An Audit of Histopathology Reports of Invasive Breast Carcinomas with Reference to Adequacy and Amendments
Download
Abstract
Background: Periodic auditing of histopathology report (HPR) of breast cancer, monitors compliance and increases the standards of reporting. This study was done to audit the completeness of breast cancer HPR in accordance with the CAP protocol and to analyse the amendments in tumour summaries.
Methods: Retrospective review of HPR of invasive breast carcinoma resections from 2016-2020. Core (CE) and non-core elements (NCE) evaluated using CAP protocols. Outcome measures analyzed: (i) overall report completeness for CE (ii) element specific completeness for all reports. Amendments were reviewed.
Results: Breast cancer reports included 246 resections. Most common histological variant was ductal type, NOS. Overall completeness was seen in 87%. Biomarker status was available in 83.1%. Reporting format was synoptic-like structured format. CAP protocol underwent five revisions between 2016-2019 with shifting of parameters between CE and NCE. Adequately represented CE: procedure, tumour size & type, Nottingham score, grade, stage, DCIS, margin status, lymph nodes and lymphovascular invasion. Less commonly reported CE: laterality, extent of involvement, size of largest metastatic deposit and extranodal extension. Frequently reported NCE: LVI, architecture/grade of DCIS, findings in adjacent breast. Biomarker reporting and treatment effect was complete in all reports. Amended reports were 4.8%. Majority were transcriptional errors.
Conclusion: Common CE were satisfactorily represented in majority. Reasons for deficiencies were frequent change in CAP protocols and flexibility in synoptic-like structured format over synoptic format. The study underscores the need for periodic auditing of breast cancer summaries to monitor gaps in CE reporting. Synoptic reporting with additional free text field will improve compliance and overcome deficiencies.
Introduction
Breast cancer is the most common cancer amongst Indian women, as per the report from the National cancer registry programme, India [1]. It is also the leading cause of mortality related to cancer, according to International Agency for Research on Cancer (IARC) statistics 2020 [2]. According to statistics released by World Health Organization (WHO) in 2020, 2.3 million women has been diagnosed to have breast cancer with 685000 deaths globally [3].
Breast cancer originates from lining epithelial cells of the ducts (85%) or lobules (15%) [3]. Risk factors of the breast cancer includes obesity, excessive alcohol intake, family history of breast cancer, history of radiation exposure and postmenopausal hormone therapy. Certain inherited gene mutations like BRCA1 and BRCA 2, greatly increase the risk of breast cancer.
The pathology report is the starting point in most oncology cases and determines the basis of further management. The histopathology report carries all the essential information that is critical for management of patients with breast cancer. An adequate pathology report plays an important role in (i) assuring completeness of surgery, (ii) knowing the risk of recurrence, (iii) administering treatment tailored to tumour characteristics, (iv) maintaining cancer registries to draw guidelines for national cancer control policies.
The audit is a systematic examination to assess the quality of pathology reporting. Improving the quality and completeness of the reports not only helps the clinicians in patient management, but also benefits the pathologist by contributing for audit and cancer registry. In this audit, we attempt to assess the completeness of histopathology reports for breast cancer reporting using the College of American Pathologist (CAP) protocol as a standard.
Materials and Methods
The study was conducted by retrospective review of HPR of invasive breast carcinoma resections over a period of 5 years from January 2016 to December 2020. All types of mastectomies were included with and without axillary lymph node dissection, while needle core biopsies, FNAC and review cases were excluded. Ethical approval was obtained from institutional ethics committee (IEC ref no: 23/2022).
The core elements and non-core elements were evaluated using the CAP protocols and listed in Table 1.
| Core Elements | Non-Core Elements |
| Procedure | Tumor site |
| Specimen laterality | DCIS size |
| Tumor size | DCIS architecture |
| Histological type | DCIS architecture |
| Histological score: | DCIS nuclear grade |
| A) Glandular differentiation | |
| B) Nuclear pleomorphism | |
| C) Mitotic rate | |
| Overall grade | DCIS necrosis |
| DCIS | LCIS |
| Extent of involvement: | DCIS distance from other margin |
| A) Skin | |
| B) Nipple | |
| C) Skeletal muscle | |
| Margin status | LVI |
| Distance from closest margin | DLVI |
| Margins involved by tumor | Additional findings |
| Margins involved by DCIS | Microcalcifications |
| Total no. of lymph nodes | Clinical history |
| No. of sentinal lymph nodes | Comments |
| No. of lymph nodes with macro metastasis | |
| No. of lymph nodes with micro metastasis | |
| No. of lymph nodes with isolated tumor cells | |
| Pathologic staging | |
| Revised Core Elements | Revised Non-Core Elements |
| (Year of inclusion in CAP) | (Year of inclusion in CAP) |
| Tumor focality (2016- 2017) | Tumor focality (from 2017) |
| Closest margin (2016- 2019 v4.3) | Closest margin (from 2019 v4.3) |
| Ancillary studies (2016-2018) | Ancillary studies (from 2018) |
| DCIS distance from closest margin (2016-2017) | DCIS distance from closest margin (from 2017) |
| Treatment effect (from 2017) | Treatment effect (2016- 2017) |
| Size of largest lymph node deposit (from 2019 v4.2) | Size of largest lymph node deposit (2016- 2019 v4.2) |
| Extranodal extension (from 2019 v4.2) | Extranodal extension (2016- 2019 v4.2) |
CAP- College of American Pathologists; DCIS- Ductal carcinoma in-situ; LCIS- Lobular carcinoma in-situ; LVI- Lymphovascular invasion; DLVI- Dermal lymphovascular invasion
The outcome measures that were evaluated included the overall report completeness for all the core elements and element specific completeness for all the reports. The overall report completeness was estimated by the proportion of reports containing all the core elements applicable for the specified time period. The element specific completeness was estimated by the proportion of reports that contained the specified element in the specific time period.Since there are no established benchmark for adequate reporting of any specific element, we considered a element as adequately represented in the surgical pathology report if it featured in > 90% of reports [4].
The breast biomarker reporting which included hormone receptors and Her2/neu, was assessed for completeness as per the CAP/ASCO guidelines. The core data elements that were assessed in the reports included (i) status of the hormone receptor, (ii) status of the internal control, (iii) status of the external control. For Her2 by immunohistochemistry, expression status was noted as negative, equivocal or positive.
A review of amended reports was also performed and the errors that lead to amended reports were categorized as follows [5]:
Type A- Minor [no effect on patient care]: This included spelling errors, typographical error (in demographics), formatting errors.
Type B- Moderate [no/ minimal effect on patient care]: Errors like defects/ omissions in HPRs that would not change management plan. This included errors of omission (elements missed on synoptic/ impression), missed lymphovascular invasion or incorrect grading, change in tumour type due to additional IHC.
Type C- Major [major discrepancies in diagnosis that would change treatment plan]: This included wrong interpretation of the nature of tumour like benign versus malignant.
Statistical analysis
The study is broadly descriptive in nature. Descriptive statistics like frequency/ percentages, was used for analyzing the compliance of reporting the various parameters.
Results
Baseline details
Retrospective record review done between 2016-2020 showed a total of 246 cases received for histopathological analysis. Of these there were 146 mastectomies and 91 cases of lumpectomies. In 9 cases (4%) the type of procedure was not mentioned. The mastectomies included 22 simple mastectomies (9%), 2 radical mastectomies (1%), 4 palliative mastectomy (1.6%) and 118 cases of modified radical mastectomies (48%). The reporting format followed was synoptic like structured format, where a checklist was followed to ensure reporting of all elements. The layout of the report was like synoptic, but the reports were dictated like a narrative report. Synoptic like structured format was used in 243 resections and in 3 cases narrative format was used.
Clinical details
The age of presentation was divided as quartiles. The 3rd quartile (50-75 years) had the maximum number of cases (55%) and there were none in the first quartile. The next frequent age range was the second quartile with 40% cases and 3% were in the 4th quartile. The age was not available in 2% of cases. There were 3 cases (1%) of carcinoma breast in males. Bilaterality was noted in 1 case (0.4%) and multifocal tumours in 17 cases (6.9%). Left side breast carcinomas (48.8%) were more common than the right (34.6%).
Histopathological details
The most common histological type was invasive carcinoma of no special type (ductal), reported in 203 cases (82.5%), followed by mucinous carcinoma (40.7%). Table 2 shows the frequency of other histological types that were reported.
| Histologic type of tumor | Frequency (%) |
| Invasive ducal cacinoma, NOS | 203 (82.5) |
| Mucinous carcinoma | 10 (40.7) |
| Invasive papillary carcinoma | 6 (2.4) |
| Invasive carcinoma with apocrine differentiation | 5 (2.3) |
| Invasive lobular cacinoma | 5 (2.3) |
| Medullary carcinoma | 3 (1.2) |
| Metaplastic carcinoma | 3 (1.2) |
| Invasive carcinoma with neuroendocrine differentiation | 3 (1.2) |
| Mixed carcinoma | 2 (0.8) |
| Infiltrating tubular carcinoma | 1 (0.4) |
| Adenoid cystic carcinoma | 1 (0.4) |
The most common histological grade was Nottingham grade 2 (52%) and score 6 (29%). Most frequently reported pathological stage was T2 (53%) and N0 (39%). The frequencies of other grades and stages are given in Figure 1.
Figure 1. The Frequencies of Tumor Grades and Stages (G represents grade, S represents score, T represents T stage and N represents node status).
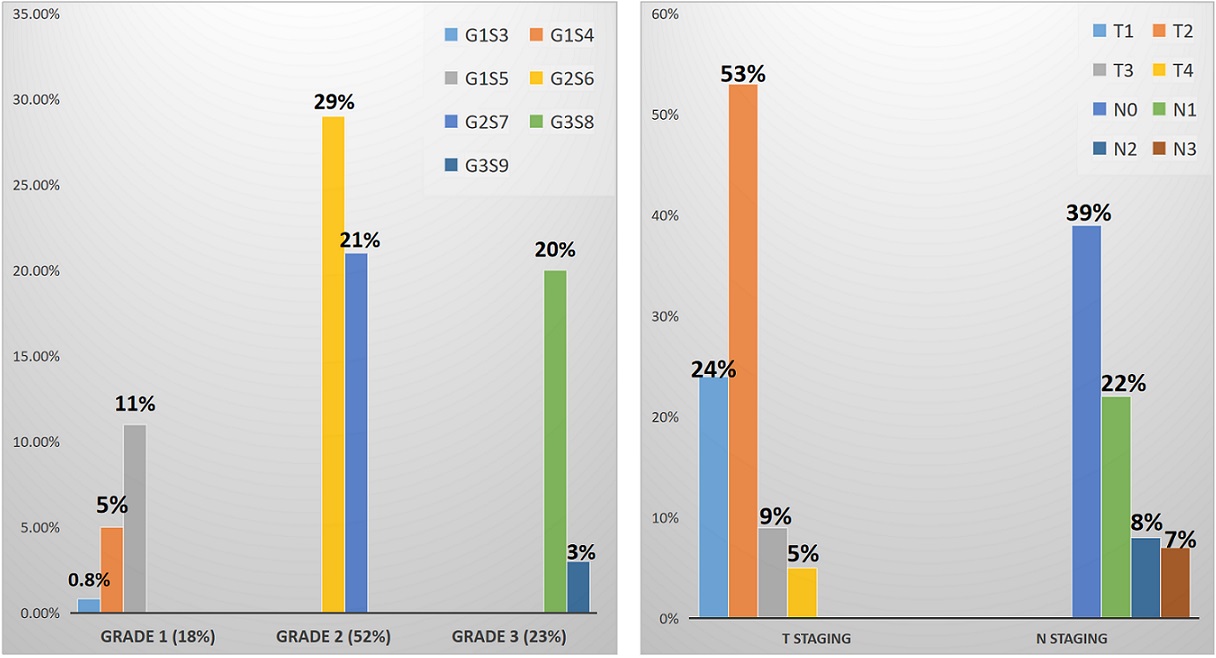
The median lymph node yield was 13 and lymphnode positivity was seen in 15 cases.
Hormone receptor status was available in 204 (83%) cases. Estrogen receptor positivity was seen in 67% of cases and Progesterone receptor positivity in 56% cases. Her2/neu was positive in 26%. Triple negativity was reported in 29% cases.
Neoadjuvant chemotherapy (NACT) was administered in 37 cases, out of which 22 cases had previous trucut breast biopsy reported in our institute.
Evaluation of the core elements studied for resection specimens
The CAP protocol for breast invasive resection and biopsy specimens has undergone five revision between 2016 -2020. A shift in elements between core (CE) and non core(NCE) was noted between the revisions. Parameters like tumour focality, ancillary studies and closest margin moved from CE to NCE in 2017, 2018 and 2019 v4.3 respectively. Similarly, treatment effect (2017), size of largest lymph node deposit and extranodal extension (2019 v4.2) moved from NCE to CE.
The overall completeness of the report was seen in 214 reports (87%), where all the core elements were adequately represented in the HPRs. When element specific completeness was studied, the most adequately represented CE was histological tumour type (99%), followed by histological grade, number of lymph nodes and margin status which were reported in 98% of the HRPs. The other adequately represented CE were: procedure, tumour size, Nottingham score and DCIS. The frequency of reporting of CE is shown in Figure 2.
Figure 2. The Frequency of Reporting of Core Elements.
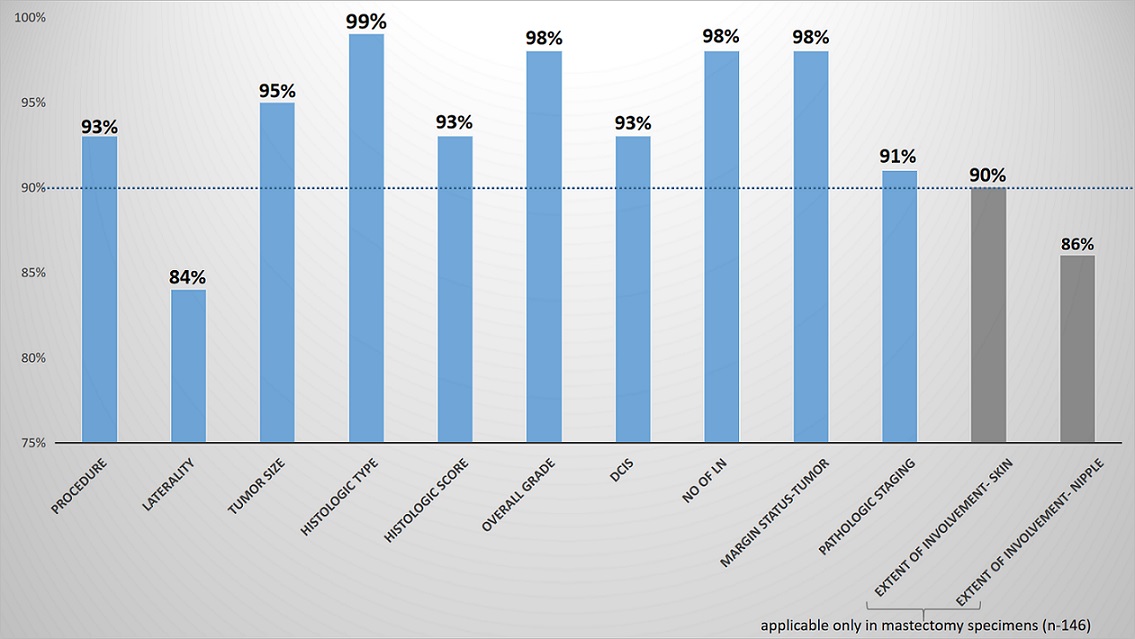
Treatment effect which came under CE from 2017 was reported in 100% of cases, whereas, size of largest lymph node deposit and extranodal extension which moved to CE from 2019 were reported only in 6.7% and 40% cases respectively. The other less commonly reported CE was laterality and extent of tumour involvement. Biomarker reporting and treatment effect was complete in all reports.
Evaluation of the non-core elements in resection specimens
We also analyzed the frequency of reporting NCE in resection specimens. Largely the NCE can be grouped under in-situ lesions and parameters related to them (DCIS and LCIS), vascular space invasion (LVI and DLVI), tumour site and focality (in mastectomy specimens). It was interesting to find that LVI and DCIS related parameters were most often reported in the HPRs in 95% and 92% respectively and parameters related to DCIS like architectural pattern and grade were also more often reported, although these elements do not form a part of the CE reporting in breast invasive reports.
Analysis of amended reports review
Of the 246 breast carcinoma cases, amended reports we sent in 12 cases (4.8%). The reasons for amendment were categorized and listed in Table 3.
| Type of error | Reason for amendment |
| A: Minor [no effect on patient care] | Transcriptional error (Spelling and related errors); n=3 Transcriptional error (Change in distance of tumor from different margins); n=1 Transcriptional errors (Omitted ancillary report); n=1 Transcriptional errors (Omitted histologic type); n=1 |
| B: Moderate [no/ minimal effect on patient care] | Transcriptional error (Change in pathological staging); n=5 |
| C: Major [major discrepancies in diagnosis that would change treatment plan] | Lab related error (Change in margin involvement by tumor after examination of deeper section); n=1 |
The most frequent error was type A (50%) and the least frequent was type C (1.83%). Type B errors contributed to 41.6%.
Discussion
The prevalence of breast carcinoma is high in Indian women and also one of the leading cause of cancer related mortality globally[2]. Appropriate patient management requires understanding of the tumour biology and behaviour by identifying histopathological parameters which reflect the same. Therefore, arises the need for a good histopathology report registering all the relevant parameters which will help not only in immediate patient management, but also in prognostication. This goal of this study was to audit the adequacy of HPR in breast carcinoma resection specimens using the CAP protocol as reference standard.
The most common procedure done in our setting was modified radical mastectomy and the most common age of presentation was the 3rd quartile (50-75 yr). In a study by Mamoon et al [6], the most common procedure type was modified radical mastectomy (94.1%) similar to the present study. The mean age of presentation was 30-39 yr (33.1%) and 41-50 yr (28.9%) in studies done by Yesufe et al [7] and Maturi et al [8] respectively, which was younger age group than our study. Bilaterality (0.4%) was rare in this study similar to that reported by Yesufe et al [7], where they reported a frequency of 0.2% of bilateral tumors. Male breast cancers were reported in 1% of the cases, which was similar to studies done by Adedayo et al (0.7%) [9], Yesufe et al (4.1%) [7] and Maturi et al (0.89%) [8]. The left side breast cancers were slightly more frequent in our population, whereas right sided breast carcinomas are more commonly reported in a study by Yesufe et al [7].
As most common histological type in our study was invasive carcinoma nos, ductal type, and the frequent grade reported was grade 2, which are similar across the world and reported by other authors [7,8,10]. The hormone receptor and Her 2 expression is known to vary amongst population groups. Majority of the tumours in our population were expressing hormone receptors, whereas other study done on Indian population by Maturi et al [8] showed low frequency of expression (ER positivity- 37.3%; PR positivity- 24.5%). A study by Daramola et al [10] on African population showed ER, PR positivity of 26.9% and 15.8% respectively. Her2/neu positivity was higher in our study (26%), when compared to a study done by Daramola et al [10] on African population (6.1%). The rate of triple negative breast cancer was 29% in our study, whereas it was 1.28% in an other study done on Indian population [8].
The audited reports show overall completeness of the report to be 87% , which is higher than the studies by Atanda et al [11], Adedayo et al [9], Kricker et al [12], Yesufe et al [7] and Toma et al [13] where the overall report completeness for the essential parameters was 2.2%, 6.1%, 21%, 61.6% and 74.3% respectively. This could probably be explained by the fact that we have used synoptic-like format for reporting, which resulted in less chances of omission of core elements. There are studies which have shown improvement in completeness of histopathology reports after implementation of a standard reporting format [4, 14, 15].
Some of the essential elements in breast carcinoma reporting are: histological type, grade, stage, margin status to evaluate the completion of resection, LVI to predict the possibility of distant metastasis and risk of recurrence and biomarker studies for planning adjuvant treatment in addition to usual chemotherapy. In the present study, the common CE, that remained throughout as CE during all the revisions of CAP protocol, were adequately reported. However, those elements which moved from CE to NCE or vice versa or newly added as CE in the CAP revisions were frequently under reported.
The adequately reported CE in our reports were procedure, tumour size, tumour type, Nottingham score, grade, DCIS, margin status, lymph nodes and stage. This finding is similar to that done by Toma et al [13], where they found histopathological tumor type (98.8%), tumor grade (92.1%) and stage (94.7%) were the adequately reported elements. In another study by Yesufe et al [7], tumor type (95.7%) and stage (94.7%) were adequately represented. In our study less commonly reported CE were laterality, size of largest lymph node metastatic deposit and extranodal extension, whereas commonly missed elements as published by Toma et al [13] were closest margin, LVI, and tumor grade. These studies has adopted protocols from their local/ national guidelines.
There are many standard protocols available like College of American Pathologists (CAP) protocol and the Royal College of Pathologists (RCP) which has minimum datasets for cancer reporting, where all the salient features required for the patient management and prognostication are listed, so that no single parameter is missed in the histopathology report. The CAP protocols also defines optional data elements which gives flexibility of omitting, as deemed necessary by the organization/local practise methods.
Several studies have demonstrated that implementing a structured synoptic reporting improves reporting of all essential elements when compared to narrative reporting format (88% v 34%) [10]. This is for the simple reason that in the synoptic formats all the pathological parameters that feature in the histopathology report will be listed, reducing the chances of missing any parameter. On the other hand, narrative reports are dictated and there are higher chance of missing some of the core parameters, since the pathologist preference play an important part in narrative reports.
Amendments were made in 4.8% of our breast cancer reports and the most common reasons for amendments was transcriptional errors, which was similar to a study done by Harrison BT [16]. The common reasons for amendments in previously published studies were editing/ change in final diagnosis, followed by omission of intra-operative consultation reports and missing essential parameter [5]. To the best of our knowledge, this is the first study from a tertiary care center in India, auditing histopathology reports of breast cancer using the widely accepted CAP protocol. Other studies in literature have used either local guidelines/ country specific guidelines for auditing the reports, which may not permit comparison across the globe.
In conclusion, our study findings are comparable to similar audits from other parts of the world. Common core elements were satisfactorily represented in majority. While common elements are most often well represented, 33% of core elements which were recent additions in the revised CAP protocols were often missed. The frequent change in CAP protocols with shifting of parameters between CE and NCE and flexibility in synoptic-like structured format were the most common reasons for the deficiencies. Implementing structured minimal criteria for synoptic histopathological reports for tumors summaries, regular training of the staffs on the updates of tumor and biomarker reporting and regular auditing of tumor summaries for completeness can improve the overall quality of histopathology reports.
Acknowledgements
Nil
Compliance with Ethical Standards
Yes. Approval obtained from institutional Ethics committee(IEC ref no: 23/2022)
Conflict of Interest
The authors declare no conflict of interest.
References
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries Sung H, Ferlay J, Siegel RL , Laversanne M, Soerjomataram I, Jemal A, Bray F. CA: a cancer journal for clinicians.2021;71(3). CrossRef
- Breast cancer now most common form of cancer: WHO taking action [Internet]. Who.int. 2022 Volkov S. Available from: https://www.who.int/news/item/03-02-2021-breast-cancer-now-most-common-form-of-cancer-who-taking-action..
- Breast cancer [Internet]. Who.int. 2021 [cited 9 July 2022] Anderson B. Available from: https://www.who.int/news-room/fact-sheets/detail/breast-cancer..
- The effects of implementing synoptic pathology reporting in cancer diagnosis: a systematic review Sluijter CE , Lonkhuijzen LRCW , Slooten H, Nagtegaal ID , Overbeek LIH . Virchows Archiv: An International Journal of Pathology.2016;468(6). CrossRef
- Quality assurance and patient safety protocols for breast and gynecologic pathology in an Academic Women’s Hospital Dabbs DJ , Stoos CT , Mallon A. Applied Cancer Research.2016;36(1). CrossRef
- Histopathology of breast carcinoma--an audit of 50 reports in Rawalpindi, Pakistan Mamoon N, Hassan U, Mushtaq S, Sharif MA . Asian Pacific journal of cancer prevention: APJCP.2010;11(1).
- Adequacy of Pathologic Reports of Invasive Breast Cancer From Mastectomy Specimens at Tikur Anbessa Specialized Hospital Oncology Center in Ethiopia Yesufe AA , Assefa M, Bekele A, Ergete W, Aynalem A, Wondemagegnehu T, Tausjø J, Assefa Tessema G, Kantelhardt EJ , Gansler T, Jemal A. Journal of Global Oncology.2018;4. CrossRef
- Study of breast cancer- a critical audit of a surgeon and pathologist at a rural cancer centre Maturi R, et al . International Journal of Contemporary Medical Research.2016;3(9):2578-2581.
- A review of breast cancer pathology reports in Nigeria Joseph AO , Li Y, Salako O, Doi S, Balogun OD , Awofeso OM , Abdulkareem F, Onitilo AA . Ecancermedicalscience.2021;15. CrossRef
- Breast Cancer Reporting in Lagos, Nigeria: Implications for Training and Education in Africa Daramola AO , Banjo AA , Bennett A, Abdulkareem F, Shaaban AM . Journal of global oncology.2016;2(6). CrossRef
- Audit of histopathology reports for breast cancer in Aminu Kano Teaching Hospital Atanda A. T., Atanda J. O.. West African Journal of Medicine.2010;29(3). CrossRef
- An audit of breast cancer pathology reporting in Australia in 1995 Kricker A., Armstrong B., Smith C., Bilous M., Camaris C., Mayer A., Psarianos T.. British Journal of Cancer.1999;80(3-4). CrossRef
- Quality of Histopathological Reporting in Breast Cancer: Results From Four South African Breast Units Toma A, O'Neil D, Joffe M, Ayeni O, Nel C, Berg E, Nayler S, Cubasch H, Phakathi B, Buccimazza I, Čačala S, Ruff P, Norris S, Nietz S. JCO global oncology.2021;7. CrossRef
- The use of a standard proforma in breast cancer reporting Mathers M. E., Shrimankar J., Scott D. J., Charlton F. G., Griffith C. D., Angus B.. Journal of Clinical Pathology.2001;54(10). CrossRef
- Closing the quality loop: facilitating improvement in oncology practice through timely access to clinical performance indicators Srigley J, Lankshear S, Brierley J, McGowan T, Divaris D, Yurcan M, Rossi R, Yardley T, King MJ , Ross J, Irish J, McLeod R, Sawka C. Journal of Oncology Practice.2013;9(5). CrossRef
- Quality Assurance in Breast Pathology: Lessons Learned From a Review of Amended Reports Harrison BT , Dillon DA , Richardson AL , Brock JE , Guidi AJ , Lester SC . Archives of Pathology & Laboratory Medicine.2017;141(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2022
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times