, the 5-year rate of distant metastasis-free survival was 59.5%. Locoregional recurrence (LRR) is an important risk factor for distant metastasis and death from breast cancer. In a recent study by Bantema-Joppe et al. 2013, which included a consecutive series of 536 young (<40 years old) breast cancer patients using a multistate survival model. Patients with Locoregional recurrence had a higher risk of distant metastases or death compared with patients without LRR [hazard ratio: 5.5; 95% CI: 2.1 to 14.5] [27].
The disease-free survival (DFS) among patients who achieved pathologic complete response (pCR) versus those who did not achieve pCR or with residual disease as seen in Figure 5 showed that the 5-year disease-free survival was 73.17% among patients with complete pathologic response after neoadjuvant treatment compared to 51.25% of those with residual disease.
Figure 5. Disease-free Survival among Patients who Achieved pCR Versus those who did not Achieve pCR.
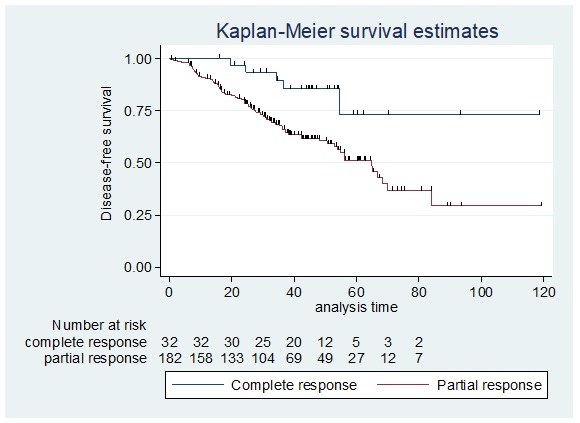
The overall 5-year disease-free survival among patients who achieved pathologic complete response was significantly higher compared to those who did not achieve pCR (Log-rank test p=0.0107). In a recent retrospective cohort by Klein et al. 2019, which involved 103 LABC patients treated for curative intent with neoadjuvant chemotherapy, surgery, and adjuvant radiotherapy with a median follow-up of 45.6 months using Kaplan-Meier curves there were significant differences observed for recurrence free survival (RFS) (p=0.015) and overall survival OS (p=0.015) among patients with complete pathologic response [28].
The disease free-survival among patients who achieved pathologic complete response according to phenotype as seen in Figure 6 showed that the 5-year overall disease-free survival rate per phenotypic subtype were as follows: Luminal, Her2 Unknown: 100%; Luminal, Her2 Negative: 0%; Luminal, Her2 Positive: 100%; Non-luminal, Her2 Positive: 66.67%; Triple negative disease: 66.67%.
Figure 6. Disease-free Survival among Patients who Achieved pCR Stratified According to Phenotype. L, Her2-U: Luminal, Her2 Unknown; L, Her2-Neg: Luminal, Her2 Negative; L, Her2-Pos: Luminal, Her2 Positive; NL, Her2-Pos: Non-luminal, Her2 Positive; Triple Neg: Triple Negative.
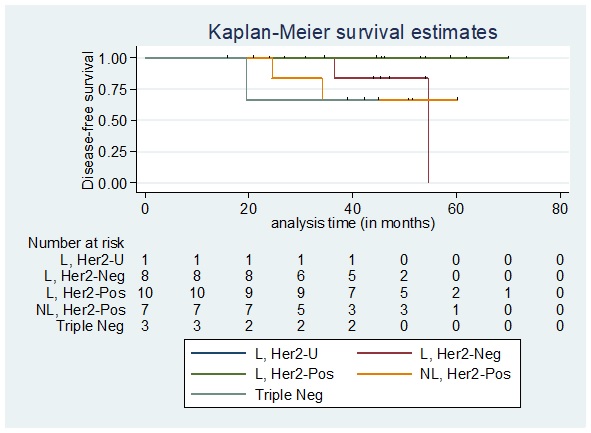
The 5-year disease free-survival among patients who achieved pathologic complete response does not significantly differ by phenotypic subtype (Log-rank test p=0.3573). In our study, most of the Luminal/HER2 negative diseases had poor survival outcomes consistent with the study by Soo Youn Bae, 2015 pointing out that single hormone receptor positivity like ER+/PR- without Her2 overexpression was associated with poorer survival. Meanwhile, among patients who did not achieve pathologic complete response the disease-free survival according to phenotype showed that the 5-year overall disease-free survival rate per phenotypic subtype were as follows: Luminal, Her2 Unknown: 0%; Luminal, Her2 Negative: 52.52%; Luminal, Her2 Positive: 57.98%; Non-luminal, Her2 Positive: 53.76%; and Triple negative disease: 15.24% (Figure 7).
Figure 7. Disease-free Survival among Patients who did not Achieve pCR Stratified According to Phenotype. L, Her2-U: Luminal, Her2 Unknown; L, Her2-Neg: Luminal, Her2 Negative; L, Her2-Pos: Luminal, Her2 Positive; NL, Her2-Pos: Non-luminal, Her2 Positive; Triple Neg: Triple Negative.
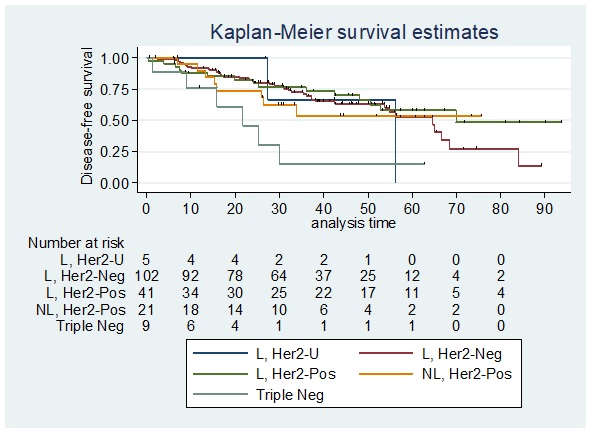
The 5-year disease free- survival among patients who did not achieved pathologic complete response does significantly differ by phenotypic subtype (Log-rank test p value=0.0363). In our study, triple negative diseases had a lower disease free-survival of 15.24% which is similar to the study by Li et al. 2014, which showed that triple-negative breast cancer has a higher locoregional recurrence rate than in patients with other molecular subtypes due to its highly heterogeneous clinical, pathologic, and molecular features. Cox regression analysis also showed that triple negative breast cancer is an independent predictor for LRRFS [hazard ratio: 4.617; 95% CI: 2.192-9.723; p < 0.001] [26].
In our study there were 15 patient deaths that were recorded, having an incidence of 6.70% (95% CI: 4.06- 10.85%). As seen in Table 5 which shows the demographic and clinical characteristics of patients by mortality, a higher proportion of patients who died exhibited disease progression (p=<0.00001) as compared to patients with no recurrence who survived.
| Characteristics | Yes | No | P value |
| (n=15) | (n=209) | ||
| n (%) | n (%) | ||
| Age (in years), median | 51.60 ± 15.99 | 50.29 ± 10.84 | 0.6623 a |
| <60 years old | 12 (80) | 164 (78) | 1.000 b |
| ≥60 years old | 3 (20) | 45 (22) | |
| Menopausal status | |||
| Pre-menopausal | 10 (67) | 136 (65) | 0.900 c |
| Post-menopausal | 5 (33) | 73 (35) | |
| AJCC staging# | |||
| IIA | 1 (7) | 24 (12) | 0.693 b |
| IIB | 2 (13) | 48 (23) | |
| IIIA | 4 (27) | 53 (24) | |
| IIIB | 8 (53) | 70 (34) | |
| IIIC | 0 | 12 (6) | |
| Histologic type | |||
| Invasive ductal carcinoma | 14 (93) | 197 (94) | 0.604 b |
| Invasive lobular | 0 | 1 (1) | |
| carcinoma | |||
| Others | 1(7) | 11 (5) | |
| Estrogen receptor (ER) status # | |||
| Positive | 9 (60) | 157 (76) | 0.213 b |
| Negative | 6 (40) | 49 (24) | |
| Progesterone receptor (PR) status # | |||
| Positive | 10 (67) | 142 (69) | 0.781b |
| Negative | 5 (33) | 63 (31) | |
| HER-2 # | |||
| Positive | 7 (47) | 77 (39) | 0.532 c |
| Negative | 8 (53) | 123 (61) | |
| Ki67 # | |||
| Positive | 3 (100) | 56 (84) | 1.000 b |
| Negative | 0 | 11 (16) | |
| Phenotypic subtype # | |||
| Luminal, Her-2 unknown | 0 | 7 (4) | 0.817 c |
| Luminal, Her-2 negative | 9 (60) | 104 (52) | |
| Luminal, Her-2 positive | 3 (20) | 51 (26) | |
| Non-Luminal, Her-2 | 3 (20) | 25 (13) | |
| positive | |||
| Triple negative | 0 | 12 (6) | |
| Adjuvant treatment # , %yes | |||
| Cytotoxic only | 1 (9) | 15 (10) | 0.168 b |
| Endocrine only | 0 | 5 (3) | |
| Radiotherapy only | 2 (18) | 71 (47) | |
| Combined | 8 (73) | 61 (40) | |
| Surgical management # | |||
| MRM with ALND | 14 (100) | 197 (99) | 1.000 b |
| BCS with ALND | 0 | 1 (1) | |
| Treatment response # | |||
| Complete | 0 | 32 (15) | 0.136 b |
| Residual | 15 (100) | 176 (85) | |
| Disease progression # | |||
| Stable | 2 (13) | 135 (68) | <0.00001* |
| Progressive | 13 (87) | 64 (32) | |
| Luminal, Her-2 unknown | 2 (3) | 5 (4) | 0.480 c |
| Luminal, Her-2 negative | 41 (53) | 71 (53) | |
| Luminal, Her-2 positive | 16 (21) | 37 (27) | |
| Non-Luminal, Her-2 positive | 10 (13) | 18 (13) | |
| Triple negative | 8 (10) | 4 (3) | |
| Adjuvant treatment # , %yes | |||
| Cytotoxic only | 8 (12) | 8 (8) | 0.005 *b |
| Endocrine only | 2 (3) | 2 (2) | |
| Radiotherapy only | 19 (28) | 54 (56) | |
| Combined | 38 (57) | 31 (33) | |
| Surgical management # | |||
| MRM with ALND | 77 (100) | 133 (99) | 1.000 c |
| BCS with ALND | 0 | 1 (1) | |
| Treatment response# | |||
| Complete | 5 (6) | 27 (18) | 0.012 *b |
| Residual | 73 (94) | 116 (81) | |
| Disease progression # | |||
| Stable | 5 (7) | 131 (96) | <0.0001 *b |
| Progressive | 72 (94) | 5 (4) |
#Missing data; aIndependent t test was used; bFisher’s exact test was used; cChi square test was used
This is consistent with studies indicating that Locoregional recurrence is an important risk factor for death from breast cancer.
As shown in Figure 8, the overall survival among patients who achieved pathologic complete response according to phenotype had no deaths recorded after 5-years for all the phenotypic subtypes.
Figure 8. Overall Survival among Patients who Achieved pCR Stratified According to Phenotype. L, Her2-U: Luminal, Her2 Unknown; L, Her2-Neg: Luminal, Her2 Negative; L, Her2-Pos: Luminal, Her2 Positive; NL, Her2-Pos: Non-luminal, Her2 Positive; Triple Neg: Triple Negative.
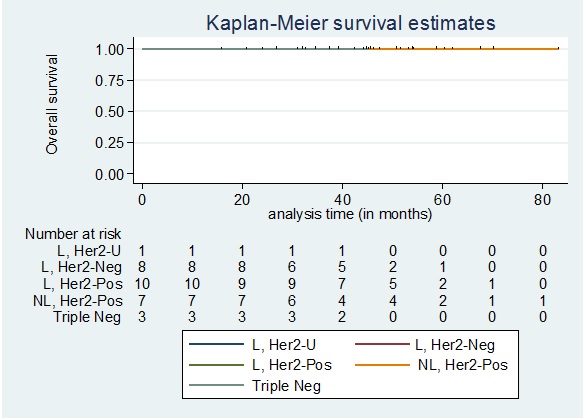
In Figure 9, the overall survival among patients who did not achieve pathologic complete response according to the phenotype showed no deaths among patients in the Luminal, Her2 Unknown and Triple negative group.
Figure 9. Overall Survival among Patients who did not Achieve pCR Stratified According to Phenotype. L, Her2-U: Luminal, Her2 Unknown; L, Her2-Neg: Luminal, Her2 Negative; L, Her2-Pos: Luminal, Her2 Positive; NL, Her2-Pos: Non-luminal, Her2 Positive; Triple Neg: Triple Negative.
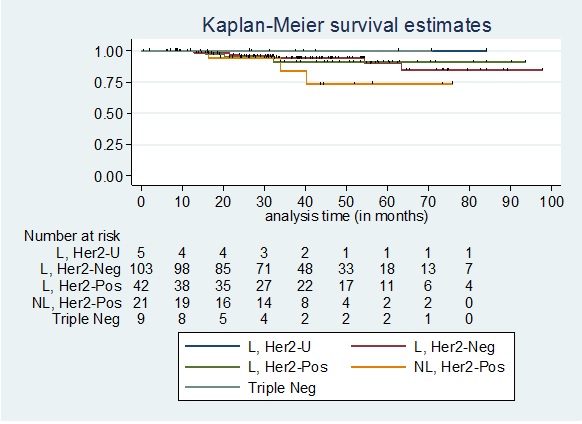
The 5-year overall survival for the 3 other phenotypic subtypes are as follows: Luminal, Her2 Negative: 90.23%; Luminal, Her2 Positive: 91.08%; Non-luminal, Her2 Positive: 73.46%. The 5-year overall survival among patients who did not achieve pathologic complete response did not significantly differ by phenotypic subtype (Log-rank test p=0.5726).
Strengths and Limitations
Our study is one of the first retrospective single-center study done on survival outcomes among patients with locally advanced breast cancer (LABC) after neoadjuvant chemotherapy. Limitations of our study include its retrospective nature which depends on the completeness and accuracy of the information from the chart review and clinical notes. Not all patients who were initially included in the previous study of Ordinario, et al. were analyzed in this study due to failure of follow-up and limited information. Pathologic markers such as Ki67 levels and Her2 status were generally not available in some patients during the conduct of the study. However, this likely did not alter our conclusions. Our study has the risk of underestimation/underreporting of mortalities since death registries were not included in the data search. There were thirteen patients who were excluded from the analysis due to lack of follow-up. In addition, the dates of diagnosis were used as surrogate for the date of treatment initiation. Our study was able to demonstrate that among LABC patients given neoadjuvant chemotherapy the goal for pathologic complete response has to be achieved in order to increase overall recurrence free survival. Most of the patients with disease recurrence had stage IIIB disease, negative for progesterone receptor, and positive for Ki67 levels. Hormone receptor positive breast tumors (ER+/ PR+) without HER2 overexpression can be an avenue for use of endocrine therapy as neoadjuvant treatment due to its activity in inhibiting the expression of ErbB-1 and ErbB-2 (epidermal growth factor receptor and HER2/ neu) signaling through ER ligand-dependent activity, and the growth promoting effects of these receptor tyrosine kinases.
Conclusion and Recommendations
Pathologic complete response has better outcomes in terms of disease-free survival compared to those who did not achieve complete pathologic response after neoadjuvant chemotherapy. Disease-free survival among patients who achieved pathologic complete response does not significantly differ among the different molecular phenotypes. The overall survival of the patients in this cohort does not differ in terms of phenotypic subtypes and mortality is seen among patients with disease progression.
For future research, aside from standard pathology, the use of other measurement tools for treatment response such as the modified response (MR) score and the Chevallier score can be used for a specific correlation with recurrence and survival rates. Likewise, since our study did not have sufficient number of deaths to study predictors of overall survival future studies can improve the sample size and longer follow-up in order to identify the prognostic factors influencing locoregional recurrence and distant metastasis using univariate and multivariate analyses.
References
- De Vita, Hellman & Rosenberg’s Cancer: Principles and Practice of Oncology, 10th edition, 1117-1152 .
- St. Luke’s Medical Center Tumor Registry. Breast Cancer Working Group. 2016. .
- Ordinario, M.O., et al. Neoadjuvant Systemic Therapy for Locally Advanced Breast Cancer in St. Luke’s Medical Center: 10-year local experience and response rates. 2017. - unpublished .
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Breast Cancer. Version 2.2017 .
- Spring L, Greenup R, Reynolds K et al: Pathological complete response after neoadjuvant chemotherapy predicts improved survival in all major subtypes of breast cancer: Systematic review and meta-analyses of over 18,000 patients. 2016 AACR Annual Meeting. Abstract 1439. April 18, 2016. .
- Philippine Council of Health Research and Development. Department of Science and Technology. http://www.pchrd.dost.gov.ph/ .
- Role of neo-adjuvant chemotherapy in locally advanced breast cancer Akhtar M., Akulwar V., Kulkarni A., Bansal A.. Indian Journal of Cancer.2015;52(3). CrossRef
- Pathologic response and long-term follow-up in breast cancer patients treated with neoadjuvant chemotherapy: a comparison between classifications and their practical application Corben Adriana D., Abi-Raad Rita, Popa Ion, Teo Clarence H. Y., Macklin Eric A., Koerner Frederick C., Taghian Alphonse G., Brachtel Elena F.. Archives of Pathology & Laboratory Medicine.2013;137(8). CrossRef
- Acidera, Anna F. A Review of Primary Systemic Chemotherapy for Locally Advanced Breast Cancer at the Ambulatory Care Unit of St. Luke’s Medical Center. St. Luke’s Medical Center. 2004. - unpublished .
- Fournier, Frances Renee. Residual Cancer Burden Among Patients with Locally Advanced Breast Cancer in St. Luke’s Medical Center: a 6-Year Study. 2015. - unpublished .
- Pattern and Predictors of Locoregional Failure in Locally Advanced Breast Cancer Following Neoadjuvant Chemotherapy and Modified Radical Mastectomy with or without Radiotherapy: A Philippine Tertiary Breast Center Experience. Global Breast Cancer Conference 2015 and 4th International Breast Cancer Symposium. - unpublished Macalindong , et al . .
- Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, et al . Lancet (London, England).2010;375(9712). CrossRef
- Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial Gianni L, Pienkowski T, Im Y, Roman L, Tseng L, Liu M, Lluch A, et al . The Lancet. Oncology.2012;13(1). CrossRef
- Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Schneeweiss A., Chia S., Hickish T., Harvey V., Eniu A., Hegg R., Tausch C., Seo J. H., Tsai Y.-F., Ratnayake J., McNally V., Ross G., Cortés J.. Annals of Oncology: Official Journal of the European Society for Medical Oncology.2013;24(9). CrossRef
- Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, Azambuja E, Aura C, Gómez H, et al . Lancet (London, England).2012;379(9816). CrossRef
- Spectrum of breast cancer in Asian women Agarwal G, Pradeep PV , Aggarwal V, Yip C, Cheung PSY . World Journal of Surgery.2007;31(5). CrossRef
- Human epidermal growth factor receptor 2 status of breast cancer patients in Asia: Results from a large, multicountry study Nirmala Pathmanathan , Jing-shu Geng , Wencai Li , Xiu Nie , Januario Veloso , Julie Hill , Philip McCloud , Michael Bilous . .
- Tamoxifen as initial sole treatment of localised breast cancer in elderly women: a pilot study Preece PE , Wood RA , Mackie CR , Cuschieri A. British Medical Journal (Clinical Research Ed.).1982;284(6319). CrossRef
- Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial Ellis MJ , Coop A, Singh B, Mauriac L, Llombert-Cussac A, Jänicke F, Miller WR , Evans DB , Dugan M, Brady C, Quebe-Fehling E, Borgs M. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2001;19(18). CrossRef
- UK and USA breast cancer deaths down 25% in year 2000 at ages 20-69 years Peto R, Boreham J, Clarke M, Davies C, Beral V. Lancet (London, England).2000;355(9217). CrossRef
- Early Breast Cancer Trialists' Collaborative Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomized trials Lancet.2000;355:1757-1770.
- Early Breast Cancer Trialists' Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomized trials Lancet.1998;352:930-942.
- Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomized trials Lancet.1998;351:1451-1467.
- Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy Haque W, Verma V, Hatch S, Suzanne Klimberg V, Brian Butler E, Teh BS . Breast Cancer Research and Treatment.2018;170(3). CrossRef
- Poor prognosis of single hormone receptor- positive breast cancer: similar outcome as triple-negative breast cancer Bae SY , Kim S, Lee JH , Lee H, Lee SK , Kil WH , Kim SW , Lee JE , Nam SJ . BMC cancer.2015;15. CrossRef
- Risk factors for locoregional recurrence after postmastectomy radiotherapy in breast cancer patients with four or more positive axillary lymph nodes Li Q, Wu S, Zhou J, Sun J, Li F, Lin Q, Guan X, Lin H, He Z. Current Oncology.2014;21(5). CrossRef
- Breast Cancer Res Treat Bantema-Joppe EJ , van den Heuvel ER , de Munck L, et al . 2013;140:577.
- Locally advanced breast cancer treated with neoadjuvant chemotherapy and adjuvant radiotherapy: a retrospective cohort analysis Klein J, Tran W, Watkins E, Vesprini D, Wright GC , Look Hong NJ , Ghandi S, Kiss A, Czarnota GJ . BMC cancer.2019;19(1). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2023
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times
- Supplementary file downloaded - 0 times