Real-World Clinical Outcomes of Low Dose versus Standard Dose Abiraterone in Patients with Metastatic Castration-Resistant Prostate Cancer: A Prospective Pragmatic Study
Download
Abstract
Introduction: Abiraterone is a standard treatment for metastatic castration-resistant prostate cancer (mCRPC). Food intake has shown significant effect on its pharmacokinetics. The aim of this study was to evaluate the efficacy and safety of standard dose abiraterone under fasting conditions versus low dose abiraterone with a low-fat meal in patients with mCRPC.
Methods: In this prospective real world study 32 patients with mCRPC were treated in two groups of 16 cases by routine clinical practice of their treating physician with low-dose abiraterone (250 mg with a low fat breakfast) or standard-dose abiraterone (1000 mg in fasting state). The changes in serum prostate specific antigen (PSA) level and PSA response rate (≥50% reduction after 12 weeks) were the primary end points.
Results: The median changes of serum PSA before and after treatment, as well as the PSA nadir were not significantly different between the two groups (P = 0.128 and P = 0.051, respectively). Despite a trend toward higher PSA response rate in the low-dose group, the difference was not statistically significant (75.0% vs. 62.5%; P = 0.704). Median serologic progression free survival (PFS) was significantly higher in the low-dose group (15 vs. 8 months; Log-rank P = 0.031). There was a trend toward lower adverse events in the low-dose group, but this difference was not statistically significant (37.5% vs. 62.5%; P = 0.289).
Conclusion: Low-dose abiraterone seems to be comparable to standard-dose abiraterone in mCRPC with 75% lower financial cost; however, this conclusion needs to be proved by further well designed and large scale studies.
Introduction
Castrate-resistant prostate cancers (CRPC) is defined as the state of clinical and/or biochemical progression of prostate cancer in the presence of very low serum testosterone levels [1]. For a long time, the standard treatment for metastatic prostate cancer has been androgen deprivation therapy (ADT) alone; but the emergence of new therapeutic agents has changed this paradigm [2, 3]. Abiraterone acetate is a selective and irreversible inhibitor of CYP17 enzyme, which is responsible for the production of extragonadal (adrenal and intratumoral) androgens [4]. Several studies have confirmed the efficacy and safety of this drug in metastatic CRPC [5-9]. Additionally, recent studies have shown that the addition of abiraterone to the standard treatment of ADT improves survival in mCRPC patients [10, 11].
Currently, abiraterone is the most widely used first-line treatment in mCRPC [12]. The standard dose (STD) of abiraterone is 1000 mg once daily, taken on an empty stomach with prednisolone 5 mg twice a day. This dose of abiraterone has improved overall survival (OS), progression-free survival (PFS), and quality of life in patients with mCRPC compared to placebo [8, 9, 11]. Early clinical studies showed that taking abiraterone with food increases the bioavailability and the minimum plasma concentration (Cmin) of this drug [13-15]. Hence, a low-dose (LD) regimen can be considered as a cost-effective approach, provided that biochemical control and disease-free survival are not compromised and there is no higher toxicity [4]. Accordingly, several clinical trials have investigated the LD regimen of abiraterone with food, considering the potential economic benefit and lower toxicity profile [13, 16]. A recent phase II study on mCRPC showed that biochemical PFS was non-inferior in LD abiraterone (250 mg/day with food) recipients compared to STD abiraterone (1000 mg/day in fasting state) recipients [16].
Although prostate cancer survival has increased due to the development of new drugs such as abiraterone acetate, universal access to treatment is not always possible, especially in low- and middle-income countries [17,18]. Recently, the National Comprehensive Cancer Network (NCCN) guideline has also considered LD abiraterone (250 mg per day with a meal) as an acceptable alternative to the STD abiraterone (1000 mg on an empty stomach) and emphasized that the use of LD abiraterone reduces treatment costs and increases patient acceptance [17].
Considering the increasing use of abiraterone, the relatively high price of this drug, and the possible economic importance of reducing the dose of abiraterone, we conducted this study with the aim of investigating the efficacy and safety of LD abiraterone versus STD abiraterone in patients with mCRPC. The main objective of this study was to compare the anticancer activity of LD abiraterone with STD abiraterone, which was done through measuring the change in serum level of prostate specific antigen (PSA), and serologic or PSA response rate (≥50% reduction after 12 weeks of treatment). Although PSA is not a clinically valid surrogate target, changes in serum PSA levels correlate with overall survival in patients treated with abiraterone [8, 9]. Secondary objectives included comparison of serologic or PSA progression-free survival (PSA PFS) as well as the severity of adverse drug reactions in LD and STD abiraterone recipients.
Materials and Methods
This pragmatic study is a non-randomized clinical study that was prospectively conducted in the real world on patients with mCRPC treated at the department of clinical oncology of Ahvaz Golestan Hospital between May 2020 and February 2023; simultaneously, we did a similar but retrospective study on patients with mCRPC who were treated at our department between March 2018 and June 2021. Some patients were common between the two studies from May 2020 to June 2021. The results of that retrospective study will be presented in another article. All patients provided written informed consent before participation in the study. This study was approved by the Research Ethics Committee of Golestan Hospital, and the Helsinki Declaration’s ethical guidelines and the rules governing patient information confidentiality were followed during the whole course of the study.
All patients with pathological diagnosis of prostate cancer with clinically and/or radiologically proven bone or visceral metastases and serological and/or radiological mCRPC diagnosis (defined as disease progression with a testosterone level of 50 ng/dL or orchiectomy) were included. Radiological disease progression was defined as the development of two or more new lesions on bone scan or the progression of a soft tissue mass on computerized tomography (CT) scan or magnetic resonance imaging (MRI) scan based on Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) [19]. Also, serological disease progression was defined by increasing PSA levels of more than 2 ng/ml and/or more than 25% above the nadir (in patients with an initial response to treatment) or the entry level (in patients with no initial response to treatment after 12 weeks) based on the criteria of the Prostate Cancer Clinical Trials Working Group [19].
Patients with Eastern Cooperative Oncology Group (ECOG) performance status of 3 or more, uncontrolled hypertension, abnormal basic electrolytes, and abnormal kidney and liver function were excluded. Prior use of abiraterone for more than 7 days before enrollment and prior use of enzalutamide or other androgen pathway inhibitors were not allowed. In addition, the use of systemic corticosteroids (apart from daily prednisone 10 mg), other hormone therapies, and any herbal medication that decreases PSA levels was also prohibited. Prior use of high-dose ketoconazole was allowed, because it does not preclude response to abiraterone despite similar mechanism of action [18]. Prior use of chemotherapy was also allowed.
The patients participating in the study were divided into two groups receiving STD abiraterone of 1000 mg daily (after at least 8 hours of overnight fasting and at least 2 hours before eating any food) or LD abiraterone of 250 mg daily (less than 30 minutes apart or with a low- fat breakfast). The patient chooses the sort of breakfast; however, while using abiraterone, patients were advised to stay away from high-fat foods (such sausages and fried dishes). All patients received oral prednisolone at a dose of 5 mg twice a day with/or without pantoprazole (depending on physician’s recommendation). The course of treatment with abiraterone and the prescribed dose of this drug was based on the routine clinical practice of their treating physician and was independent of the patient’s decision to participate in the study. All patients obtained abiraterone from the pharmacy and received this medicine on an outpatient basis. The patients were visited in the 1st, 2nd, and 4th weeks and then monthly to assess potential treatment-related side effects and response to treatment. Basic serum electrolytes, complete blood count (CBC), PSA, kidney and liver function tests were performed before enrolment and monthly thereafter.
The main objective of this study was to compare the anticancer activity of LD abiraterone with STD abiraterone, which was done through measuring the change in serum level of PSA, and serologic or PSA response rate (≥50% reduction after 12 weeks of treatment). Secondary objectives included comparison of serologic or PSA progression-free survival (PSA PFS) as well as the severity of adverse drug reactions in LD and STD abiraterone recipients. For patients with stable disease, PSA PFS was taken into account at the time of the last patient’s assessment. Treatment-related side effects were assessed based on the Common Terminology Criteria for Adverse Events-version 5 (CTCAE v5.0) and were graded from 1 to 5 according to their severity [20]. Based on PSA response rate (our primary end point) of 30% with STD abiraterone according to the randomized phase III trial conducted by de Bono et al. [9], and applying the sample size formula with type 1 error of 5% and test power of 80%, a sample size equal to 16 cases per each group was calculated. Frequency and percentage were used to describe qualitative variables data, whereas mean, standard deviation, median, and interquartile range (IQR) were used to describe quantitative variables data. Shapiro-Wilk was used to determine if the data were normal. To ascertain the association between qualitative variables, the chi-square test (also known as Fisher’s exact test) was used. Independent t-test (or Mann-Whitney non-parametric test) and Wilcoxon analysis were used to compare quantitative variables between two groups. The Kaplan-Meier curve and Log-rank test were used to calculate the survival rate of individuals. Statistical analyses were carried out using Statistical Package for the Social Sciences version 22 (SPSS v22) software, (SPSS Inc., Chicago, IL, U.S.A). We regarded p-value of ≤0.05 for statistical significance.
Results
Between May 2020 and February 2023, all patients with mCRPC treated at the department of clinical oncology of Ahvaz Golestan Hospital were enrolled, and after exclusion of 7 patients, 18 patients assigned to STD and LD groups. Two patients in each group withdrew shortly after treatment, and 16 patients in each group were treated per protocol (Figure 1).
Figure 1. Study diagram. MCRPC, metastatic castration-resistant prostate cancer; LD, low dose; STD, standard dose.
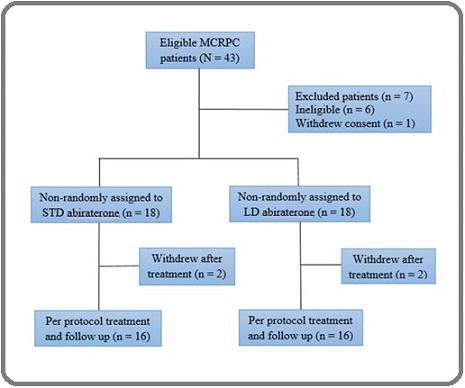
Despite non- randomization, patient characteristics such as age, location of metastasis, and ECOG performance status were well balanced, without any significant differences between the two groups, representing a typical mCRPC population (male only, older age, and predilection of bone metastasis) in each treatment arm (Table 1).
| Characteristics | STD (n = 16) | LD (n = 16) | P-value |
| Age (years) | |||
| Mean ± SD | 69.13±7.07 | 65.63±11.23 | 0.300 * |
| Location of metastasis | |||
| Bone, n (%) | 15 (93.8) | 14 (87.5) | |
| Viscera, n (%) | 1 (6.3) | 2 (12.5) | 0.544 ** |
| ECOG performance status | |||
| Score 0, n (%) | 11 (68.8) | 11 (68.8) | |
| Score 1, n (%) | 5 (31.3) | 4 (25.0) | |
| Score 2, n (%) | 0 (0) | 1 (6.3) | 0.574 ** |
STD, standard dose; LD, low dose; ECOG, Eastern Cooperative Oncology Group; * Independent t test; ** Chi-square test
All patients in both groups received abiraterone in the pre-chemotherapy setting and no patient has received previous high-dose ketoconazole in both groups.
After 12 weeks of treatment, median PSA level significantly decreased from the baseline in the STD group (median PSA change 5.50 ng/ml; P = 0.004) and the LD group (median PSA change 2.50 ng/ml; P < 0.0001). There were no significant differences in serum PSA levels at baseline (median PSA 9.67 vs. 3.59 ng/ml; P = 0.073) and after 12 weeks of treatment (median PSA 2.43 vs. 0.70 ng/ml; P = 0.224), as well as the PSA nadir (median PSA 1.35 vs. 0.26 ng/ml; P = 0.051), between two groups (Table 2).
| Variable | STD (n = 16) | LD (n = 16) | P-value |
| PSA (ng/ml) before treatment | |||
| Mean ± SD | 11.74 ± 13.61 | 32.16 ± 15.50 | |
| Median (IQR) | 9.67 (4.62 – 23.77) | 3.59 (2.27 – 13.87) | 0.073 * |
| PSA (ng/ml) after 12 weeks | |||
| Mean ± SD | 8.86 ± 6.89 | 22.76 ± 9.41 | |
| Median (IQR) | 2.43 (0.07 – 9.85) | 0.70 (0.29 – 9.12) | 0.224 * |
| PSA changes (ng/ml) after 12 weeks | |||
| Mean ± SD | 9.08 ± 6.72 | 10.18 ± 6.09 | |
| Median (IQR) | 5.50 (2.26 – 10.63) | 2.50 (1.23 – 4.85) | 0.128 * |
| P-value | 0.004 ** | < 0.0001 ** | – |
| PSA (ng/ml) nadir | |||
| Mean ± SD | 8.03 ± 5.45 | 18.50 ± 5.90 | |
| Median (IQR) | 1.35 (0.43 – 8.72) | 0.26 (0.08 – 0.91) | 0.051 ** |
| PSA response, n (%) | |||
| 10 (62.5) | 12 (75.0) | 0.704 *** | |
| Serologic (PSA) PFS (months) | |||
| Mean ± SD | 9.62 ± 5.63 | 14.06 ± 6.53 | |
| Median (IQR) | 8.00 (4.25 – 14.75) | 15.00 (10.25 – 19.75) | 0.031 **** |
PSA: Prostate-specific antigen; STD: standard dose; LD: low dose; PSA response: ≥50% reduction of PSA level after 12 weeks; PFS: Progression free survival; SD: Standard deviation; IQR: Interquartile range; * Mann-Whitney test; ** Wilcoxon test; *** Fisher exact test; **** Log Rank (Mantel-Cox)
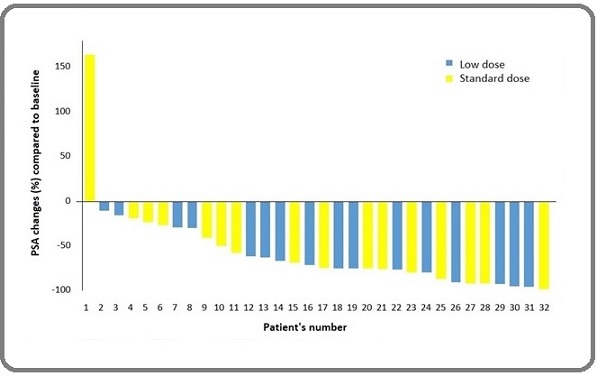
There were also no significant differences in median serum PSA changes from baseline until 12 weeks after therapy (median PSA change 5.50 vs. 2.50 ng/ml; P = 0.128). Despite that there was a trend toward more PSA response in the LD group than STD group, but this difference was not statistically significant (75.0% vs. 62.5%; P = 0.704). No patient in the LD group, and only one patient in the STD group developed PSA progression after 12 week (Figure 2).
Figure 2. Waterfall Plot Showing the Percentage of PSA Changes after 12 Weeks of Treatment Compared to the Baseline.
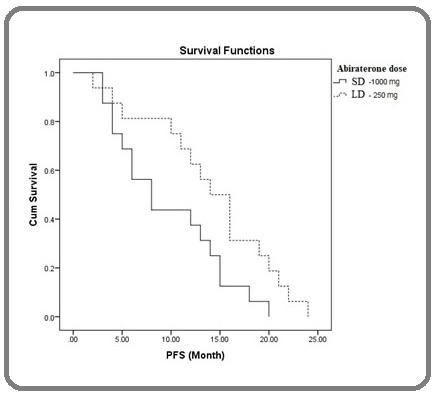
Median serologic PFS in the LD group was significantly higher than the STD group (15 vs. 8 months; Log-rank P = 0.031) (Figure 3).
Figure 3. Kaplan-Meier Plot of Time to Progression Showing Serologic Progression-free Survival (PSA PFS) .
In overall, adverse events were observed in 10 cases (62.5%) of the STD group and 6 cases (37.5%) of the LD group (P=0.289). Although this difference is not statistically significant, there appears to be a trend toward fewer side effects in the LD group. These adverse events were hypertension, cardiac arrhythmia, peripheral edema, fatigue, constipation, hypokalemia, flushing, insomnia, dyspepsia, arthralgia, and myalgia (Table 3).
| Side effects | CTCAE Grade | STD (n = 16) | LD (n = 16) | P-value* |
| Hypokalemia, n (%) | 1 | 1 (6.3) | 0 | 1 |
| Hypertension, n (%) | 3 | 0 | 1 (6.3) | 1 |
| Edema, n (%) | 1 | 2 (12.5) | 2 (12.5) | 1 |
| Cardiac arrhythmia, n (%) | 1 | 0 | 1 (6.3) | 1 |
| Hot flash, n (%) | 1 | 1 (6.3) | 2 (12.5) | 1 |
| Constipation, n (%) | 1 | 4 (25.0) | 3 (18.8) | 1 |
| Dyspepsia, n (%) | 1 | 0 | 1 (6.3) | 1 |
| Fatigue, n (%) | 1 | 3 (18.8) | 2 (12.5) | 1 |
| Insomnia, n (%) | 1 | 2 (12.5) | 1 (6.3) | 1 |
| Arthralgia, n (%) | 1 | 1 (6.3) | 1 (6.3) | 1 |
| Myalgia, n (%) | 1 | 1 (6.3) | 0 | 1 |
CTCAE, Common Terminology Criteria for Adverse Events; STD, standard dose; LD, low dose. * Fisher's exact test
All adverse events were grade 1 according to CTCAE v5.0 except one case of grade 3 hypertension in the LD group. There were no chest pain, headache, diarrhea, fever, skin rash, cough, dyspnea, nasopharyngitis, hypernatremia, hypocalcaemia, hypophosphatemia, hyperglycemia, or liver dysfunction in both groups.
Discussion
This prospective non-randomized pragmatic study suggests that LD abiraterone has a similar efficacy to the SD abiraterone in the treatment of mCRPC and almost equally affects the PSA level. Even it seems that LD abiraterone may have a slightly better, but non-significant, effect on reducing serum PSA levels and PSA response rates. Pharmacokinetic studies have shown that the bioavailability and serum concentration of abiraterone increase in the presence of food, without increasing the toxicity of the drug. [14, 15, 21, 22]. Additionally, based on the findings of the current study, LD abiraterone taken with food can have the same efficacy as the STD abiraterone with an empty stomach in terms of PSA PFS and can be used as a cost-effective strategy to lower treatment costs. Due to the high cost of abiraterone, prescribing LD abiraterone can increase access to treatment by reducing treatment costs, improve treatment compliance, and prevent treatment interruptions [16, 17]. Recently a phase II randomized clinical trial on patients with mCRPC by Shaherose et al. [13] showed that LD abiraterone (250 mg with a high-fat meal) was comparable to STD abiraterone (1000 mg fasting) in terms of PSA response. Mean PSA response (reduction more than or equal to 50% after 12 weeks) was 68% in the STD group and 82% in the LD group. At 12 weeks, 2 out of 35 individuals in the STD group and none of the patients in the LD group had PSA progression. Another phase II randomized clinical trial on patients with CRPC by Szmulewitz et al. [16] showed that after 12 weeks of treatment, the serum PSA level decreased more in the LD group (250 mg/day with a low-fat breakfast) than in the STD group (1000 mg/day), though these differences were not statistically significant. The PSA response rates (≥50% reduction) in the LD group and the STD group were 58% and 50%, respectively. A retrospective study on patients with mCRPC at the Princess Margaret Cancer Centre by Leibowitz et al. [23] demonstrated a non-significant difference in PSA response rate at 12 weeks (41% vs. 26%, p = 0.23) in the STD abiraterone (1000 mg) and LD abiraterone (250 or 500 mg) cohorts, respectively. In a chemo-naive sub-group of patients, there was also a non-significant difference in PSA response rate at 12 weeks (51% vs. 27%, p = 0.11) in the STD abiraterone and LD abiraterone cohorts, respectively. The outcomes of these studies are in line with our study’s findings in terms of PSA response rate.
According to earlier studies, between 35% to 42% of individuals receiving abiraterone at a constant dosage of 1000 mg per day in fasting state, do not achieve the efficacy threshold of a Cmin ≥8.4 ng/ml, and are thus at risk of lower treatment efficacy [24-26]. Additionally, it has been shown that taking abiraterone with meals significantly raises the drug’s Cmin [14, 24]. On the other hand, a prospective observational study on mCRPC patients by Carton et al showed that patients with Cmin ≥8.4 ng/mL had longer PFS (12.2 months vs. 7.4 months; P = 0.044) [26]. In a real-world retrospective cohort on mCRPC patients by van Nuland et al. [25], patients with Cmin ≥8.4 ng/mL had a longer PSA independent PFS (16.9 vs. 6.1 months; P = 0.033) and a better prognosis. Median PSA PFS in the study of Leibowitz et al. [23] was not different significantly (LD 5.6 vs. STD 4.4 months; P = 0.31) between the two groups. Median PSA PFS in the study of Szmulewitz et al. [16] was similar (8.6 months) in the two groups. Median PSA PFS in the LD group of our study (15 months) was significantly higher than the STD group (8 months), which was not in line with the above mentioned studies. There are some potential explanations for this, including the fact that all patients in our study were chemo-naïve but in most above mentioned studies, there were sub-groups of patients who has received previous chemotherapy or high-dose ketoconazole. Another justification is that although in most above mentioned studies PSA PFS was examined, in some of them PFS (both serologic and radiologic) or PSA independent PFS was examined.
In our study, adverse events were observed in 10 cases (62.5%) of the STD group and 6 cases (37.5%) of the LD group, which was higher in the STD group non-significantly (P=0.289). Abiraterone has been linked in previous studies to adverse effects such as fatigue, nausea, vomiting, hypertension, elevated liver enzymes, flushing, arthralgia, myalgia, and hypokalemia [4, 6, 11, 13]. Shaherose et al. [13] observed adverse events such as fatigue (13 vs. 12 cases), hypertension (12 vs. 10 cases), hypokalemia (11 patients in each arm), and fluid retention (9 vs. 3 cases) in the STD group (35 patients) and LD group (35 patients), respectively; which were not significantly different. The adverse events of the study by Szmulewitz et al. [16] were similar in both groups. Overall, adverse events observed in at least 15% of patients in both groups. There were non-significantly more patients with grade 3 or higher events in the LD group (32.4% v 17.6%; P = 0.26). Furthermore, a phase I/II clinical trial on patients with mCRPC by Chi et al. [14] showed that STD abiraterone (1000 mg) with different modifications in prandial/fasting states do not alter abiraterone safety in short-term. Although abiraterone area under plasma concentration–time curve (AUC) was 2-fold higher with a high-fat meal and similar with a low- fat meal versus modified fasting state; however, adverse events (all grade ≤3) were similar.
According to Petrioli et al.’s study [27], treating elderly patients (over 85 years old) with advanced CRPC by a lower dose of abiraterone acetate (750 mg per day) with concomitant low dose prednisolone was effective and well tolerated. The side effects related to the treatment were all grade 1 or 2 toxicities, including hypertension (15%), cardiac disorders (11.5%), peripheral edema (15%), fatigue (19%), and hypokalemia (7.5%).
The economic benefits of using LD abiraterone are of great interest [4, 16, 17, 23]. According to a review and report by Dey et al. on the available literature, in the United States, the estimated retail cost of STD abiraterone is approximately 10 thousand US Dollars per month. If LD abiraterone can be used instead, the cost would be a quarter of this, and the average lifetime cost saving per patient would be more than 120 thousand and 250 thousand US Dollars in mCRPC and metastatic castration-sensitive prostate cancer (mCSPC), respectively. The estimated annual cost savings for Medicare using LD abiraterone would be approximately 700 million US Dollars. Also, in India, the estimated retail cost of STD abiraterone by the cheapest generic drug is approximately 110 US Dollars per month, the average lifetime financial gain per patient would be nearly 1360 and 2700 US Dollars in mCRPC and mCSPC, respectively. To put this in context, the per capita gross national income in India (in 2020-2021) is approximately 1900 US Dollars [4]. In a cost-effectiveness analysis of the Science and Cost Cancer Consortium survey to evaluate the use of abiraterone in India, Patel et al. showed that the cost savings for all patients with metastatic prostate cancer in India by using LD abiraterone would be 180 million US Dollars. The cost saving per patient would be 3640 US Dollars, equal to approximately 2.5 times the average per capita income in India [17]. According to the pharmaceutical statistics of the Food and Drug Organization of Iran, from April 2017 to March 2018, the market value of abiraterone was about 7 billion IRI Rials with the numerical value of 12,600 capsules (250 mg) of abiraterone, 100% of which were imported. Also, from April 2018 to March 2019, the market value of abiraterone was about 153 billion IRI Rials with the numerical value of 379,800 capsules (250 mg) of abiraterone, more than 53% of the numerical value of abiraterone was domestically produced (less than 42% of the market value) and less than 47% were imported (more than 58% of the market value). In recent years, the trend of abiraterone consumption and also the ratio of domestic generic drug consumption to imported drug has been increasing, so that from April 2022 to March 2023, the market value of this drug in Iran was 2300 billion IRI Rials with the numerical value of 2,400,000 capsules (250 mg) and 885,000 capsules (500 mg), more than 99% of the numerical value and market value of this drug in Iran was domestically produced [28]. If we use LD abiraterone instead of STD abiraterone in Iran, the total cost will be a quarter and the average financial profit per year will be more than 1700 billion IRI Rials (4 million US Dollars). This strategy would be attractive for countries with limited resources.
In conclusion, the results of the present study suggest that LD abiraterone with a low-fat breakfast has a similar efficacy as the STD abiraterone in fasting state, with non-significantly better toxicity profile, and significantly better PSA PFS. Therefore, LD abiraterone with a low-fat breakfast could be considered as an effective, affordable, and safe method in the treatment of mCRPC patients in order to reduce high treatment costs and possible side effects caused by the drug. Because we did not evaluate radiologic PFS and overall survival (OS), our study was unable to meet fully validated CRPC survival end points. However, there were an association between PSA PFS and OS in previous studies on mCRPC [29]; therefore, PSA PFS outcomes of our study is impressive. Due to the limitations of the present study, such as the small sample size and the lack of randomization, these conclusions needs to be proved by further well designed and large scale studies with longer follow-up.
Highlights
• There was a non-significant trend toward higher PSA response in the LD abiraterone group (75.0% vs. 62.5%; P = 0.704).
• There was statistically significant higher median serologic PFS in the LD abiraterone group (15 vs. 8 months; Log-rank P = 0.031).
• There was a non-significant trend toward lower adverse events in the LD abiraterone group (37.5% vs. 62.5%; P = 0.289).
• LD abiraterone with a low-fat breakfast could be considered as an effective, affordable, and safe method in the treatment of mCRPC patients.
Declaration of interest
The authors declare no conflict of interest.
Funding
This research was funded by the Cancer Research Center of Ahvaz Jundishapur University of Medical Sciences (project registration number CRC-0123).
Author contributions
All authors contributed to the study conception and design, but the main contributor was Sasan Razmjoo. Material preparation, data collection and analysis were performed by Faride Karimi, Sasan Razmjoo, Seyed Mohammad Hosseini, Ali Bagheri, and Maryam Feli. The first draft of the manuscript was written by Faride Karimi and Sasan Razmjoo and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki and approved by the Research Ethics Committee of Golestan Hospital (approved ID IR.AJUMS.HGOLESTAN.REC.1401.185).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Acknowledgments
This article is taken from Faride Karimi’s residency thesis (dissertation registration number D-4922) at Ahvaz Jundishapur University of Medical Sciences. The authors express their gratitude to the staff of the department of clinical oncology and clinical research development center of Golestan Hospital, especially Elham Farhadi, who collaborated in data collection and analysis.
References
- Definition of Castrate Resistant Prostate Cancer: New Insights Morote J, Aguilar A, Planas J, Trilla E. Biomedicines.2022;10(3). CrossRef
- Metastatic Prostate Cancer: Treatment Options. Oncology, 2022;100(1):48-59. Achard V, Putora PM , Omlin A, Zilli T, Fischer S. .
- New Insights and Emerging Therapeutic Approaches in Prostate Cancer Licitra F, Giovannelli P, Di Donato M, Monaco A, Galasso G, Migliaccio A, Castoria G. Frontiers in Endocrinology.2022;13. CrossRef
- Is Low-Dose Abiraterone for Prostate Cancer An Attractive Strategy for Limited Resource Settings? Dey T, Goyal S, Periasamy K, Madan R. Indian Journal of Medical and Paediatric Oncology.2022;43(1):40-46.
- Serum androgens as prognostic biomarkers in castration-resistant prostate cancer: results from an analysis of a randomized phase III trial Ryan CJ , Molina A, Li J, Kheoh T, Small EJ , Haqq CM , Grant RP , Bono JS , Scher HI . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2013;31(22). CrossRef
- Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer Danila DC , Morris MJ , Bono JS , Ryan CJ , Denmeade SR , Smith MR , Taplin M, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2010;28(9). CrossRef
- Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response Ryan CJ , Shah S, Efstathiou E, Smith MR , Taplin M, Bubley GJ , Logothetis CJ , et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2011;17(14). CrossRef
- Abiraterone in metastatic prostate cancer without previous chemotherapy Ryan CJ , Smith MR , Bono JS , Molina A, Logothetis CJ , Souza P, Fizazi K, et al . The New England Journal of Medicine.2013;368(2). CrossRef
- Abiraterone and increased survival in metastatic prostate cancer Bono JS , Logothetis CJ , Molina A, Fizazi K, North S, Chu L, Chi KN , et al . The New England Journal of Medicine.2011;364(21). CrossRef
- Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy James ND , Bono JS , Spears MR , Clarke NW , Mason MD , Dearnaley DP , Ritchie AWS , et al . The New England Journal of Medicine.2017;377(4). CrossRef
- Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin Al, Alekseev BY , Özgüroğlu M, et al . The New England Journal of Medicine.2017;377(4). CrossRef
- Prescribing Patterns of Oral Antineoplastic Therapies Observed in the Treatment of Patients With Advanced Prostate Cancer Between 2012 and 2014: Results of an Oncology EMR Analysis Malangone-Monaco E, Foley K, Varker H, Wilson KL , McKenzie S, Ellis L. Clinical Therapeutics.2016;38(8). CrossRef
- 159MO Low-dose abiraterone with fatty food versus standard dose abiraterone in metastatic castration-resistant prostate cancer Shaherose S., Charu GK , Santa A., Rajappa S. J., Mohan K., Boyella P. K.. Annals of Oncology.2022;33. CrossRef
- Food effects on abiraterone pharmacokinetics in healthy subjects and patients with metastatic castration-resistant prostate cancer Chi KN , Spratlin J, Kollmannsberger C, North S, Pankras C, Gonzalez M, Bernard A, et al . Journal of Clinical Pharmacology.2015;55(12). CrossRef
- Food enhanced pharmacokinetics for clinical translation of low dose abiraterone acetate in metastatic castration-resistant prostate cancer Meenu M SR , Seth A, Das T, Valpandian , Arya DS . J Cancer Sci Clin Ther.2020;4(3):314-324.
- Prospective International Randomized Phase II Study of Low-Dose Abiraterone With Food Versus Standard Dose Abiraterone In Castration-Resistant Prostate Cancer Szmulewitz RZ , Peer CJ , Ibraheem A, Martinez E, Kozloff MF , Carthon B, Harvey RD , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2018;36(14). CrossRef
- Low-Dose Abiraterone in Metastatic Prostate Cancer: Is It Practice Changing? Facts and Facets Patel A, Tannock IF , Srivastava P, Biswas B, Gupta VG , Batra A, Bhethanabhotla S, et al . JCO global oncology.2020;6. CrossRef
- Sequential use of the androgen synthesis inhibitors ketoconazole and abiraterone acetate in castration-resistant prostate cancer and the predictive value of circulating androgens Kim W, Zhang L, Wilton JH , Fetterly G, Mohler JL , Weinberg V, Morse A, et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2014;20(24). CrossRef
- Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group Scher HI , Halabi S, Tannock I, Morris M, Sternberg CN , Carducci MA , Eisenberger MA , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2008;26(7). CrossRef
- services USDohah. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. National Cancer Institute 2017.
- Population pharmacokinetic analysis of abiraterone in chemotherapy-naïve and docetaxel-treated patients with metastatic castration-resistant prostate cancer Stuyckens K, Saad F, Xu XS , Ryan CJ , Smith MR , Griffin TW , Yu MK , et al . Clinical Pharmacokinetics.2014;53(12). CrossRef
- Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy Ryan CJ , Smith MR , Fong L, Rosenberg JE , Kantoff P, Raynaud F, Martins V, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2010;28(9). CrossRef
- Abiraterone acetate in metastatic castration-resistant prostate cancer: a retrospective review of the Princess Margaret experience of (I) low dose abiraterone and (II) prior ketoconazole Leibowitz-Amit R, Seah J, Atenafu EG , Templeton AJ , Vera-Badillo FE , Alimohamed N, Knox , et al . European Journal of Cancer (Oxford, England: 1990).2014;50(14). CrossRef
- Concomitant intake of abiraterone acetate and food to increase pharmacokinetic exposure: real life data from a therapeutic drug monitoring programme Groenland SL , Nuland M, Bergman AM , Feijter JM , Dezentje VO , Rosing H, Beijnen JH , Huitema ADR , Steeghs N. European Journal of Cancer (Oxford, England: 1990).2020;130. CrossRef
- Exposure-response analyses of abiraterone and its metabolites in real-world patients with metastatic castration-resistant prostate cancer Nuland M., Groenland S. L., Bergman A. M., Steeghs N., Rosing H., Venekamp N., Huitema A. D. R., Beijnen J. H.. Prostate Cancer and Prostatic Diseases.2020;23(2). CrossRef
- Relation between plasma trough concentration of abiraterone and prostate-specific antigen response in metastatic castration-resistant prostate cancer patients Carton E., Noe G., Huillard O., Golmard L., Giroux J., Cessot A., Saidu N. E. B., et al . European Journal of Cancer (Oxford, England: 1990).2017;72. CrossRef
- Reduced Dose of Abiraterone Acetate with Concomitant Low-dose Prednisone in the Treatment of ≥ 85 Year-old Patients with Advanced Castrate-resistant Prostate Cancer Petrioli R, Francini E, Fiaschi AI , Laera L, Miano ST , De Rubertis G, Roviello G. Anticancer Research.2015;35(5).
- Food and Drug Administration of Iran: Available at: https://publicbi.fda.gov.ir/Reports/powerbi/Iran_Drug_Report1401?rs:embed=true. [Accessed 20 June 2024] .
- Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916 Hussain M, Goldman B, Tangen C, Higano CS , Petrylak DP , Wilding G, Akdas AM , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2009;27(15). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2024
Author Details