Comparison among Various Prognostic Pathologic Markers Revealed Significantly Poorer Prognosis in Non-basal-like than in Basal-like Triple Negative Breast Cancer
Download
Abstract
Objective: To find the incidence and prognosis of basal-like (TNB) and non-basal-like (TNN) in triple negative breast cancer.
Methods: Triple negative breast cancer (TN) cases were retrospectively reviewed and biomarkers studied on tissue microarray for ER, PR, HER2, Ki67, basal cytokeratins (CK5/6, CK14, CK17), EGFR, CD117, p63, p53, vimentin, CK7, CK8/18, CK19, BCL2, p16, WT1, and cyclin D1. The patients were reclassified according to ER, PR, HER2, Ki67 index, basal CKs and EGFR results into: lumA, lumB, HER2+, TNB and TNN.
Results: From 2007 to 2010, there were 193 female patients (120 TNB, 17 TNN, 42 lumB, and 14 HER2+). There were 184 (95.3%) invasive ductal carcinoma (IDC). All 17 TNN were IDC. High grade histology accounted for 71.5%. The median follow up time was 62.93 months. There were no significant differences in age, histologic grade, and tumor size between TNB and TNN while TNN had significant number of higher stage (p=0.028), axillary lymph node metastasis of more than 3 nodes (p=0.005) and lower disease free survival (DFS, p= 0.004) and overall survival (OS, p=0.001). TNN also had the poorest prognosis among the four subtypes.Tumor size and axillary nodal involvement were the independent predictors for DFS and OS. Absence of EGFR expression was an independent factor for lower DFS and OS in TN. Absence of CK8/18 expression was an independent factor for lower DFS in any combined group and OS in the combined TN and lumB group. Absence of p16 expression was an independent factor for lower DFS and OS of all cohort.
Conclusion: TNN accounted for12.3% of TN and had poorer prognosis. Tumor size and axillary nodal involvement were the independent predictors for DFS and OS. Absence of EGFR, CK8/18 or p16 expression had influence on survival in TN or combined group.
Introduction
Breast cancer is the most common cancer in most countries, including Thailand, and it has been classified according to gene expression profiling into 5 subtypes [1]. Immunohistochemical (IHC) markers of specific gene expression products were then proposed [1-3] with some modification in the St. Gallen consensus on the primary therapy of early breast cancer [4] to classify breast cancer as a practical basis for treatment, as follows: luminal A (lumA), luminal B (lumB), HER2+ (HER2+), and triple-negative (TN). TN subtype is subgrouped into [1] basal-like TN (TNB) when it is immunophenotypically negative ER, PR, and HER2 with at least any basal cytokeratin (basal CK; CK5, CK14, CK17) or epidermal growth factor receptor (EGFR) expression, and [2] non-basal-like TN (TNN) when it is immunophenotypically negative ER, PR, HER2, basal CK, and EGFR.
Of the 5 subtypes, TN is usually poorly differentiated, has aggressive clinical behavior, and has a poor prognosis. Moreover, therapy for TN subtype is limited due to the lack of hormone receptors, and HER2 expression of the tumor. Several studies have investigated the predictive/ prognostic factors of this breast cancer subtype, especially factors that could lead to targeted therapies.
We previously classified 100 Thai breast cancer patients recruited during 2002-2004 according to the results of IHC study and found a prevalence of TNB and TNN of 15% and 14%, respectively [5]. We found no significant difference in age, tumor size, lymphovascular invasion (LVI), nodal metastasis, microvessel density, expression of vascular endothelial growth factor (VEGF), or survival between subtypes. Moreover, we were unable to find significant clinical difference between TNB and TNN due to our small sample size. Comparison between TN and non-TN revealed significantly higher tumor grade, mitotic count expressed by Ki-67 index, p53, and vimentin expression, and decreased overall survival (OS) in TN subtype compared to non-TN subtype [6, 7].
In the current retrospective study of a larger population, we focused on the identification of TNB and TNN using biomarkers, clinical outcomes, and predictive/prognostic factors in the TN subtype.
Materials and Methods
The protocol for this study was approved by the Institutional Review Board of the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand [COA no. R635/2553(EC3)]. We performed a retrospective study of archival formalin-fixed paraffin-embedded (FFPE) breast cancer specimens previously reported ER-, PR-, and HER2- (IHC 0/1+) or equivocal (IHC 2+) during 2007 to 2010. Cases with adequate tissue for study were enrolled, and the histology and prognostic/ predictive factors of the retrieved tumors were reviewed by 3 pathologists, TC, NS and MW.
Construction of tissue microarray (TMA) and IHC study Three cores of 2 mm diameter FFPE tissue from each case and 6 controls (breast cancer with ER+, PR+, HER2+; breast cancer with p53+; fibroadenoma; appendix; lung; placental tissue) were constructed averaging 24 cases per one recipient block. Cases with HER2 IHC2+ were embedded in a separate recipient block. Each block was cut and stained for ER, PR, and HER2, markers of basal-like subtype [three basal CKs (CK5, CK14, and CK17) and EGFR], CD117, p63, p53, vimentin, Ki67, CK7, CK8/18, CK19, BCL2, p16, WT1, and cyclin D1. Dual in situ hybridization (DISH) for HER2 was performed on the block with HER2 IHC2+ by using the Ventana HER2 Dual ISH DNA Probe Cocktail Assay. Details of the antibodies used and the criteria for interpretation are provided in Table 1.
| Antibody | Clone | Source | Dilution | Positive criteria |
| ER | SP1 | Ventana | prediluted | ≥1% nuclear staining |
| PR | 1.00E+02 | Ventana | prediluted | ≥1% nuclear staining |
| HER2 | 4B5 | Ventana | prediluted | Score 3+, strong, complete membrane staining in ›10% of invasive tumor cells |
| CK5/6 | D5&16B4 | Cell Marque | 0.111111111 | ≥1% cytoplasmic and membrane staining |
| CK14* | LL002 | Novocastra | 0.180555556 | ≥1% cytoplasmic and membrane staining |
| CK17* | E3 | Novocastra | 0.111111111 | ≥1% cytoplasmic and membrane staining |
| EGFR | 5B7 | Ventana | prediluted | ≥1% membrane staining |
| CD117 | C-kit | DAKO | 0.736111111 | ≥10% membrane staining |
| p63 | 4A4 | DAKO | prediluted | Any nuclear staining |
| p53 | D0-7 | Cell Marque | 0.388888889 | ≥10% nuclear staining |
| Vimentin | V9 | DAKO | 0.388888889 | At least 10% cytoplasmic staining |
| Ki-67 | MIB1 | DAKO | 0.25 | Counted from at least 200 tumor cells, expressed in% |
| CK7 | OV-TL12/30 | Cell Marque | 0.736111111 | ≥1% cytoplasmic and/or membrane staining; score# |
| CK8/18 | SP3 | Thermo Scientific | 0.111111111 | ≥1% cytoplasmic and/or membrane staining; score# |
| CK19 | A53-B/A2.26 | Thermo Scientific | 0.25 | ≥1% cytoplasmic and/or membrane staining; score# |
| BCL2 | 124 | DAKO | 0.111111111 | ≥1% cytoplasmic membrane staining; score# |
| p16 | E6H4 | Ventana | prediluted | Cytoplasmic and nuclear staining; score# |
| WT1 | 6F-H2 | Cell Marque | 0.388888889 | Any nuclear staining Cytoplasmic staining, score# |
| Cyclin D1 | SP4 | Thermo Scientific | 0.111111111 | Any nuclear staining; score# |
All were performed using an automated immunostainer. *Performed from the Institute of Pathology, Ministry of Public Health, Thailand; #score=(intensity,1-3)+(quantity,1-5); intensity, 1=mild, 2=intermediate, 3=strong; quantity, 0=no positive cell, 1=<1, 2=≥1-10, 3=>10-33, 4=>33- 66, 5=>66-100% positive cells
All stained slides were scanned using an Aperio ScanScope (Leica Biosystems, Wetzlar, Germany), and IHC interpretation was performed manually on the scanned slides by TC and NS who were blinded to the clinical outcomes. The HER2 DISH was interpreted by NS. ER, PR, and HER2 results were evaluated according to the ASCO/CAP guidelines [8, 9]. Patients were re-classified into 5 groups as follows: 1) lumA (ER+ and/ or PR+, HER2-, and Ki-67 ≤14%), 2) lumB (ER+ and/or PR+, and HER2+ or ER+ and/or PR+, HER2-, and Ki-67 >14%), 3) HER2+ (ER-, PR-, and HER2+), 4) TNB (ER-, PR-, and HER2- with at least any CK5+, CK14+, CK17+, or EGFR+), and 5) TNN (ER-, PR-, HER2-, CK5-, CK14-, CK17-, and EGFR-).
Of 1,561 breast cancer patients (1,163 cases with predictive factor study) who underwent mastectomy or wide excision at Siriraj Hospital during 2007 to 2010, there were 276 patients with ER-, PR-, or HER2- (score 0/1+) or equivocal (score 2+) documented in the pathology reports. After review, 203 patients with adequate tissue for study were proceeded for TMA construction and IHC staining. A small number of cases with ER+/PR+ and HER2+ were identified. Twenty-four patients with equivocal HER2 status and HER2+ (IHC3+) had HER2 DISH performed, which revealed positivity in 18 patients (14 patients HER2 IHC3+ on pathology review, and 4 patients with HER2 IHC2+).
Pathological staging parameters, including tumor size, LVI, and nodal status, were obtained from review of the pathology reports. Clinical data, including age, mode of treatment, and survival status (recurrence and metastasis/ death), were retrieved from medical records, patient communication, and Thai population database.
Statistical analysis
Descriptive statistics were used to summarize demographic data, clinical data, and Ki67 index. Chi- square test was used to compare biomarker expressions between TNB and TNN, and between TN and non-TN (lumB and HER2). P-values were adjusted for multiple comparisons using a modified Bonferroni correction method.
For survival analysis, the follow-up period was defined as the time from the operation date to the date of the last visit/observation or death. Disease-free survival (DFS) was defined as the time between the date of the operation and the date of relapse (recurrence/metastasis). Overall survival (OS) was defined as the time between the date of the operation and the date of death. Log-rank test was used to estimate and compare survival between groups. A p-value of <0.05 was considered statistically significant.
Results
According to the IHC results of ER, PR, HER2, basal CK, and EGFR, there were 121 TNB, 17 TNN, 42 lumB, and 14 HER2+ patients with complete follow-up data. A total of 193 female patients were clinicopathologically assessed. The clinical, histologic, and IHC studies are summarized in Tables 2 and 3.
| TNB no.of cases | TNN no.of cases | LumB no.of cases | HER2 no.of cases | P (overall TNB vs TNN vs lumB) | P (TN vs lumB) | P TN vs nonTN | |
| (120, 62.2%) | (17, 8.8%) | (42, 21.8 %) | (14, 7.3%) | ||||
| Mean age (years) | 52.1 | 59.9 | 49.12 | 54.21 | 0.013 (overall) | 0.053 | 0.199 |
| (95% CI) | (49.8-54.5) | (52.6-67.2) | (45.7-52.5) | (44.98-63.45) | 0.013 | ||
| (min, max) | (27, 86) | (37, 88) | (32, 80) | (26, 85) | (lumB vs TNN) | ||
| Histologic subtype | |||||||
| IDC | 113 (94.2) | 17 (100) | 40 (95.2) | 14 (100) | |||
| ILC | 0 | 0 | 1 (2.4) | 0 | |||
| Metaplastic carcinoma | 5 (4.2) | 0 | 0 | 0 | |||
| Adenosquamous carcinoma | 1 (0.8) | 0 | 0 | 0 | |||
| Medullary-like features | 1 (0.8) | 0 | 0 | 0 | |||
| Mucinous carcinoma | 0 | 0 | 1 (2.4) | 0 | |||
| Histologic grade (IDC) | |||||||
| 1 | 3 (2.6) | 0 | 0 | 0 | |||
| 2 | 32 (28.1) | 6 (35.3) | 7 (17.5) | 3( 21.4) | |||
| 3 | 79 (69.3) | 11 (64.7) | 33 (82.5) | 11 (78.6) | 0.381 | 0.195 | 0.156 |
| Tumor size, mean | 3.13 | 4.67 | 3.14 | 2.41 | |||
| (95% CI) | (2.73-3.53) | (2.28-7.06) | (2.59-3.68) | (1.92-2.89) | |||
| (min, max) | (0.8-19.0) | (1.2-19.0) | (0.5-10.0) | (1.5-4.0) | |||
| ≤2 cm | 40 (33.3) | 3 (17.6) | 14 (33.3) | 7 (50) | |||
| 2-5 cm | 70 (58.3) | 11 (64.7) | 25 (59.5) | 7 (50) | |||
| > 5 cm | 10 (8.3) | 3 (17.6) | 3 (7.1) | 0 | 0.569 | 0.888 | 0.53 |
| LVI (+/-)(%+) | 40/79 (33.6) | 6/11 (35.3) | 16/26 (38.1) | 9/5 (64.3) | 0.871 | 0.612 | 0.169 |
| Axillary node status | |||||||
| Negative | 81 (67.5) | 9 (52.9) | 24 (57.1) | 6 (42.9) | |||
| Positive nodes 1-3 | 17 (14.2) | 2 (11.8) | 15 (35.7) | 4 (28.6) | |||
| ≥4 | 22 (18.3) | 6 (36.3) | 3 (7.1) | 4 (28.6) | 0.005 | 0.003 | |
| Perinodal invasion (+/-) (%+) | 18/13 (60) | 0/7 | 10/7 (58.8) | 3/4 (42.9) | 0.016 | 0.432 | 0.602 |
| Staging | |||||||
| 1 | 26 (21.7) | 1 (5.9) | 8 (19.0) | 4 (28.6) | |||
| 2 | 70 (58.3) | 7 (41.2) | 28 (66.7) | 6 (42.9) | |||
| 3 | 20 (16.7) | 6 (35.3) | 4 (9.5) | 3 (21.4) | |||
| 4 | 4 (3.3) | 3 (17.6) | 2 (4.8) | 1 (7.1) | 0.028 | 0.02 | 0.758 |
| Staging group | |||||||
| 1+2 | 96 (80.0) | 8 (47.1) | 36 (85.7) | 10 (71.4) | |||
| 3+4 | 24 (20.0) | 9 (52.9) | 6 (14.3) | 4 (28.6) | 0.004 | 0.002 | 0.345 |
| Surgery | |||||||
| Wide excision+SLNB | 21 (17.5) | 1 (5.4) | 3 (7.14) | 0 | |||
| Wide excision+ALND | 16 (13.3) | 1 (5.4) | 2 (4.8) | 1 (7.1) | |||
| TM+SLNB | 37 (30.8) | 3 (17.6) | 7 (16.7) | 2 (14.3) | |||
| TM+SLNB+ LD flap | 7 (5.8) | 1 (5.4) | 2 (48) | 0 | |||
| MRM | 39 (32.5) | 11 (64.7) | 28 (66.7) | 11 (78.6) | |||
| Neo-adjuvant therapy (total, 174) | 10 | 6 | 6 | 0 | |||
| Anthracycline-based | 9 (90.0) | 3 (50.0) | 5 (83.3) | ||||
| Anthracycline-based + taxane | 0 | 1(16.7) | 0 | ||||
| Others | 1 (10.0) | 2 (33.3) | 1 (16.7) | ||||
| Chemotherapy*(total, 174) | 98 | 14 | 33 | 13 | |||
| Anthracycline-based | 66 (67.3) | 6 (42.9) | 23 (69.7) | 8 (61.5) | |||
| Anthracycline-based + taxane | 14 (14.3) | 5 (35.7) | 5 (15.2) | 3 (23.1) | |||
| Others | 18 (18.4) | 3 (21.4) | 5 (15.2) | 2 (15.4) | |||
| None | 8 (7.5) | 3 (17.6) | 5 (13.2) | 0 | |||
| Radiation | 53 (44.2) | 9 (52.9) | 17 (40.5) | 2 (21.4) | |||
| Recurrence (+/-)(%+) | 32/88 (26.7) | 10/7 (58.8) | 14/28 (33.3) | 5/9 (35.7) | 0.26 | 0.743 | 0.009 |
| Status | |||||||
| Alive, disease free | 79 (65.8) | 5 (29.4) | 28 (66.7) | 7 (50.0) | |||
| Alive with disease | 5 (4.2) | 1 (5.9) | 2 (4.8) | 0 (0.0) | |||
| Dead | 36 (30.0) | 11 (64.7) | 12 (28.6) | 7 (50.0) | 0.057 | 0.723 | 0.754 |
Abbreviations: TNB, triple negative basal-like; TNN, triple negative non-basal-like; TN, triple negative breast cancer, TNB&TNN; lumB, luminal B; IDC, invasive ductal carcinoma; LVI, lymphovascular invasion; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; TM, total mastectomy; LD, latissimus dorsi; MRM, modified radical mastectomy; *, neoadjuvant and postoperative
| Antibody | Total no. | TNB | TNN | LumB | HER2 | P | P | P | P | |
| (193, 100%) | no.of cases (120, 62.2%) | no.of cases (17, 8.8%) | no.of cases (42, 21.8%) | no.of cases (14, 7.3%) | All subtypes | (TNB, TNN and lumB) | (TN vs lumB) | (TN vs Non-TN) | ||
| ER | Negative | 120 (100) | 17 (100) | 25 (59.5) | 14 (100) | |||||
| Positive | 0 | 0 | 17 (40.5) | 0 | ||||||
| PR | Negative | 120 (100) | 17 (100) | 10 (23.8) | 14 (100) | |||||
| Positive | 0 | 0 | 32 (76.2) | 0 | ||||||
| HER2 | Negative | 120 (100) | 17 (100) | 38 (90.5) | 0 | |||||
| Positive | 0 | 0 | 4 (9.5) | 14(100) | ||||||
| Ki-67 | 45.7 | 46.9 | 41.61 | 49.11 | 30.13 | |||||
| (95%CI, SD) | (43.05-48.35, 18.66) | (43.41-50.38, 19.28) | (33.72-49.49, 15.34) | (43.63-54.59, 17.58) | (23.24-37.02, 11.94) | 0.005 | 0.373 | 0.394 | 0.528 | |
| Ki-67 | ≤14 | 3 (1.6) | 2 (1.7) | 0 | 0 (2.4) | 1 (7.1) | ||||
| >14-30 | 41 (21.2) | 23 (19.2) | 6 (35.3) | 5 (11.9) | 7 (50.0) | |||||
| >30-50 | 72 (38.9) | 44 (36.7) | 5 (29.4) | 18 (42.9) | 5 (35.7) | 0.038 | 0.524 | 0.499 | 0.879 | |
| >50 | 77 (39.9) | 51 (42.5) | 6 (35.3) | 19 (45.2) | 1 (7.1) | |||||
| CK5/6 | Negative | 82 (42.5) | 37 (30.8) | 17 (100) | 16 (38.1) | 12 (85.7) | ||||
| Positive | 111 (57.5) | 83 (69.2) | 0 | 26 (61.9) | 2 (14.3) | 0.945 | 0.177 | |||
| CK14 | Negative | 110 (57.0) | 62 (51.7) | 17 (100) | 19 (45.2) | 12 (85.7) | ||||
| Positive | 83 (43.0) | 58 (48.3) | 0 | 23 (54.8) | 2 (14.3) | 0.157 | 0.769 | |||
| CK17 | Negative | 93 (48.4) | 48 (40.0) | 16 (100) | 20 (47.6) | 9 (64.3) | ||||
| Positive | 99 (51.6) | 72 (60.0) | 0 | 22 (52.4) | 5 (35.7) | 0.949 | 0.551 | |||
| EGFR | Negative | 67 (34.7) | 31 (25.8) | 17 (100) | 12 (28.6)) | 8 (57.1) | ||||
| Positive | 126 (65.3) | 89 (74.2) | 0 | 30 (71.4) | 6 (42.9) | 0.437 | 0.852 | |||
| CD117 | Negative | 124 (64.2) | 66 (55.0) | 13 (76.5) | 31 (73.8) | 14 (100) | ||||
| Positive | 69 (35.8) | 54 (45.0) | 4 (23.5) | 11 (26.2) | 0 | 0.002 | 0.04 | 0.06 | 0.003 | |
| p63 | Negative | 153 (79.3) | 100 (83.3) | 17 (100) | 26 (61.9) | 10 (71.4) | ||||
| Positive | 40 (20.7) | 20 (16.7) | 0 | 16 (38.1) | 4 (28.6) | 0.003 | 0.001 | 0.001 | 0.001 | |
| p53 | Negative | 92 (47.7) | 63 (52.5) | 7 (41.2) | 16 (38.1) | 6(42.9) | ||||
| Positive | 101 (52.3) | 57 (47.5) | 10 (58.8) | 26 (61.9) | 8 (57.1) | 0.379 | 0.23 | 0.14 | 0.136 | |
| Vimentin | Negative | 119 (61.7) | 74 (61.7) | 12 (70.6) | 20 (47.6) | 13 (92.9) | ||||
| Positive | 74 (38.3) | 46 (38.3) | 5 (29.4) | 22 (52.4) | 1 (7.1) | 0.02 | 0.151 | 0.066 | 0.618 | |
| CK7 | Negative | 19 (9.9) | 12 (10.0) | 2 (11.8) | 4 (9.8) | 1 (7.1) | ||||
| # score ≥3 | Positive | 173 (90.1) | 108 (90.0) | 15 (88.2) | 37 (90.2) | 13 (92.9) | 0.98 | 0.971 | 0.931 | 0.813 |
| CK8/18 | Negative | 13 (6.8) | 8 (6.7) | 4 (23.5) | 1 (2.4) | 0 (0.0) | ||||
| # score ≥3 | Positive | 179 (93.2) | 112 (93.3) | 13 (76.5) | 40 (97.6) | 14 (100.0) | 0.02 | 0.017 | 0.172 | 0.084 |
| BCL2 | Negative | 121 (63) | 70 (58.3) | 16 (94.1) | 21 (51.2) | 14 (100.0) | ||||
| # score >0 | Positive | 71 (37) | 50 (41.7) | 1 (5.9) | 20 (48.8) | 0 (0.0) | <0.001 | 0.008 | 0.185 | 0.911 |
| p16 | Negative | 28 (14.6) | 14 (11.7) | 4 (23.5) | 6 (14.6) | 4 (28.6) | ||||
| # score >0 | Positive | 164 (85.4) | 106 (88.3) | 13 (76.5) | 35 (85.4) | 10 (71.4) | 0.25 | 0.395 | 0.806 | 0.371 |
| # score 3-6 | 83 (52.9) | 58 (56.3) | 7 (58.3) | 11 (32.4) | 7 (87.5) | |||||
| # score 7-8 | 74 (47.1) | 45 (43.7) | 5 (41.7) | 23 (67.6) | 1 (12.5) | 0.017 | 0.046 | 0.013 | 0.129 | |
| WT1 | Negative | 86 (44.8) | 48 (40.0) | 7 (41.2) | 19 (46.3) | 12 (85.7) | ||||
| # score ≥3 | Positive | 106 (55.2) | 72 (60.0) | 10 (58.8) | 22 (53.7) | 2 (14.3) | 0.013 | 0.776 | 0.48 | 0.041 |
| WT1 | Negative | 188 (97.9) | 116 (96.7) | 17 (100.0) | 41 (100.0) | 14 (100.0) | ||||
| nuclear stain | Positive | 4 (2.1) | 4 (3.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.484 | 0.372 | 0.268 | 0.2 |
| Cyclin D1 | ||||||||||
| # score 0 | 47 (24.4) | 28 (23.3) | 9 (52.9) | 9 (21.4) | 1 (7.1) | |||||
| # score >0 | 145 (75.1) | 92 (76.7) | 8 (47.1) | 32 (76.2) | 13 (92.9) | 0.019 | 0.027 | 0.516 | 0.198 |
Abbreviations: TNB, triple negative basal-like; TNN, triple negative non-basal-like; TN, triple negative breast cancer; LumB, luminal B. #score=(intensity, 1-3)+(quantity,1-5); intensity, 1=mild, 2=intermediate, 3=strong; quantity, 0=no positive cell, 1=<1, 2=≥1-10, 3=>10-33, 4=>33-66, 5=>66-100% positive cells
Overall characteristics of included cases (Table2)
The mean age at diagnosis was 52.3±13.1 years (range: 26-88 years, median: 51 years). There were 184 (95.3%) invasive ductal carcinomas, not otherwise specified (IDC, NOS), 1 invasive lobular (ILC), 5 metaplastic, 1 adenosquamous, 1 medullary-like, and 1 mucinous carcinoma with micropapillary-like feature. High-grade tumor accounted for 71.5%. Tumor size ranged from 0.5 to 19 cm (mean: 3.22±2.4 cm). Modified radical mastectomy was performed in 89 (46.1%) patients. Total mastectomy with sentinel lymph node (SNL) biopsy with or without axillary node dissection was performed in 59 (30.6%) patients. SLN was performed in 55.4%, and 9.3% were positive. Among 193 patients, 32, 103, 6, and 52 patients had stage 1, 2, 3, and 4 at diagnosis, respectively. Positive axillary nodes were found in 37.8% of patients, of which 50.8% had perinodal invasion. LVI was observed in 37.3% of all patients. Neoadjuvant chemotherapy was given to 11.4% of patients, and 81.9% received postoperative chemotherapy. Less than half of the patients (46.5%) received radiation therapy. The follow-up time ranged from 0.53 to 96.53 months (median follow-up: 62.93 months). Of those, 10.9% and 30.3% had locoregional recurrence and distant metastasis, respectively; 61.7% were alive and disease-free; 31.1% had relapse or metastasis during the course of their disease; and, 34.7% died of their disease.
Associations between clinicopathological parameters and subtypes
Patients with TNN breast cancer had significantly older age than those with lumB breast cancer (p=0.013).
No significant difference in tumor size was found among the TNB, TNN, and lumB subtypes. LumB was associated with the lowest proportion of more than 3 axillary node involvement, while TNN was associated with the highest proportion (p=0.005), and so did TN when compared with lumB (p=0.003) (Table 2). Forty-eight patients had metastasis at the time of diagnosis. TNN had higher stages than TNB or lumB (p=0.028), and so did TN versus lumB (p=0.020).
One-third of TNN and 8.3% of TNB received neoadjuvant treatment, whereas no HER2+ received neoadjuvant chemotherapy. Of 174 patients with known history of chemotherapy treatment, 90.8% received neoadjuvant and/or postoperative adjuvant chemotherapy, including 92.5% of TNB, 82.4% of TNN, 86.8% of lumB, and all HER2+. Of those, 81.6%, 78.6%, 84.8%, and 84.6% received anthracyclines either singly or in combination with others, respectively. TN had a significantly higher recurrence rate than non-TN.
Biomarker expressions among different subtypes
IHC of biomarkers according to the 4 reclassified subtypes are summarized in Table 3. ER, PR, and HER2 of lumB were expressed in 40.5%, 76.2%, and 9.5%, respectively.
Basal-like biomarker expressions
Basal CKs: Both TNB and lumB contained basal CKs (CK 5/6, CK14, or CK17) in a significant number. They were found in 35.7% of HER2+. Among these, CK5/6 had greater sensitivity (70.0%) for detecting basal-like subtype when compared with other CKs. When all three were used, 81.7% of TNB could be diagnosed.
EGFR: This protein was detected in nearly equal proportions in TNB and lumB (74.2% and 71.4%, respectively), and in less than half of HER2+. It was insignificantly more prevalent than CK5/6 (18.3% of TNB and 9.5% of lumB had positive EGFR alone). With the use of CK5/6 and EGFR, 98.3% of TNB could be detected.
CD117: The distribution of CD117 expressions was significantly different with no expression in HER2+, low frequency in TNN (23.5%), and higher frequency in lumB and TNB (26.2% and 45.0%, respectively).
p63: This protein had low sensitivity (15.8% positive) when compared with basal CKs for detecting TNB. In addition, it was not expressed in TNN. It was present in all 5 metaplastic and adenosquamous carcinomas, and in one-third of lumB; however, it declined significantly when TN (TNB and TNN) subtype was compared with lumB (13.9% vs. 38.1%, respectively).
Vimentin: This marker was expressed in only 10% of HER2 (p=0.020) while nearly 30-50% of tumors in the three remaining subtypes.
p53: This protein was expressed in more than 50% of tumors in all subtypes without significant difference in distribution.
Ki-67 index: Prolonged storage of tissue caused diminished staining, so repeat staining with increased concentration was performed. Ki67 index was high in all subtypes with a median value of 45%, except for the HER2+ subtype, which had a significantly lower Ki67 index (30.13%, p=0.005).
CD34: There was no expression of this protein in tumor cells, including spindle cells in metaplastic carcinoma. No stromal cell staining was observed.
CK7: Only 5 patients had no expression of this marker. Expression was similar in all subtypes with approximately 90% positive when using a cutoff score of 3.
CK8/18: Only 3 patients had no CK8/18 expression. Expression among subtypes (score of at least 3) was significantly different with highest expression of 100% in HER2+, and lowest expression of 76.5% in TNN subtypes. Three patients had no CK7 expression, but had CK8/18 expression.
CK19: Six patients had no CK19 expression. One of them had negative CK7 expression, and two had neither CK7 nor CK8/18 expression. All patients with negative CK19 also had negative BCL2. (data not shown in Table 3) BCL2: There was no BCL2 expression in 63%, and less than 10% expression in 20.8% of patients. HER2+ subtype had no expression regardless of cutoff score. When using a cutoff score of less than 10%, TNN subtype also had no expression.
p16: There was no expression in 14.6%, and diffuse strong expression in 47.1% of all patients. There was no significant difference in high p16 expression between TNB and TNN. Diffuse strong expression was less in HER2+ subtype, and was significantly increased in lumB when compared with those with non-diffuse staining (Table 3, p16 score 3-6 vs. score 7-8).
Cyclin D1: All types with any positive tumor cell ranged from 47.1% in TNN to 92.9% in HER2+ subtype. Only 2.1% of all cases were strongly positive.
WT1: HER2+ subtype had the lowest cytoplasmic expression of WT1 (14.3% when using score 3 as the cutoff), whereas TNB, TNN, and lumB subtypes had a similar proportion of positive WT1. Localization of WT1 in the nucleus was observed in only 4 patients belonging to the TNB group, and all of them died of their disease. Negative BCL2 was found in these 4 patients, and 3 of them had negative p16, luminal CKs, or cyclin D1. There was no association between cytoplasmic/cell membrane staining intensity or percentage of staining and nuclear staining of WT1.
Comparison of biomarker expressions between TN (TNB and TNN) and non-TN (lumB and HER2)
Ki67 index was not significantly different between the TN and non-TN groups. Increased CD117 expression was significantly found in TN, while p63 expression was more common in non-TN.
Survival analysis according to subtypes and biomarker expressions (Tables 4-5) (Figure 1).
There were 10 patients with distant metastasis at the time of diagnosis, so only 116 TNB, 14 TNN, 40 lumB, and 13 HER2 were analyzed for DFS. The mean follow- up time was 72.44±2.61 months. The median follow-up time was 62.93 months (range: 0.53-96.5 months). Loco- regional recurrence occurred in 21 patients, and 53 patients had distant metastasis. Sixty-six deaths occurred during the follow-up period (Table 2).
The clinical parameters significantly associated with lower DFS and OS were tumor size larger than 5 cm, grade 3 tumor, and presence of axillary nodal involvement. Furthermore, patients with higher stage had significantly lower OS (Table 4).
| Clinicopathologic feature/Breast cancer subtype | DFS | OS | ||||||
| Case | Event | 5-year survival | p | Case | Event | 5-year survival | p | |
| Age (years) | ||||||||
| ≤50 | 91 | 30 | 0.687 | 96 | 32 | 0.699 | ||
| >50 | 92 | 34 | 0.66 | 0.767 | 97 | 34 | 0.671 | 0.808 |
| Tumor size | ||||||||
| ≤2 cm | 63 | 19 | 0.702 | 64 | 19 | 0.741 | ||
| 2-5 cm | 108 | 37 | 0.696 | 114 | 36 | 0.711 | ||
| >5 cm | 12 | 8 | 0.333 | 0.006 | 15 | 11 | 0.267 | <0.001 |
| Tumor grade | ||||||||
| 1 & 2 | 47 | 22 | 0.515 | 49 | 22 | 0.562 | ||
| 3 | 128 | 40 | 0.714 | 0.048 | 136 | 43 | 0.715 | 0.108 |
| LVI | ||||||||
| No | 115 | 32 | 0.757 | 120 | 32 | 0.762 | ||
| Yes | 67 | 32 | 0.531 | 0.013 | 72 | 34 | 0.56 | 0.008 |
| Axillary nodal involvement | ||||||||
| No | 118 | 28 | 0.785 | 120 | 25 | 0.809 | ||
| Yes | 65 | 36 | 0.473 | <0.001 | 73 | 41 | 0.483 | <0.001 |
| Staging | ||||||||
| 1, 2 | 150 | 36 | 0.787 | |||||
| 3, 4 | 43 | 30 | 0.33 | <0.001 | ||||
| TNB vs TNN | ||||||||
| TNB | 116 | 37 | 0.701 | 0.004 | 120 | 36 | 0.728 | 0.001 |
| TNN | 14 | 9 | 0.343 | 17 | 11 | 0.343 | ||
| TN vs LumB | ||||||||
| TN | 130 | 46 | 0.661 | 0.296 | 137 | 47 | 0.679 | 0.389 |
| LumB | 40 | 12 | 0.749 | 42 | 12 | 0.737 | ||
| TN vs nonTN | ||||||||
| TN | 130 | 46 | 0.661 | 0.514 | 137 | 47 | 0.679 | 0.722 |
| nonTN | 53 | 18 | 0.697 | 56 | 19 | 0.694 | ||
| TNB vs LumB | ||||||||
| TNB | 116 | 37 | 0.749 | 0.53 | 120 | 37 | 0.735 | 0.606 |
| LumB | 40 | 12 | 0.701 | 42 | 12 | 0.737 |
Abbreviations: TNB, triple negative basal-like; TNN, triple negative non-basal-like; TN, triple negative breast cancer; lumB, luminal B; LVI, lymphovascular invasion
DFS: When compared with TNB, TNN had significantly lower DFS (p=0.004, Table 4; Figure 1).
Figure 1. Disease Free Survival (DFS) and Overall Survival (OS) According to Subtypes.
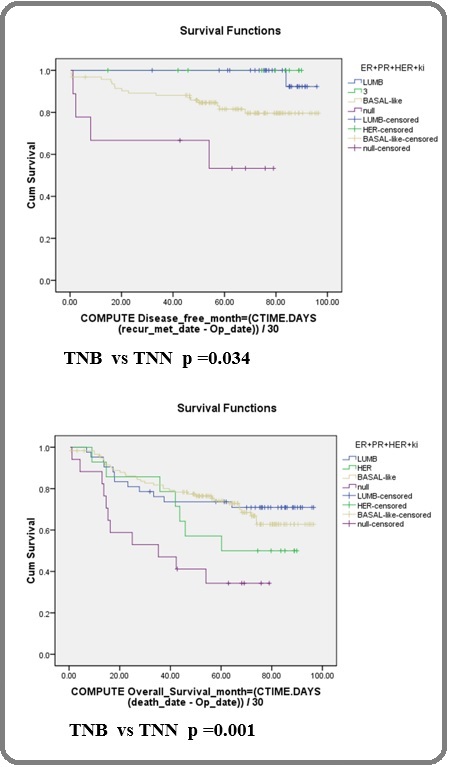
In TNN subtype, patients with a CK7 score ≤3 or CK8/18 score ≤3 who had disease recurrence before 5 years had significantly lower DFS (p<0.001) (Table 5). In the TN group, an absence of EGFR or cyclin D1 was significantly associated with lower DFS (p=0.014 and p=0.026, respectively) (Table 5). OS: TNN had a significantly higher death rate than TNB (p=0.001, Table 4; Figure 1). No significant difference was observed when OS between lumB and TNB or between non-TN and TN were compared (Table 4). In TN, the patients with absence of EGFR or CK8/18 had significantly lower OS (p=0.005 and p=0.045, respectively, Table 5). Only one patient in the TN group had a cyclin D1 score ≥7, and that patient died at 14.6 months. Four patients who had positive WT1 nuclear staining died, and three of those died before 15 months after diagnosis. Other biomarkers, including basal CKs, BCL2, p16 (in varying cut off levels), and WT1 (cytoplasmic staining), in each subtype and overall cases had no significant influence on survival (Table 5).
| Subtype | Biomarker status | DFS | OS | |||||||
| Case | Event | 5-year survival | p | Case | Event | 5-year survival | p | |||
| TNB | CK5/6 | - | 36 | 10 | 0.771 | 0.522 | 37 | 9 | 0.829 | 0.354 |
| + | 80 | 27 | 0.67 | 83 | 27 | 0.686 | ||||
| CK14 | - | 61 | 21 | 0.702 | 0.763 | 62 | 19 | 0.749 | 0.888 | |
| + | 55 | 16 | 0.699 | 58 | 17 | 0.706 | ||||
| CK5/14/17 | - | 22 | 5 | 0.816 | 0.262 | 22 | 5 | 0.909 | 0.295 | |
| + | 94 | 32 | 0.674 | 98 | 31 | 0.687 | ||||
| EGFR | # score 0,1 | 63 | 20 | 0.719 | 0.847 | 64 | 19 | 0.72 | 0.887 | |
| 2,3 | 53 | 17 | 0.677 | 56 | 17 | 0.739 | ||||
| EGFR/CKs | - | 2 | 1 | 0.5 | 0.751 | 2 | 1 | 0.5 | 0.713 | |
| + | 114 | 36 | 0.704 | 118 | 35 | 0.733 | ||||
| CK7 | # score ≤3 | 12 | 2 | 0.917 | 0.166 | 12 | 1 | 0.917 | 0.081 | |
| >3 | 104 | 35 | 0.674 | 108 | 35 | 0.706 | ||||
| CK8/18 | # score ≤3 | 8 | 2 | 0.75 | 0.565 | 8 | 2 | 0.75 | 0.601 | |
| >3 | 108 | 35 | 0.696 | 112 | 34 | 0.726 | ||||
| P16 | - | 13 | 6 | 0.615 | 0.289 | 14 | 6 | 0.571 | 0.304 | |
| + | 103 | 31 | 0.711 | 106 | 30 | 0.749 | ||||
| WT1 | - | 44 | 13 | 0.717 | 0.62 | 45 | 11 | 0.778 | 0.251 | |
| + | 72 | 24 | 0.69 | 75 | 25 | 0.698 | ||||
| Cyclin D1 | - | 27 | 11 | 0.619 | 0.247 | 28 | 11 | 0.679 | 0.227 | |
| + | 89 | 26 | 0.726 | 92 | 25 | 0.743 | ||||
| TNN | CK7 | # score ≤3 | 2 | 2 | 0 | <0.001 | 2 | 2 | 0 | <0.001 |
| >3 | 12 | 7 | 0.4 | 15 | 9 | 0.289 | ||||
| CK8/18 | # score ≤3 | 4 | 4 | 0 | <0.001 | 4 | 4 | 0 | 0.001 | |
| >3 | 10 | 5 | 0.48 | 13 | 7 | 0.449 | ||||
| p16 | - | 3 | 3 | 0 | 0.363 | 4 | 3 | 0.25 | 0.846 | |
| + | 11 | 6 | 0.455 | 13 | 8 | 0.385 | ||||
| WT1 | - | 5 | 3 | 0.4 | 0.543 | 7 | 4 | 0.429 | 0.312 | |
| + | 9 | 6 | 0.333 | 10 | 7 | 0.3 | ||||
| Cyclin D1 | - | 8 | 6 | 0.25 | 0.102 | 9 | 6 | 0.333 | 0.712 | |
| + | 6 | 3 | 0.5 | 8 | 5 | 0.375 | ||||
| TN | CK7 | # score ≤3 | 14 | 4 | 0.786 | 0.49 | 14 | 3 | 0.786 | 0.302 |
| >3 | 116 | 42 | 3644 | 123 | 44 | 0.666 | ||||
| CK8/18 | - | 3 | 2 | 0.333 | 0.052 | 3 | 2 | 0.333 | 0.045 | |
| + | 127 | 44 | 0.699 | 134 | 45 | 0.687 | ||||
| CK8/18 | # score ≤3 | 12 | 6 | 0.5 | 0.201 | 12 | 6 | 0.5 | 0.19 | |
| >3 | 118 | 40 | 0.677 | 125 | 41 | 0.696 | ||||
| EGFR | - | 44 | 21 | 0.516 | 0.014 | 47 | 23 | 0.506 | 0.005 | |
| + | 86 | 25 | 0.737 | 90 | 24 | 0.773 | ||||
| BCL2 | # score 0-2 | 92 | 33 | 0.677 | 0.934 | 98 | 34 | 0.681 | 0.945 | |
| >2 | 38 | 13 | 0.656 | 39 | 13 | 0.681 | ||||
| p16 | - | 16 | 9 | 0.492 | 0.085 | 18 | 9 | 0.494 | 0.204 | |
| + | 114 | 37 | 0.686 | 119 | 38 | 0.709 | ||||
| WT1 | - | 49 | 16 | 0.682 | 0.497 | 52 | 15 | 0.728 | 0.196 | |
| + | 81 | 30 | 0.649 | 85 | 32 | 0.649 | ||||
| Cyclin D1 | - | 35 | 17 | 0.532 | 0.026 | 37 | 17 | 0.593 | 0.063 | |
| + | 95 | 29 | 0.71 | 100 | 30 | 0.711 | ||||
| All | CK8/18 | - | 3 | 2 | 0.333 | 0.03 | 3 | 2 | 0.333 | 0.032 |
| + | 179 | 61 | 0.678 | 189 | 64 | 0.689 | ||||
| EGFR | - | 63 | 27 | 0.584 | 0.08 | 67 | 29 | 0.58 | 0.047 | |
| + | 120 | 37 | 0.722 | 126 | 37 | 0.743 | ||||
| p16 | - | 25 | 13 | 0.517 | 0.06 | 28 | 14 | 0.497 | 0.069 | |
| + | 157 | 50 | 0.698 | 164 | 52 | 0.716 | ||||
| CyclinD1 | - | 44 | 21 | 0.539 | 0.013 | 47 | 22 | 0.574 | 0.025 | |
| + | 138 | 42 | 0.715 | 145 | 44 | 0.72 |
Abbreviations: TNB, triple negative basal-like; TNN, triple negative non-basal-like; TN, triple negative breast cancer; lumB, luminal B; All, all cases. #score=(inten sity,1-3)+(quantity,1-5); intensity, 1=mild, 2=intermediate, 3=strong; quantity, 0=no positive cell, 1=<1, 2=≥1-10, 3=>10-33, 4=>33-66, 5=>66-100% positive cells
Effects of treatment on survival
Breast conserving surgery
Of 45 patients, 10 of 37 TNB, 1 of 2 TNN, 0 of 5 lumB, and 1 HER2+ had recurrence within 5 years, and 9 of 37 TNB, 1 of 2 TNN, 0 of 5 lumB, and 1 HER2+ died within 5 years (p=0.191 and p=0.019, respectively). However, the results of this analysis should be interpreted with caution due to the small number of subjects.
Total mastectomy
Of 145 patients with exclusion of stage 4 breast cancer, 37.8 % of 82 TNB, 69.2% of 13 TNN, 37.8% of 37 lumB, and 46.2% of 13 HER2+ had recurrence within 5 years, and of 148 patients, 33.7% of 83 TNB, 66.3% of 15 TNN, 32.4% of 37 lumB, and 46.2% of 13 HER2+ died within 5 years. TNN had significantly lower DFS and OS when compared with TNB or lumB (p=0.027 and p=0.021, respectively). No significant difference in DFS or OS was observed between TNB and lumB.
Chemotherapy
TN patients and overall patients in our cohort who received chemotherapy had significantly increased DFS, but not significantly increased OS (all patients p=0.028 and p=0.121, respectively, TN patients p<0.001 and p=0114, respectively). Among the overall cohort, anthracycline-based alone or in combination with taxane was used in 81% of patients who received neoadjuvant chemotherapy, in 77.8% who received postoperative chemotherapy, and in 82.3% who received neoadjuvant and postoperative chemotherapy (81.6% of TNB, and 78.6% of TNN) (Tables 2 and 4).
Radiotherapy
Patients who received radiotherapy had significantly lower DFS and OS (p=0.03 and p=0.011, respectively). In TN subtype, receiving radiotherapy did not result in significantly different DFS or OS. However, patients with TN breast cancer who underwent lumpectomy and received subsequent radiotherapy had significantly increased DFS (p=0.029). In multivariate analysis, patients who received radiotherapy had borderline significantly increased DFS, but not OS (adjusted for age, types of surgery, and stages).
Multivariate analysis
Overall, multivariate analysis by Cox linear regression model showed that among clinicopathological parameters, tumor size and axillary nodal involvement were the independent prognostic factors for DFS (hazard ratio [HR]: 1.154, 95% confidence interval [CI]: 1.030-1.293, p=0.013, and HR: 2.497, 95% CI: 1.411-4.420, p=0.002, respectively), and for OS (HR: 1.162, 95% CI: 1.084- 1.245, p<0.001, and HR: 2.628, 95% CI: 1.488-4.643, p=0.001, respectively). In TNN subtype, CK8/18 was the independent predictor for better DFS and OS (HR: 0.040, 95% CI: 0.002-0.758, p=0.032, and HR: 0.090, 95% CI: 0.011-0.717, p=0.023, respectively). In TN subtype, positive CK8/18 (score >0) and positive EGFR (score >0) were the independent predictors for DFS (HR: 0.045, 95% CI: 0.005-0.405, p=0.006, and HR: 0.491, 95% CI: 0.262- 0.921, p=0.027, respectively). CK8/18 (score >0) and positive EGFR (score >0) were the independent predictors for OS (HR: 0.060, 95% CI: 0.007-0.512, p=0.010, and HR: 0.489, 95% CI: 0.263-0.909, p=0.024, respectively).
Discussion
This study, which had a larger sample size than our previous study, found 11.9% TN, of which 87.7% were TNB. This is quite different from the previous study that reported 25% TN, of which 56% were TNB [5]. This difference between studies may be explained by the larger size of our study cohort, differences in criteria for evaluating predictors or markers, and different clones of antibodies. The current positive criteria used were for the most part similar to those used in studies of IHC markers for basal-like breast cancer against a gene expression profile gold standard [10-12].
The prevalence of TNB over TNN in the current study was quite high (87.7%). With comparable thresholds of biomarker expression, this was similar to the finding of Taliano, et al. [13] (89.1%), lower (80.6% of 352 TN cases) than the finding of Levva, et al. [12], and lower (81.06% of 391 TN cases) than the finding of Prat, et al. [14]. We could find significant differences in DFS or OS between TNB and TNN, even with increased survival in the former. These findings were in agreement with those from a study by Choi, et al. [15], but contrary to the findings of other studies [16, 17]. The better prognosis of TNB could be related to its good response to chemotherapy [14] and its heterogeneity of disease [18]. This might suggest the need to differentiate the TNN from the TNB subgroup.
TNN also had the poorest prognosis among the four subtypes/subgroups (TNB, TNN, lumB, and HER2+), which was also reported by Choi, et al. [15]. Most lumB in our study had high Ki67 index, and approximately 10% of those had HER2 amplification. These findings may be explained by the archived pathology report diagnosis of TN breast cancer that was then revised to low positive ER or PR after our review. These types of lumB breast cancer have some phenotypes of TN breast cancer (high grade nuclei, high Ki67 index) that are considered to be subgroup with poor prognosis close to the TN cancer.
Regarding clinicopathological factors, the patients with TNN were significantly older than lumB breast cancer patients, and there was a trend toward TNN being older than TNB. Most patients in our cohort had high histologic grade, and all TNN were IDC, NOS whereas non-IDC, NOS including metaplastic and medullary-like carcinomas, were TNB subtype. There was no significant difference in tumor size, LVI, lymph node metastasis, or perinodal invasion among subtypes, except for TNN, which had a higher proportion of more than 3 metastatic axillary lymph nodes compared to lumB, and had the least perinodal extension. TNN also had higher staging (stages 3 and 4) and a higher death rate when compared with others. The higher staging of TNN could be one of the explanations for the observed worse prognosis.
Regarding the clinical prognostic factors, the commonly known adverse prognostic factors, tumor size larger than 5 cm and presence of axillary nodal involvement, were also the independent predictors for lower DFS and/or OS in this study. The presence of LVI or higher stage was associated with decreased OS.
Maximal tumor size was an adverse prognostic factor for both DFS and OS by univariate analysis in TNN if the tumor was more than 5 cm; however, in TNB, it only adversely affected OS.
Prevalence of biomarkers and their influence on survival in TNB, TNN, and TN
There is no single biomarker that is specific to any breast cancer subtype. Each can be more or less found in different subtypes, but their strength of expression varies. For example, basal CK expression is greater in TNB and lumB, but less in HER2+; EGFR is more commonly found in TNB, lumB, and HER2+; CD117 is more commonly found in TNB, TNN, and lumB; and p63 is more prevalent in non-TN than in TN. In our study, the combination of EGFR and CK5/6 can diagnose more than 98% of TNB. Thus, in routine practice, we suggest the using of both EGFR and CK5/6 for distinguishing between TNB and TNN.
All biomarker expressions studied in this cohort had no influence on either the DFS or OS of TNB, including basal CKs and EGFR at different cutoffs. This finding is similar to and different from some previous studies [15-17]. However, in some groups or combinations of subtypes, EGFR score >0, CK8/18 score >0, cyclin D1 score >0, and p16 score >0 were independent factors for DFS and/or OS.
EGFR expression is more commonly present in TNB than in other subtypes, and according to many studies is associated with adverse prognosis [19-21]. However, different biomarker cutoffs, even for hormone receptors, HER2 status, and EGFR, have to be considered. In the present study of biomarkers, EGFR expression was found in 74.2%, which is consistent with other studies that reported a range of 42-76% [19, 20, 22, 23], and close to the 71.6% reported from a study in the molecular activity and immunohistochemistry of EGFR [12]. That group also found the EGFR and EGFR/p53 immunophenotypes to be associated with favorable outcomes in TN patients receiving anthracyclines and/or taxanes. Using a comparable EGFR cutoff, our study also found the presence of an EGFR score >0 to be an independent factor for DFS and OS not only in the TN group, but also in the combined group of TN and lumB. These results may be explained by the adjuvant treatments with anthracycline- based and/or taxane in our cohort, which were as high as 81.6%, 78.6%, 84.8%, and 84.6% in the TNB, TNN, lumB, and HER2+ groups, respectively.
Luminal CKs: The weak or absent expression of luminal CKs (CK 7, CK8, and CK18) were previously reported to be associated with recurrence/metastasis and OS [24-26]. Our study demonstrated that loss of CK7 or CK8/18 expression had an adverse effect on DFS and OS in TNN. In the TN group, loss of CK8/18 expression was also associated with lower OS, and a trend toward lower DFS. In multivariate analysis, all patients except for HER2 subtype with a loss of CK8/18 expression had lower DFS and OS (all patients, including HER2+, had only decreased OS). These findings also support those from the previous studies. The explanation for this could be related to the dedifferentiation of tumor cells leading to proliferation and progression of the tumor [24, 26].
Among all luminal CKs (CK7, CK8/18, and CK19), loss of CK19 expression was found in 3.1% of the patients, while loss of CK8/18 was found in 1.5% of patients. As such, when using CK19 as the primer in polymerase chain reaction for detection of metastatic carcinoma in sentinel lymph node, there could be some false negativity, especially in high-grade carcinoma.
BCL2: The protein product of the BCL2 (B-cell lymphoma-2) gene is commonly known as its anti- apoptotic activity. The BCL2 family includes anti-apoptotic regulators, pre-apoptotic regulators, and antiapoptotic proteins. Interactions among this family of proteins could result in cell apoptosis [27, 28]. In breast cancer, the expression ranged upward to 54.4% [29], with the lowest expressions of 11.4-18.3% observed in HER2+ subtype [29, 30] and commonly in HR+/HER2- [29]. In our study of the TN group, we found low or no expression in the TNN and HER2+ subtypes, which is similar to a previous study that found no expression in HER2+ subtype, and less presence in TNN than in TNB subtype [31]. The expression of BCL2 in TN (predominantly in TNB) was 37.3% or 20.4% according to different cutoffs. The frequency of the expression was comparable to that reported from other studies [30, 32]. Regarding its effect on survival, there is some controversy regarding the role of BCL2 as a prognostic marker. It was shown to be a favorable prognosticator in overall breast cancers, and an independent prognostic marker in HR+/ HER2- subtype [29, 30]. Multivariate analysis in another study revealed independent association between BCL2 and unfavorable outcome in the non-basal subgroup [32], and in TN patients treated with anthracycline-based adjuvant chemotherapy [21]. In the present study, we found no prognostic influence of this protein on TNB or lumB.
These findings are in agreement with those reported from two previous studies [29, 30]. In the present study, we found no significant influence of BCL2 on survival among the overall cohort, or in any of the subtypes.
p16: This marker is a tumor suppressor protein that acts as a cyclin-dependent kinase (CDK) inhibitor that inactivates CDK4/6, which inhibits phosphorylation of the retinoblastoma protein and leads to arrest of the cell cycle. Our study demonstrated high expression of p16 in lumB without any prognostic significance, whereas loss of expression was an independent prognostic indicator for lower DFS and OS among the whole cohort. This is in agreement with a study in breast cancer cell line that postulated that loss of p16 expression could reduce the response of ER-negative breast cancer cells to chemotherapy by conferring cancer stem cell-like properties [33]. Another study reported association between diffusely positive p16 and increased survival in no-special type TN patients treated with neoadjuvant chemotherapy [34]. We did not find this association. Our group is currently investigating the relationship between p16 expression and in retinoblastoma proteins in this cohort.
Cyclin D1: This marker is a protooncogene expression that is present in and shorting the G1 phase of the cell cycle via the cyclin D1 and CDK 4/6 complex pathway resulting in uncontrolled proliferation. It also interacts with a variety of other transcription factors, including ER, androgen receptor, and histone deacetylases and acetylases, which suggests that cyclin D1 plays an important role in the regulation of transcription [35, 36]. Cyclin D1 was shown to express in about half of all invasive carcinomas [37], and it was found to positively correlate with ER and HER2 expression [38]. In our cohort, it was expressed in 47.1% of TNN, and in 92.9% of HER2+. The results of cyclin D1 expression were not different from the previous reports. The prognostic/ predictive role of cyclin D1 expression has not yet been fully elucidated. Higher expression of cyclin D1 was shown to improve tumor-free survival [38]. Alternatively, higher expression of cyclin D1 was reported to be associated with increased risk of breast cancer death in ER positive cases [39]. Regarding ER-negative breast cancer, there is still some controversy on overexpression of cyclin D1 with poor or good DFS and OS [40, 41]. In univariate analysis, cyclin D1 expression was significantly associated with better DFS in TN, and with better DFS and OS among all cases in our entire cohort without HER2+; however, those significant associations did not survive multivariate analysis. With other cutoffs, there was no significant correlation. Comparison with others or conclusion of the outcome results were quite difficult as there was some difference in the cutoffs and the treatments used.
WT1: This marker is a product of WT1 (Wilms’ tumor), which was originally known as tumor suppressor gene [42]. It has a role in proliferation, angiogenesis, and cancer induction epithelial-to-mesenchymal transition [43]. Whether it acts as an oncogenic or tumor suppressing factor in breast cancer is still unclear. Expression of this gene was found in 10-30% of breast cancer, and it showed increased expression in ER-positive tumors [43]. WT1 cytoplasmic protein expression was detected in 48.5%, and was found to be more prevalent in ER-positive tumors [44]. Nuclear staining was much less expressed in up to 7% [45]. WT1 expression was reported to be associated with both improved [46] and worse outcomes [47]. In a recent study by Artibani [43], it was concluded that WT1 played a role in regulating the epithelial-mesenchymal balance of breast cancer cells, and that WT1-expressing tumors were mainly associated with a mesenchymal phenotype. WT1 expression was correlated with CYP3A4 levels, and associated with poorer response to taxane treatment [43]. In our study, which was limited to TN, cytoplasmic expression of WT1 was found in 55.2% of all patients of the cohort, predominantly in TN and lumB. Less expression of both WT1 and vimentin was found in HER2+. This may be explained by WT1’s role in epithelial-mesenchymal transition. However, we could not find any correlation between cytoplasmic expression and the outcome in any subgroup. For the nuclear staining, it was found in only 4 TNB and all died of their disease. Although the number of positive cases was small, all had poor OS. They had negative p16 and negative luminal CK which were associated with poor outcomes according to our study.
Effects of treatment on survival
Due to the small number of patients who underwent breast-conserving treatment (37 TNB and 2 TNN), comparison between these two subtypes could not be confidently performed. Our study confirmed the poorer prognosis of TNN over TNB or lumB in patients who underwent total mastectomy. There was no significant difference in DFS or OS between TNB and lumB. According to the European Society for Medical Oncology (ESMO), cytotoxic chemotherapy is the standard treatment for TN, and the choice of regimen should be determined after consideration of several disease-related factors (histology, biomarkers, staging, previous therapies, and response) and patient-related factors (patient preference, biological age, menopausal status, comorbidities, and socioeconomic and psychological factors) [48, 49]. Cytotoxic chemotherapy, especially taxane and anthracycline-based combination chemotherapy, has been the standard treatment for TN [48, 50]. In our study, 81.6% of TNB and 78.6% of TNN received these chemotherapy regimens. TN patients and overall patients in our cohort who received chemotherapy had significantly increased DFS, but not OS. The patients in our cohort received standard treatment and the result of the outcome could be comparable to others.
Patients who received radiotherapy had significantly lower DFS and OS. This might be due to radiotherapy administration in more advanced loco-regional disease. TN breast cancer patients who underwent breast-conserving surgery and who received radiotherapy had significantly increased DFS. Multivariate analysis revealed that patients who received radiotherapy had borderline significantly increased DFS, but not OS. This finding supports the role of radiotherapy as local control for TN subtype breast cancer.
In conclusion, among TN breast cancers, the incidence of TNN in Thai patients was 12.3%, which is much lower than that reported from Western countries, and the prognosis was significantly poorer when compared with TNB. Tumor size and axillary nodal involvement were the independent predictors for DFS and OS. Among the biomarkers studied, absence of EGFR expression was an independent factor for lower DFS and OS in TN, and in combined TN and lumB subtype. Absence of either CK8/18 or p16 expression was an independent factor for decreased DFS of the whole cohort, and for decreased OS in the combined TN and lumB group.
Acknowledgments
The authors gratefully acknowledge Chomkwan Atthapoch, Rapeephan Cholkate, and Jiranun Sangsakul for preparation of the tissue microarray; Chomkwan Atthapoch for slide sectioning; Siwaporn Klamkhlai for immunohistochemistry staining; Tiranan Talalag and Phensri Niamyim for manuscript preparation; and, Suthipol Udompunthurak of the Research Group and Research Network Division, Research Department, Faculty of Medicine Siriraj Hospital, Mahidol University for his assistance with some aspects of the statistical analysis.
Conflict of interest declaration
All authors declare no personal or professional conflicts of interest, and no financial support from the companies that produce and/or distribute the drugs, devices, or materials described in this report.
Funding disclosure
This study was supported by a Siriraj Grant for Research Development from the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (grant no. COA no. R635/2553(EC3).
References
- Molecular portraits of human breast tumours Perou C. M., Sørlie T., Eisen M. B., Rijn M., Jeffrey S. S., Rees C. A., Pollack J. R., et al . Nature.2000;406(6797). CrossRef
- Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications Sørlie T., Perou C. M., Tibshirani R., Aas T., Geisler S., Johnsen H., Hastie T., et al . Proceedings of the National Academy of Sciences of the United States of America.2001;98(19). CrossRef
- Breast cancer classification and prognosis based on gene expression profiles from a population-based study Sotiriou C, Neo SY, McShane LM , Korn EL , Long PM , Jazaeri A, Martiat P, et al . Proceedings of the National Academy of Sciences of the United States of America.2003;100(18). CrossRef
- Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011 Goldhirsch A., Wood W. C., Coates A. S., Gelber R. D., Thürlimann B., Senn H.-J.. Annals of Oncology: Official Journal of the European Society for Medical Oncology.2011;22(8). CrossRef
- Clinicopathologic features of breast carcinomas classified by biomarkers and correlation with microvessel density and VEGF expression: a study from Thailand Chuangsuwanich T, Pongpruttipan T, O-Charoenrat P, Komoltri C, Watcharahirun S, Sa-Nguanraksa D. Asian Pacific journal of cancer prevention: APJCP.2014;15(3). CrossRef
- Vimentin as a poor prognostic factor for triple-negative breast cancer Yamashita N, Tokunaga E, Kitao H, Hisamatsu Y, Taketani K, Akiyoshi S, Okada S, et al . Journal of Cancer Research and Clinical Oncology.2013;139(5). CrossRef
- P53 and Ki-67 as prognostic markers in triple-negative breast cancer patients Pan Y, Yuan Y, Liu G, Wei Y. PloS One.2017;12(2). CrossRef
- American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer Hammond MEH , Hayes DF , Dowsett M, Allred DC , Hagerty KL , Badve S, Fitzgibbons PL , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2010;28(16). CrossRef
- Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update Wolff AC , Hammond MEH , Hicks DG , Dowsett M, McShane LM , Allison KH , Allred DC , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2013;31(31). CrossRef
- Breast cancer subtypes and the risk of local and regional relapse Voduc KD , Cheang MCU , Tyldesley S, Gelmon K, Nielsen TO , Kennecke H. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2010;28(10). CrossRef
- A survey of immunohistochemical biomarkers for basal-like breast cancer against a gene expression profile gold standard Won JR , Gao D, Chow C, Cheng J, Lau SYH , Ellis MJ , Perou CM , Bernard PS , Nielsen TO . Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2013;26(11). CrossRef
- Prognostic Evaluation of Epidermal Growth Factor Receptor (EGFR) Genotype and Phenotype Parameters in Triple-negative Breast Cancers Levva S, Kotoula V, Kostopoulos I, Manosou K, Papadimitriou C, Paradopoulou K, Lakis S, et al . Cancer Genomics & Proteomics.2017;14(3). CrossRef
- Calretinin expression in high-grade invasive ductal carcinoma of the breast is associated with basal-like subtype and unfavorable prognosis Taliano RJ , Lu S, Singh K, Mangray S, Tavares R, Noble L, Resnick MB , Yakirevich E. Human Pathology.2013;44(12). CrossRef
- Response and survival of breast cancer intrinsic subtypes following multi-agent neoadjuvant chemotherapy Prat A, Fan C, Fernández A, Hoadley KA , Martinello R, Vidal M, Viladot M, et al . BMC medicine.2015;13. CrossRef
- Triple-negative, basal-like, and quintuple-negative breast cancers: better prediction model for survival Choi YL, Oh E, Park S, Kim Y, Park Y, Song K, Cho EY , et al . BMC cancer.2010;10. CrossRef
- Use of immunohistochemical markers can refine prognosis in triple negative breast cancer Tischkowitz M, Brunet JS, Bégin LR , Huntsman DG , Cheang MCU , Akslen LA , Nielsen TO , Foulkes WD . BMC cancer.2007;7. CrossRef
- Triple-negative breast cancer: distinguishing between basal and nonbasal subtypes Rakha EA , Elsheikh SE , Aleskandarany MA , Habashi HO , Green AR , Powe DG , El-Sayed ME , et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2009;15(7). CrossRef
- Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies Lehmann BD , Bauer JA , Chen X, Sanders ME , Chakravarthy AB , Shyr Y, Pietenpol JA . The Journal of Clinical Investigation.2011;121(7). CrossRef
- Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma Nielsen TO , Hsu FD , Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2004;10(16). CrossRef
- Invasive ductal carcinoma of the breast with the "triple-negative" phenotype: prognostic implications of EGFR immunoreactivity Viale G, Rotmensz N, Maisonneuve P, Bottiglieri L, Montagna E, Luini A, Veronesi P, et al . Breast Cancer Research and Treatment.2009;116(2). CrossRef
- BCL2 is an independent predictor of outcome in basal-like triple-negative breast cancers treated with adjuvant anthracycline-based chemotherapy Bouchalova K, Svoboda M, Kharaishvili G, Vrbkova J, Bouchal J, Trojanec R, Koudelakova V, et al . Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine.2015;36(6). CrossRef
- Basal cytokeratin and epidermal growth factor receptor expression are not predictive of BRCA1 mutation status in women with triple-negative breast cancers Collins LC , Martyniak A, Kandel MJ , Stadler ZK , Masciari S, Miron A, Richardson AL , Schnitt SJ , Garber JE . The American Journal of Surgical Pathology.2009;33(7). CrossRef
- Androgen receptor, EGFR, and BRCA1 as biomarkers in triple-negative breast cancer: a meta-analysis Zhang L, Fang C, Xu X, Li A, Cai Q, Long X. BioMed Research International.2015;2015. CrossRef
- Down-regulated expression of cytokeratin 18 promotes progression of human breast cancer Woelfle U, Sauter G, Santjer S, Brakenhoff R, Pantel K. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2004;10(8). CrossRef
- Cytokeratin and vimentin expression in breast cancer Vora HH , Patel NA , Rajvik KN , Mehta SV , Brahmbhatt BV , Shah MJ , Shukla SN , Shah PM . The International Journal of Biological Markers.2009;24(1). CrossRef
- Relationship of CK8/18 expression pattern to breast cancer immunohistochemical subtyping in Egyptian patients Aiad HA , Samaka RM , Asaad NY , Kandil MA , Shehata MA , Miligy IM . Ecancermedicalscience.2014;8. CrossRef
- The Bcl-2 family: roles in cell survival and oncogenesis Cory S, Huang DCS , Adams JM . Oncogene.2003;22(53). CrossRef
- The Role of BCL2 Family of Apoptosis Regulator Proteins in Acute and Chronic Leukemias Tzifi F, Economopoulou C, Gourgiotis D, Ardavanis A, Papageorgiou S, Scorilas A. Advances in Hematology.2012;2012. CrossRef
- Bcl-2 is a highly significant prognostic marker of hormone-receptor-positive, human epidermal growth factor receptor-2-negative breast cancer Seong MK, Lee JY, Byeon J, Sohn YJ, Seol H, Lee JK, Kim EK, Kim HA, Noh WC . Breast Cancer Research and Treatment.2015;150(1). CrossRef
- Prognostic Influence of BCL2 on Molecular Subtypes of Breast Cancer Hwang K, Han W, Kim J, Moon H, Oh S, Song YS , Kim YA , Chang MS , Noh D. Journal of Breast Cancer.2017;20(1). CrossRef
- Apoptosis-, proliferation, immune function-, and drug resistance- related genes in ER positive, HER2 positive and triple negative breast cancer Kolacinska A., Chalubinska J., Zawlik I., Szymanska B., Borowska-Garganisz E., Nowik M., Fendler W., et al . Neoplasma.2012;59(4). CrossRef
- Prognostic significance of Bcl-2 expression in non-basal triple-negative breast cancer patients treated with anthracycline-based chemotherapy Choi JE , Kang SH , Lee SJ , Bae YK . Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine.2014;35(12). CrossRef
- Loss of p16 expression is associated with the stem cell characteristics of surface markers and therapeutic resistance in estrogen receptor-negative breast cancer Arima Y, Hayashi N, Hayashi H, Sasaki M, Kai K, Sugihara E, Abe E, et al . International Journal of Cancer.2012;130(11). CrossRef
- P16 but not retinoblastoma expression is related to clinical outcome in no-special-type triple-negative breast carcinomas Bogina GS , Lunardi G, Marcolini L, Brunelli M, Bortesi L, Marconi M, Coati F, et al . Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2014;27(2). CrossRef
- Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle Musgrove E. A., Lee C. S., Buckley M. F., Sutherland R. L.. Proceedings of the National Academy of Sciences of the United States of America.1994;91(17). CrossRef
- Cyclin D1 in breast cancer pathogenesis Arnold A, Papanikolaou A. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2005;23(18). CrossRef
- Prognostic significance of cyclin D1 protein expression and gene amplification in invasive breast carcinoma Ortiz AB , Garcia D, Vicente Y, Palka M, Bellas C, Martin P. PloS One.2017;12(11). CrossRef
- Positive expression of cyclin D1 is an indicator for the evaluation of the prognosis of breast cancer Guo L, Liu S, Jakulin A, Yilamu D, Wang B, Yan J. International Journal of Clinical and Experimental Medicine.2015;8(10).
- High expression of cyclin D1 is associated to high proliferation rate and increased risk of mortality in women with ER-positive but not in ER-negative breast cancers Ahlin C, Lundgren C, Embretsén-Varro E, Jirström K, Blomqvist C, Fjällskog M. Breast Cancer Research and Treatment.2017;164(3). CrossRef
- Overexpression of cyclinD1 predicts for poor prognosis in estrogen receptor-negative breast cancer patients Umekita Y, Ohi Y, Sagara Y, Yoshida H. International Journal of Cancer.2002;98(3). CrossRef
- Cyclin D1 in invasive breast carcinoma: favourable prognostic significance in unselected patients and within subgroups with an aggressive phenotype Mylona E, Tzelepis K, Theohari I, Giannopoulou I, Papadimitriou C, Nakopoulou L. Histopathology.2013;62(3). CrossRef
- High Wilms' tumor 1 mRNA expression correlates with basal-like and ERBB2 molecular subtypes and poor prognosis of breast cancer Qi X, Zhang F, Yang X, Fan L, Zhang Y, Liang Y, Ren L, et al . Oncology Reports.2012;28(4). CrossRef
- WT1 expression in breast cancer disrupts the epithelial/mesenchymal balance of tumour cells and correlates with the metabolic response to docetaxel Artibani M, Sims AH , Slight J, Aitken S, Thornburn A, Muir M, Brunton VG , et al . Scientific Reports.2017;7. CrossRef
- WT1, estrogen receptor, and progesterone receptor as markers for breast or ovarian primary sites in metastatic adenocarcinoma to body fluids Lee BH , Hecht JL , Pinkus JL , Pinkus GS . American Journal of Clinical Pathology.2002;117(5). CrossRef
- Prognostic significance of RSPO1, WNT1, P16, WT1, and SDC1 expressions in invasive ductal carcinoma of the breast Choi EJ , Yun JS , Jeon EK , Won HS , Ko YH , Kim SY . World Journal of Surgical Oncology.2013;11. CrossRef
- High expression of Wilms' tumor suppressor gene predicts poor prognosis in breast cancer patients Miyoshi Y, Ando A, Egawa C, Taguchi T, Tamaki Y, Tamaki H, Sugiyama H, Noguchi S. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2002;8(5).
- The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer Cardoso F, Loibl S, Pagani O, Graziottin A, Panizza P, Martincich L, Gentilini O, et al . European Journal of Cancer (Oxford, England: 1990).2012;48(18). CrossRef
- 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)† Cardoso F., Senkus E., Costa A., Papadopoulos E., Aapro M., André F., Harbeck N., et al . Annals of Oncology: Official Journal of the European Society for Medical Oncology.2018;29(8). CrossRef
- Triple-negative breast cancer Foulkes WD , Smith IE , Reis-Filho JS . The New England Journal of Medicine.2010;363(20). CrossRef
- Prognostic significance of Wilms Tumor 1 (WT1) protein expression in breast cancer Celaletdin C, Kalender ME , Paydas S, et al . Gaziantep Med J.2011;17:67-72. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2024
Author Details