Promoter Hypermethylation of ATG16L2, TFAP2A, EBF2, Calcitonin, ABL1 Kinase Domain T315I Mutation Association with Imatinib Therapy Resistance and Median Survival in CML Patients of North-East India
Download
Abstract
Background and Objectives: Chronic myeloid leukemia (CML) initiation and progression is regulated by epigenetic and genetic alterations. Imatinib therapy resistance in CML patients is important clinical issue. To understand association of kinase domain mutation and promoter hypermethylation of genes with imatinib therapy resistance hold significance in CML patients of North-East India. This study is a hospital based cross sectional study.
Methods: A total of Sixty three (n=63) CML patients undergone imatinib mesylate were enrolled for the study. ABL kinase domain T315I mutation was analyzed by Allele specific- PCR (AS-PCR) and confirmed by sequencing. Promoter hypermethylation was analyzed by Methylation specific PCR (MS-PCR). The Chi square test and Fisher exact test used in SPSS ver19.
Results: Tyrosine Kinase domain mutation T315I was found in 30.1% (19/63). The promoter hypermethylation of Calcitonin, ATG16L2, TFAP2A, EBF2 gene was detected in 42.9% (n=27), 28.6% (n=18), 38.1% (n=24), 27% (n= 17) CML patients respectively. Median relapse free survival was 21 months and statistically significant for CML patients without T315I mutation compared to patients with T315I mutation who has 12 months median relapse free survival (p=0.005). Median survival was 19 months for patients without EBF2 promoter hypermethylation and also statistically significant compared to CML patients with promoter hypermethylation (12 months) (p=0.026).
Interpretation and Conclusions: We conclude that among imatinib resistant CML patients of North- East India harbouring T315I mutation of ABL1 Kinase domain and promoter hypermethylation of EBF2 gene have significantly lower median relapse free survival.
Introduction
In chronic myeloid leukemia (CML), Philadelphia chromosome is the most common cytogenetic abnormality arising due to reciprocal translocation t (9; 22) (q34; q11) between the Abelson leukemia virus (ABL) oncogene on chromosome 9 and the breakpoint cluster region (BCR) gene on chromosome 22 [1]. BCR-ABL fusion oncogene through signaling pathways as STAT, MAPK/ERK cascades, PI3K/AKT/mTOR plays an important role in the pathogenesis of CML [2]. BCR-ABL tyrosine kinase inhibitor (TKI), imatinib mesylate (IM), is first line of treatment in the CML patients [3]. However patients have shown primary resistance and significant number of patients developed acquired/secondary resistance to IM treatment [4]. Resistance to imatinib therapy may be affected by BCR-ABL-dependent and /or BCR-ABL- independent mechanisms [5].
CML progression towards blast crisis requires accumulation of epigenetic and genetic alterations along with BCR-ABL1 translocation [6]. Presences of point mutations threonine-to-isoleucine exchange at amino acid position 315 (T315I) in the BCR-ABL1 kinase domain is found as the most prevalent mechanism along with other ABL kinase domain mutations of resistance to imatinib [7,8]. Aberrant DNA methylation play an important role in CML progression where gene promoter hypermethylation predominates compared to hypomethylation. Epigenetic deregulation of expression of various genes act as crucial drivers in promoting Leukemic stem cells (LSC) self-renewal potential leads to advanced phase of CML [9]. In CML patients with BCR promoter hypermethylation have better response to IM therapy [10].
An autophagy related gene, ATG16L2 (autophagy related 16-like 2) promoter hypermethylation resulted in significantly decreased major molecular response (MMR) to IM treatment [11]. Enhanced frequency of promoter hypermeythylation of TFAP2A (transcription factor AP2 alpha) and EBF2 (early B cell factor 2) was observed in CML blast crisis phase patients compared to chronic phase [11]. Calcitonin gene promoter hypermethylation was found to be associated with progression of CML disease phase [12].
There is lack of data about T315I mutations and promoter hypermethylation of ATG16L2, TFAP2A, EBF2, Calcitonin genes in CML patients of North-East India with reference to imatinib therapy resistance.
Materials and Methods
Diagnosed CML patients treated with imatinib mesylate (IM) as frontline treatment were enrolled for this study with informed written consent. The study was reviewed and approved by institutional ethics committee (BBCI/IEC-22/03) and conducted from Oct 2014 to Sep 2017. The patients were followed for at least 12 months with imatinib dose of 400 mg per day.
Patient Clinical assessment
For each CML patient, diagnosis was confirmed by hematological as well as BCR-ABL1 fusion gene molecular analysis. The response to imatinib therapy was evaluated based on the assessment of hematologic and molecular responses according to NCCN recommendations [7].
Extraction of genomic DNA from Whole blood
Whole blood genomic DNA was extracted from leukocytes using QIAamp DNA Blood Mini Kit (Qiagen,Germany) following the manufacturer’s instructions. The quantity and quality of the DNA was assessed by Biophotometer plus (Eppendorf, Germany) at 260, 280nm wavelength and 1% agarose (Thermoscientific, USA) gel electrophoresis (SCIEPLAS, UK).
Isolation of total RNA and preparation of cDNA
1 ml peripheral blood or Bone marrow aspirate used with QIAamp RNA Blood Mini Kit (Qiagen, Germany) following the manufacturer’s instructions. 1µg of Total RNA was used for cDNA synthesis using High-Capacity cDNA Reverse Transcription Kits with RNase inhibitor (Thermofisher Scientific, USA) following the manufacturer’s instructions.
Quantitative BCR-ABL1 transcripts quantification
BCR ABL1 major p210 b2a2 or b3a2 transcripts quantification was done using ipsogen® BCR-ABL1 Mbcr IS-MMR kit ( Qiagen, Germany) in peripheral blood samples or bone marrow of chronic myeloid leukemia (CML) patients as per the manufacturer’s protocol (BioRad, USA).
Allele specific-PCR (AS-PCR) to Detect T315I mutation
Allele specific-PCR (AS-PCR) for detection of T315I mutation in imatinib resistant CML patient’s samples was performed using previously described PCR primer sequences [13].
AS-PCR performed on Proflex Thermal Cycler (Thermoscientifc, USA) in 25μL mixture of 2.5μL cDNA template, 12.5μL EmeraldAmp MAX PCR Master mix (Takara, Japan) 4 pmol each of Mutant T315I & Wild type primers, and 2pmol of β-actin primers. Thermal profile of reaction- 1 cycle of 95°C 5min, followed by 35 of cycles 94°C 30 sec, 50°C 40 sec, 72°C 1 min, and 1 cycle of 72°C 7 min. PCR products size were: 158 bp T315I mutant, 374 bp T315WT, and 540 bp β-actin (Figure 1).
Figure 1. Agarose Gel Image AS-PCR Showing Amplification of T315I Mutation in CML Samples (band at 158 bp) and wild type (band at 374) (M, 100 bp ladder; NTC, negative control).
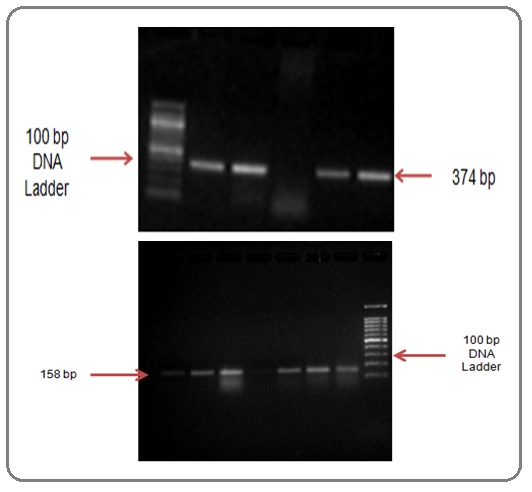
The PCR products were resolved on a 2.5% agarose gel (ThermoScientific, USA) stained with ethidium bromide (0.625mg/ml, ThermoScientific, USA) to image in GelDOC XR system (BioRad). The positive control for T315I mutation and Negative control were used.
Detection of BCR-ABL KD mutation by DNA sequencing
The primary PCR step was performed using a pair of primers designed to cover BCR-ABL gene. Two micrograms of cDNA template were amplified in a total volume of 20 μL with the following constituents, 0.3 U of Maxima Hotstart Taq DNA polymerase (ThermoFisher Scientific, USA), 10X Maxima Hotstart buffer, 1.5 mmol/L of MgCl2, 0.2 mmol/L of each dNTPs, 10 pmol of each primers (Forward primers: B2A F 5’-ACAGCATTCCGCTGACCATCAATAAG-3’ and Reverse primer: B2AR5’- ATGG TCCAG AG G ATCG CTCTCT-’3) as previously described. A secondary PCR step for amplification of Kinase Domain amino acid codon 206-428 using the internal two primer pairs, were designed to amplify two partially overlapping fragments consisting of fragment 1 Forward primers: ABL1F (5’-TGGTTCATCATCATTCAACGGTGG-3’) and Reverse primers: ABL1R ( 5 ’-TCTGAGTGGCCATGTACAGCAGC-’ 3 ), and fragment 2 Forward primers: ABL2F (5’-TCATGACCTACGGGAACCTC-3’) and Reverse primers: ABL2R (5’-ATACTCCAAATGCCCAGACG-’3) as previously described. For sequencing, the PCR products were purified using the Qiaquick PCR purification kit (Qiagen, USA) following the manufacturer’s protocol. Sequencing with forward and/or reverse primers in secondary PCR steps was carried out by the ABI3730XL DNA analyzer (Applied Biosystems, USA) using ABI BigDye terminator cycle sequencing kits (Applied Biosystems, USA). The results were compared with the WT ABL1 (accession no. NM_005157.3).
Promoter methylation analysis by MS-PCR
2μg of genomic DNA was modified by EpiTect Bisulfite Kit (Qiagen,Germany) as per the manufacturer’s instructions. The EpiTect Bisulfite Kit protocol in brief comprises steps: bisulfite-mediated conversion of unmethylated cytosines; binding of the converted single-stranded DNA to the membrane of an EpiTect spin column; washing; desulfonation of membrane-bound DNA; washing of the membrane-bound DNA to remove desulfonation agent; and elution of the pure, converted DNA from the spin column. The eluted DNA was used for the analysis of DNA methylation by Methylation Specific PCR (MS-PCR).
Methylation specific PCR:A dual primer pair (specific for methylated and unmethylated DNA sequences) was used to ascertain the relative amounts of methylated and unmethylated strands status for genes – ATG16L2, Calcitonin, TFAP2A and EBF2. In 25μL mixture of 3μL Converted DNA template, 12.5μL EmeraldAmp MAX PCR Master mix (Takara, Japan), respective genes methylated and unmethylated Forward primers and Reverse primers 4 pmol were used. Primer sequence, annealing, PCR products size details are in mentioned in (Table 1).
| Gene | Forward (5’-3’) | Reverse (5’-3’) | Ta | Size (bp) |
| Calcitonin - M | TTTTTAAGTAGTCGGGATTATAGGC | ATTTATAATAAACCGAACGCGAT | 60 | 190 |
| Calcitonin – U | TTTAAGTAGTTGGGATTATAGGTGT | AAATTTATAATAAACCAAACACAAT | 60 | 190 |
| ATG16L2 M | AAGGTTATTGATAGGTAGAGTTCGG | AAAACAAAAAAATAACCACTACGAA | 62 | 147 |
| ATG16L2 U | AAGGTTATTGATAGGTAGAGTTTGG | AAAACAAAAAAATAACCACTACAAA | 62 | 147 |
| EBF2 M | GTTTTTTTCGGTGTTTAGTTGC | ATACAACCATACAAAACTCTCCGTT | 58 | 112 |
| EBF2 U | TGTGTTTTTTTTGGTGTTTAGTTGT | ATACAACCATACAAAACTCTCCATT | 58 | 115 |
| TFAP2A M | TTTATCGTCGGTAGTTAGTATTTTGC | TCCAAAACATTTTCATAAATCGAC | 56 | 108 |
| TFAP2A U | ATTGTTGGTAGTTAGTATTTTGTGT | TTCCAAAACATTTTCATAAATCAAC | 56 | 106 |
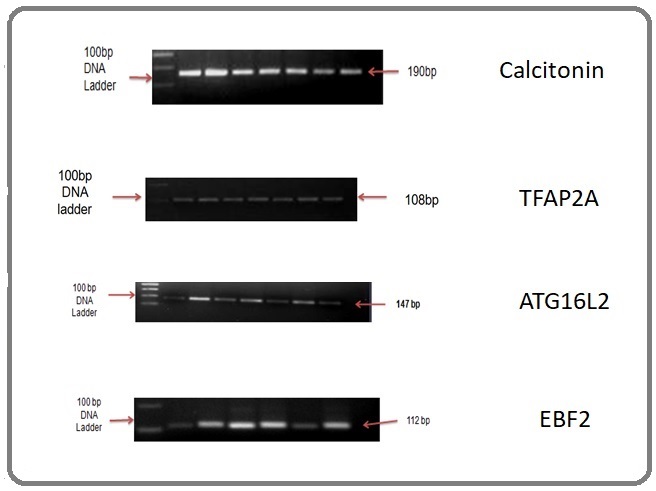
The thermal profile was as follows: 95°C 10min, followed by 40 cycles of denaturation at 95°C 20 sec, annealing (Table 2) 45 sec, extension at 72°C 45 sec, and final extension at 72°C 5 min. PCR products of ATG16L2, Calcitonin, TFAP2A and EBF2 were 147bp, 190bp, 108bp and 112bp respectively.
| T315I mutation & promoter hypermethylation | HR | P-Value | 95.0% CI for Exp(B) | ||
| Lower | Upper | ||||
| T315I Mutation | Negative | 1 | |||
| Positive | 2.181 | 0.013* | 1.181 | 4.029 | |
| Calcitonin | Negative | 1 | |||
| Positive | 1.301 | 0.379 | 0.724 | 2.337 | |
| ATG16L2 | Negative | 1 | |||
| Positive | 1.437 | 0.242 | 0.783 | 2.638 | |
| TFAP2A | Negative | 1 | |||
| Positive | 1.431 | 0.234 | 0.793 | 2.581 | |
| EBF2 | Negative | 1 | |||
| Positive | 2.014 | 0.033* | 1.056 | 3.839 |
*Statistically Significant
The PCR products were resolved in 2.5% agarose gel (Figure 2).
Figure 2. Agarose Gel Image for MS PCR of Calcitonin Gene (190bp methylated), TFAP2A Gene (108 bp methylaed), ATG6L2 Gene (147 bp methylated), EBF2 Gene (112bp mehylated).
Statistical analysis
The data acquired during the period was tested by appropriate statistical methods for significance using SPSS ver19 software. The Chi square test and Fisher exact test used to test the statistical significance of T315I mutation and promoter hypermethylation ATG16L2, Calcitonin, TFAP2A and EBF2 with imatinib resistance. Where appropriate, 95% confidence intervals (CI) was computed and the level of statistical significant test was set at 0.05. For median survival comparison, Kaplan–Meier curves were plotted and compared using the log-rank test.
Results
Clinicopathological characteristics
At the time of CML diagnosis, patients in Clinical Phase were as follows- 81% (n=51) chronic, 11.1% (n=7) accelerated and 7.9% (n=5) blast phase. Imatinib treatment responding patients (till last follow up) were 28.6% (n=18) and Imatinib treatment resistant were 71.4% (n=45).
Response To Imatinib Treatment with Respect To T315I Mutation and Promoter Hypermethylation
Among CML patients, tyrosine Kinase domain mutation T315I was found in 30.1% (19/63). The promoter hypermethylation of Calcitonin, ATG16L2, TFAP2A, EBF2 genes were detected in 42.9% (n=27), 28.6% (n=18), 38.1% (n=24), 27% (n= 17) CML patients respectively.
Response To Imatinib Treatment with Respect To T315I Mutation and Promoter Hypermethylation
Among imatinib Resistant CML patients, T315I gene mutation was found to be 40.0% (18/45). Promoter hypermethylation of ATG16L2- 35.5% (16/45), TFAP2A- 46.6% (21/45), Calcitonin- 51.1 %( 23/45) and EBF2- 33.3% (15/45). T315I mutation in CML patients with Chronic phase- 23.5 %( 12/51), Accelearted phase- 57.1% (4/7) and Blast phase-60% (3/5).
Secondary imatinib resistance was 73.7% in patients with mutation of T315I gene (14/19) whereas promoter hypermethylation of Calcitonin-74.1% (20/27), ATG16L2-77.8% (14/18), TFAP2A-75.0% (18/24) and EBF2-70.6% (12/17) in patients with secondary imatinib resistance. The resistance frequency to imatinib treatment was found to be statistically significant and 2 times higher in patients with T315I mutations (HR=2.181, CI 1.18-4.03, P=0.013) and EBF2 promoter hypermethylation (HR=2.014, CI 1.06-3.84, P=0.033). A moderate level of increasing failure rate but not statistically significant was also observed for the patients with hypermethylation in calcitonin (HR=1.301, CI 0.724-2.337, P=0.379), ATG16L2 (HR =1.437, CI 0.783- 2.638, p=0.242) and TFAP2 (HR=1.431, CI 0.793-2.581, P=0.234) genes (Table 2).
Association of Relapse Free Survival with T315I Mutation and Promoter Hypermethylation
Median relapse free survival was 21 months and statistically significant for CML patients without T315I mutation compared to patients with T315I mutation who has 12 months median relapse free survival (p=0.005). Median survival was 19 months for patients without EBF2 promoter hypermethylation and also statistically significant compared to CML patients for patients with promoter hypermethylation (12 months) (p=0.026). Patients with Calcitonin, ATG16L2, TFAP2A promoter Hypermethylation have reduced relapse free survival compared to patients without promoter hypermethylation but this association was not statistically significant (Table 3, Figure 3).
| Median Estimate in Months | 95% Confidence Interval | P-Value | ||
| Overall Relapse Free Survival | Lower Bound | Upper Bound | ||
| 17 | 12.86 | 21.14 | ||
| T315I Mutation | ||||
| Negative | 21 | 13.45 | 28.55 | 0.005* |
| Positive | 12 | 8.89 | 15.11 | |
| Calcitonin Promoter Hypermethylation | ||||
| Negative | 19 | 14.6 | 23.4 | 0.367 |
| Positive | 12 | 7.28 | 16.72 | |
| ATG16L2 Promoter Hypermethylation | ||||
| Negative | 19 | 13 | 25 | 0.229 |
| Positive | 14 | 6.02 | 21.98 | |
| TFAP2A Promoter Hypermethylation | ||||
| Negative | 19 | 15.29 | 22.71 | 0.221 |
| Positive | 12 | 6.44 | 17.56 | |
| EBF2 Promoter Hypermethylation | ||||
| Negative | 19 | 13.61 | 24.39 | 0.026* |
| Positive | 12 | 7.46 | 16.54 |
*Statistically Significant
Figure 3. Median Relapse Survival Time with Respect to T315I Mutation and Promoter Hypermethylation of Caleitotion, ATG16L2, TFAP2A and EBF2.
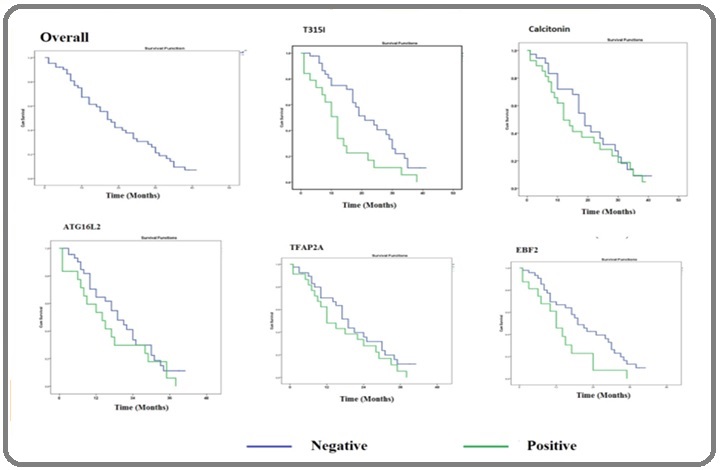
Discussion
Imatinib provides an effective and durable therapy for CML, as shown in 6 year follow-up of phase III International Randomized IRIS study that this agent induced complete hematologic response (CHR) in the majority (98%) of newly diagnosed patients in chronic phase of the disease and Complete Molecular Response (CMR) in about 87% of patients [3]. According to the results in the 8th year of the IRIS study, the administration of imatinib could not be continued because over long period about one third of patients with CML-Chronic phase developed resistance or intolerance [14].
In present study among the resistant 45 patients, 3 have primary resistance and remaining 42 patients developed secondary resistance in the due course of imatinib therapy. We found secondary resistance was high among the patients with mutation of T315I (73.7%, 14/19). An Indian study found resistance or relapses in 38% of the CML patients [15]. Another Indian study shown 32.2% patient has detectable kinase domain mutations. The most common mutation was T315I in 31% patients [16]. Primary and secondary resistance was very high in the patients registered as imatinib treated old cases [17]. It was also shown 33% CML patients had not achieved optimal IM response [18]. It has been reported that up to 40% of patients with CP CML become resistant to imatinib [19,20]. Cortes et al. showed frequency of BCR-ABL1 mutations 54% (61/112) out of which 16% were T315I mutation [21]. Horvat et al found T315I mutation in 17% of CML patients and concluded T315I mutation detection as essential for treatment of choice in T315I mutation carriers for stem-cell transplantation [22]. A Korean study reported frequency of BCR-ABL1 mutations 63% (70/111) out of which 24% were T315I mutation [23]. A retrospective study has identified T315I mutation in 50% of cases in imatinib resistant CML patients [24]. T315I mutation detection in CML patients is clinically useful to optimize outcomes for treatment failure or suboptimal response to imatinib [25]. Yap et al. found 22.7% IM resistant CML patients harbor T315I mutation [26]. The variations in frequency of T315I in different population may be due the differences in patient characteristics, methodology of detection of T315I mutation etc.
Our study data shows that median survival was poor in patients with T315I mutation which is in agreement of the earlier study observations with variation in survival status. Latin American LeukemiaNet (LALNET) study cohort found that CML patients with BCR-ABL1 mutations and particularly T315I mutations had lower Progression-free (PFS) and overall survival (OS) (43% vs. 65% and 47% vs. 72%) at 5 years period [27]. Regardless of the stage of the CML, median survival was poor in patients with BCR-ABL1 kinase domain mutations [28, 29].
Dunwel et al demonstrated first time that ATG16L2 was methylated in 69 % of CML patients. Patients with Methylated ATG16L2 had significant reduction in major molecular response (MMR) at 12 or 18 month. Promoter hypermethylation of TFAP2A and EBF2 more prominent in blast crisis (BC) compared to chronic phase (CP) [11]. Nelkin et al found that aberrant DNA methylation of Calcitonin was 6% in chronic phase, 63% in accelerated phase and 92% in blast crisis [12]. Our study has shown reduced median survival in patients with methylated EBF2, ATG16L2, TFAP2A, Calcitonin compared to unmethylated patients samples.
In present study we found that T315I mutation and promoter hypermethylation of four genes studied together accounted for approx 73% of genetic plus epigenetic alterations in imatinib resistant patients. EBF promoter hypermethylation has significant relationship with reduction in median survival in imatinib resistant patient in our study.
Apart from ABL1 kinase domain mutations other mechanisms can cause resistance in CML. Immature leukemic cells (stem cells) may exhibit intrinsic (BCR/ABL-independent) resistance [30]. Four second generation ABL TKI dasatinib, nilotinib, bosutinib and bafetinib were developed to overcome imatinib-resistance. Identification of type of BCR-ABL1 mutation in TKI-resistant patients is very important [31]. According to NCCN guidelines, mutational analysis should be performed in patients with disease progression after first-line TKI treatment. But no second generation BCR-ABL TKIs can inhibit T315I mutation positive CML. So a third-generation BCR-ABL1 TKI ponatinib is already used in clinic [32]. A lot of novel agents which can override BCR-ABL1 KD mutations including T315I are being developed but their prohibitive cost remain limiting factor [33]. Measuring plasma imatinib level has been suggested as tool for drug compliance assessment [34]. Molecular monitoring for BCR-ABL1 along with ABL1 kinase domain mutational analysis before administering second generation or third generation TKIs help in better management of patients harboring these mutations. Resistance against imatinib therapy is becoming important clinical problem in the treatment of CML patients of North-East India.
In our study many of the resistant patients have shown higher frequency of T315I mutation and promoter hypermethylation of Calcitonin, ATG16L2, TFAP2A, EBF2. Imatinib treatment resistant patients without T315I mutation and promoter hypermethylation of Calcitonin, ATG16L2, TFAP2A, EBF2 may have other kinase domain mutations or promoter hypermethylation which were not analyzed by us. Promoter hypermethylation status of EBF2 along with calcitonin, ATG16L2 and TFAP2 genes may also help in imatinib therapy response assessment in North East Indian CML patients. Detection of T315I mutations in CML patients of North-East India may be valuable during imatinib therapy.
In conclusion, we would like to conclude from current study data that imatinib resistant CML patients harbouring T315I mutation of ABL1 Kinase domain and promoter hypermethylation of EBF2 gene of North- East India has significantly lower median relapse free survival. Future studies may be required in large cohort of CML patients of North-East India to assess the impact of other mutations of ABL1 kinase domain and BCR- ABL1 independent mechanism which may be leading to imatinib resistance.
Conflicting Interest
(If present, give more details): All Authors declare there exist no competing interest with regard to present study.
Acknowledgements
We are grateful to Indian Council of Medical Research for financial assistance (Grant No. 5/7/1163/2014-RCH). We are thankful to Dr Nikhil Patkar, Department of Hematopathology, Tata Memorial Centre, Mumbai, for providing T315I mutation positive control.
References
- Localization of the c-ab1 oncogene adjacent to a translocation break point in chronic myelocytic leukaemia Heisterkamp N., Stephenson J. R., Groffen J., Hansen P. F., Klein A., Bartram C. R., Grosveld G.. Nature.1983;306(5940). CrossRef
- Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia Steelman L. S., Abrams S. L., Whelan J., Bertrand F. E., Ludwig D. E., Bäsecke J., Libra M., et al . Leukemia.2008;22(4). CrossRef
- Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia Druker BJ , Guilhot F, O'Brien SG , Gathmann I, Kantarjian H, Gattermann N, Deininger MWN , et al . The New England Journal of Medicine.2006;355(23). CrossRef
- Molecular monitoring Soverini S, Rosti G, Baccarani M, Martinelli G. Current hematologic malignancy reports.2014;9(1). CrossRef
- Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy Rossari F, Minutolo F, Orciuolo E. Journal of hematology & oncology.2018;11(1). CrossRef
- Chronic myeloid leukemia: mechanisms of blastic transformation Perrotti D, Jamieson C, Goldman J, Skorski T. The Journal of clinical investigation.2010;120(7). CrossRef
- NCCN Guidelines Insights: Chronic Myeloid Leukemia, Version 1.2017 Pallera A, Altman JK , Berman E, Abboud CN , Bhatnagar B, Curtin P, DeAngelo DJ , et al . Journal of the National Comprehensive Cancer Network: JNCCN.2016;14(12). CrossRef
- Detection of ABL1 kinase mutations in Philadelphia-positive patients exhibiting an inadequate molecular response using restriction fragment mass polymorphism and its clinical significance: a single-center experience in Korea Cho Yu , Kim So , Chi Hs , Park Sj , Jang S, Park Cj , Seo Ej , et al . International journal of laboratory hematology.2013;35(6). CrossRef
- Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival Hamilton A, Helgason gv , Schemionek M, Zhang B, Myssina S, Allan EK , Nicolini FE , et al . Blood.2012;119(6). CrossRef
- Increased BCR promoter DNA methylation status strongly correlates with favorable response to imatinib in chronic myeloid leukemia patients Youngil Koh , Dae-Young Kim , Sung-Hyo Park , Hyang-Min Byun , Inho Kim , Sung-Soo Yoon , et al . Oncology Letters.2011;2(1). CrossRef
- A genome-wide screen identifies frequently methylated genes in haematological and epithelial cancers Dunwell T, Hesson L, Rauch TA , Wang L, Clark Re , Dallol A, Gentle D, et al . Molecular cancer.2010;9. CrossRef
- Abnormal methylation of the calcitonin gene marks progression of chronic myelogenous leukemia Nelkin Bd , Przepiorka D, Burke Pj , Thomas ED , Baylin SB . Blood.1991;77(11).
- A single-tube allele specific-polymerase chain reaction to detect T315I resistant mutation in chronic myeloid leukemia patients Wongboonma W, Thongnoppakhun W, Auewarakul C. Journal of hematology & oncology.2011;4. CrossRef
- International Randomized Study of Interferon Vs STI571 (IRIS) 8-Year Follow up: Sustained Survival and Low Risk for Progression or Events in Patients with Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CML-CP) Treated with Imatinib Deininger M, O’Brien SG , Guilhot F, et al . Blood (ASH Annual Meeting Abstracts).2009;114:1126.
- Report of chronic myeloid leukemia in chronic phase from Tata Memorial Hospital, Mumbai, 2002-2008 Parikh P. Indian Journal of Medical and Paediatric Oncology : Official Journal of Indian Society of Medical & Paediatric Oncology.2013;34(3). CrossRef
- Kinase domain mutations and responses to dose escalation in chronic myeloid leukemia resistant to standard dose imatinib mesylate Rajappa S, Mallavarapu KM , Gundeti S, Paul TR , Jacob RT , Digumarti R. Indian Journal of Medical and Paediatric Oncology: Official Journal of Indian Society of Medical & Paediatric Oncology.2013;34(3). CrossRef
- Report of chronic myelogenous leukemia in chronic phase from, Asian Institute of Oncology, Mumbai, 2002-2010 Bansal S, Advani SH . Indian Journal of Medical and Paediatric Oncology: Official Journal of Indian Society of Medical & Paediatric Oncology.2013;34(3). CrossRef
- Management of imatinib-resistant patients with chronic myeloid leukemia Bhamidipati PK , Kantarjian H, Cortes J, Cornelison AM , Jabbour E. Therapeutic advances in hematology.2013;4(2). CrossRef
- Dasatinib early intervention after cytogenetic or hematologic resistance to imatinib in patients with chronic myeloid leukemia Quintás-Cardama A, Cortes JE , O'Brien S, Ravandi F, Borthakur G, Liu D, Bleickardt E, Chen T, Kantarjian HM . Cancer.2009;115(13). CrossRef
- Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial Mahon F, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, Legros L, Charbonnier A, Guerci A, Varet B, Etienne G, Reiffers J, Rousselot P. The Lancet. Oncology.2010;11(11). CrossRef
- Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors Cortes J, Jabbour E, Kantarjian H, Yin CC , Shan J, O'Brien S, Garcia-Manero G, et al . Blood.2007;110(12). CrossRef
- Clinical significance of T315I ABL kinase domain mutation detection in patients resistant to imatinib mesylate therapy Horvat I, Antolic MR , Zadro R, Sertić D, Labar B. Biochemia Medica.2010;20(1). CrossRef
- Analysis of Bcr-Abl kinase domain mutations in Korean chronic myeloid leukaemia patients: poor clinical outcome of P-loop and T315I mutation is disease phase dependent Kim S, Kim D, Kim D, Goh H, Jang S, Lee J, Kim W, Kweon I, Park S. Hematological Oncology.2009;27(4). CrossRef
- Epidemiologic study on survival of chronic myeloid leukemia and Ph(+) acute lymphoblastic leukemia patients with BCR-ABL T315I mutation Nicolini Fe , Mauro Mj , Martinelli G, Kim Dw , Soverini S, Müller MC , Hochhaus A, et al . Blood.2009;114(26). CrossRef
- Prevalence of BCR-ABL T315I Mutation in Malaysian Patients with Imatinib-Resistant Chronic Myeloid Leukemia Mat Yusoff Y, Abu Seman Z, Othman N, Kamaluddin NR , Esa E, Zulkiply NA , Abdullah J, Zakaria Z. Asian Pacific journal of cancer prevention : APJCP.2018;19(12). CrossRef
- Primary imatinib resistance in chronic myeloid leukemia patients in a developing country: BCR-ABL kinase domain mutations or BCR-ABL independent mechanisms? Yap E, Tumian NR , Azma RZ , Sharifah NA , Salwati S, Hamidah NH , Elias MH , Wong CI . The Malaysian journal of pathology.2017;39(2).
- BCR-ABL mutations in chronic myeloid leukemia treated with tyrosine kinase inhibitors and impact on survival Pagnano KBB , Bendit I, Boquimpani C, De Souza CA , Miranda ECM , Zalcberg I, Larripa I, et al . Cancer Investigation.2015;33(9). CrossRef
- Characteristics and outcome of chronic myeloid leukemia patients with E255K/V BCR-ABL kinase domain mutations Naqvi K, Cortes JE , Luthra R, O'Brien S, Wierda W, Borthakur G, Kadia T, et al . International Journal of Hematology.2018;107(6). CrossRef
- Outcomes of 219 chronic myeloid leukaemia patients with additional chromosomal abnormalities and/or tyrosine kinase domain mutations Xue M, Cheng J, Zhao J, Zhang S, Jian J, Qiao Y, Liu B. International journal of laboratory hematology.2019;41(1). CrossRef
- Chronic myeloid leukemia stem cells possess multiple unique features of resistance to BCR-ABL targeted therapies Jiang X., Zhao Y., Smith C., Gasparetto M., Turhan A., Eaves A., Eaves C.. Leukemia.2007;21(5). CrossRef
- Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants Redaelli S, Piazza R, Rostagno R, Magistroni V, Perini P, Marega M, Gambacorti-Passerini C, Boschelli F. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2009;27(3). CrossRef
- AP24534, a Pan-BCR-ABL Inhibitor for Chronic Myeloid Leukemia, Potently Inhibits the T315I Mutant and Overcomes Mutation-Based Resistance O’Hare T, Shakespeare WC , Zhu X, Eide CA , Rivera VM , Wang F, Adrian LT , et al . Cancer cell.2009;16(5). CrossRef
- BCR-ABL Point Mutations and TKI Treatment in CML Patients Kimura S, Ando T, Kojima K. J Hematol Transfus.2014;2:1022.
- Utility of the trough plasma imatinib level monitoring at two time points in patients with the chronic myeloid leukemia-chronic phase Sharma SV , Kumar S, Vijayakumar AR , Seth T, Mishra P, Mahapatra M, Sazawal S, Velpandian T, Saxena R. Journal of Cancer Research and Therapeutics.2014;10(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2023
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times